Drake G.W.F. (editor) Handbook of Atomic, Molecular, and Optical Physics
Подождите немного. Документ загружается.

LV
List of Abbreviations
2P/2H two-particle/two-hole
A
AA average atom
ACT activated complex theory
ADDS angular distribution by Doppler
spectroscopy
ADO average dipole orientation
AES Auger electron spectroscopy
AI adiabatic ionization
AL absorption loss
ALS advanced light source
AMO atomic, molecular, and optical
ANDC arbitrarily normalized decay curve
AO atomic orbital
AOM acoustooptic modulator
AS active space
ASD atomic spectra database
ASF atomic state functions
ATI above threshold ionization
AU absorbance units
B
BEA binary encounter approximation
BEC Bose–Einstein condensate
(or condensation)
BF body-fixed
BI Bell’s inequality
BL Bethe log
BO Born–Oppenheimer
BS Bethe–Salpeter
BW Brillouin–Wigner
C
CARS coherent anti-Stokes Raman scattering
CAS complete active space
CASPT complete active space perturbation
theory
CAUGA Clifford algebra unitary group approach
CC coupled cluster
CCA coupled cluster approximation
CCC convergent close coupling
CCD coupled cluster doubles
CCO coupled-channels optical
CDW continuum distorted wave
CEAS cavity enhanced absorption spectroscopy
CES cavity enhanced spectroscopy
CES constant energy surface
CETS cavity enhanced transmission
spectroscopy
CFCP free–free molecular Franck–Condon
CG Clebsch–Gordan
CH Clauser–Horne
CI configuration interaction
CIS constant ionic state
CL constant log
CM center-of-mass
CMA cylindrical mirror analyzer
COA classical oscillator approximation
CODATA Committee on Data for Science and
Technology
COIL chemical-oxygen-iodine
COLTRIMS cold-target recoil-ion momentum
spectroscopy
CP central potential
CPA chirped-pulsed-amplification
CQC classical-quantal coupling
CRDS cavity ring-down spectroscopy
CSDA continuous slowing down approximation
CSF configurational state functions
CTF common translation factor
CTMC classical trajectory Monte Carlo
CW continuous wave
CW-CRDS continuous-Wave Cavity Ring-Down
Spectroscopy
CX charge exchange
CXO Chandra X-ray Observatory
D
DB detailed balance
DCS differential cross sections
DDCS doubly differential cross sections
DF Dirac–Fock
DFB distributed feedback
DFS decoherence free subspace
DFT discrete Fourier transform
DFWM degenerate four wave mixing
DLR dielectronic recombination
DODS different orbitals for different spins
DR dielectronic recombination
DSPB distorted wave strong potential Born
approximation
E
EA excitation-autoionization
EBIT electron beam ion traps
EBS eikonal Born series
LVI List of Abbreviations
ECP effective core potential
ECS exterior complex scaling
EEDF electron energy distribution functions
EOM equation of motion
EPR Einstein–Podolsky–Rosen
ESM elastic scattering model
ESR experimental storage ring
EUV extreme ultraviolet
EW-CRDS evanescent-wave CRDS
EXAFS extended X-ray absorption fine structure
F
FBA first Born approximation
FCPC full-core plus correlation
FEL free-electron lasers
FFT fast Fourier transform
FID free induction decay
FIR far-infrared
FM frequency modulation
FOTOS first-order theory for oscillator strengths
FS fine-structure
FT Fourier transform
FTIR Fourier transform infrared spectroscopy
FTMS Fourier transform mass spectrometry
FTS Fourier transform spectroscopy
FUSE far ultraviolet spectroscopic explorer
FUV far ultraviolet
FVP Fermi virtual photon
FWHM full width at half maximum
G
GBT generalized Brillouin’s Theorem
GFA Green’s function approach
GGA generalized gradient approximation
GHZ Greenberger, Horne, Zeilinger
GI gauge invariant
GIB guided ion beam
GOME global ozone monitoring experiment
GOS generalized oscillator strength
GPE Gross–Pitaevskii equation
GRPAE generalized random phase
approximationwith exchange
H
HEDP high energy-density physics
HF Hartree–Fock equations
HF Hellman–Feynman
HG harmonic generation
HOM Hong–Ou–Mandel
HREELS high resolution electron energy loss
spectroscopy
HRTOF H-atom Rydberg time-of-flight
HUM Hylleraas–Undheim–MacDonald
theorem
I
IC intermediate coupling
ICF inertial confinement fusion
ICOS integrated cavity output spectroscopy
ICSLS international conference on spectral line
shapes
IERM intermediate energy R-matrix
IPCC intergovernmental panel on climate
change
IPES inverse photoemission spectroscopy
IPIR independent-processes and
isolated-resonance
IPM independent particle model
IPP impact parameter picture
IR irreducible representations
IR infrared
IRI international reference ionosphere
IRREP irreducable representation
ISO infrared space observatory
J
JB Jeffrey–Born
K
KS Kohn–Sham
KTA potassium titanyl arsenate
KTP potassium titanyl phosphate
L
LieA Lie algebras
LA linear algebraic
L-CETS locked cavity enhanced transmission
spectroscopy
LDA local density approximation
LEED low energy electron diffraction
LER laser electric resonance
LG Lie groups
LHC left-hand circular
LHV local hidden variable
LIF laser-induced-fluorescence
LIGO laser interferometer gravitational-wave
observatory
LISA laser interferometer space antenna
LL Landau–Lifshitz
LM Levenberg–Marquardt
LMR laser magnetic resonance
LPT laser photodetachment threshold
LRL Laplace–Runge–Lenz
LTE local thermodynamic
equilibrium
LYP Lee, Yang, and Parr
LZ Landau–Zener
List of Abbreviations LVII
M
MBE molecular beam epitaxy
MCP microchannel plate
MDAL minimum detectable absorption loss
MBPT many-body perturbation theory
MCDHF multiconfigurational Dirac–Hartree–Fock
MCHF multiconfiguration Hartree–Fock
MCSCF multiconfigurational self-consistent field
MEMS microelectromechanical systems
MFP mean free path
MIGO matter–wave interferometric
gravitational-wave observatory
MIM metal-insulator-metal
MKSA meters, kilograms, seconds, and amperes
MM Massey–Mohr
MMCDF multichannel multiconfiguration
Dirac–Fock
MO molecular orbital
MOPA master oscillator power amplifier
MOT magneto-optical trap
MOX molecular orbital X-radiation
MP2 second order Møller–Plesset perturbation
theory
MP3 third order Møller–Plesset perturbation
theory
MPI multiphoton ionization
MQDT multichannel quantum defect theory
MR multireference
MR-SDCI multireference singles/doubles
configuration interaction
MUV middle ultraviolet
N
NAR nonadiabatic region
NEP noise-equivalent power
NEXAFS near-edge X-ray absorption fine structure
NDIR non-dispersive infrared
NFS nonfine-structure
NICE-OHMS noise-immune, cavity-enhanced optical
heterodyne molecular spectroscopy
NIM normal incidence monochromator
NIST National Institute of Standards and
Technology
NMR nuclear magnetic resonance systems
NNS nearest-neighbor energy level spacings
NR nonrelativistic
NRQED NR quantum electrodynamics
O
OAO-2 orbiting astronomical observatory
OB ordinary Bremsstrahlung
OBE optical Bloch equations
OBK Oppenheimer–Brinkman–Kramers
OCP one-component plasma
OHCE one-and-a-half centered expansion
OMI ozone monitoring instrument
OPO optical parametric oscillator
P
P-CRDS pulsed-cavity ringdown spectroscopy
PADDS angular distribution by Doppler
spectroscopy
PAH polycyclic aromatic hydrocarbon
PBS polarizing beam splitters
PCDW projectile continuum distorted wave
approximation
PDM phase diffusion model
PEC potential energy curves
PES photoelectron spectroscopy
PES potential energy surface
PH/HP particle–hole/hole–particle
PI photoionization
PID particle identification
PIMC path-integral Monte Carlo
PMT photomultiplier tubes
PNC parity nonconservation
PPT positive partial transposes
PR polarization radiation
PSD postion senitive detectors
PSS perturbed stationary state
PT perturbation theory
PWBA plane wave Born approximation
PZT piezo-electric transducer
Q
QCD quantum chromodynamics
QED quantum electrodynamics
QIP quantum information processing
QKD quantum key distribution
QMC quantum Monte Carlo
QND quantum nondemolition
QS quasistatic
QSS quasi-steady state
R
RATIP relativistic atomic transition and
ionization properties
RDC ring-down cavity
READI resonant excitation auto-double ionization
REC radiative electron capture
REDA resonant excitation double autoionization
REMPI resonance-enhanced multiphoton
ionization
RES rotational energy surface
RHC right-hand circular
RHIC relativistic heavy ion collider
LVIII List of Abbreviations
RIMS recoil-ion momentum spectroscopy
RMI relativistic mass increase
RMPS R-matrix with pseudostates
RNA Raman–Nath approximation
RPA random-phase approximation
RPA retarding potential analyzer
RPAE random phase approximation with
exchange
RR radiative recombination
RRKM Rampsberger–Rice–Karplus–Marcus
RSE radial Schrödinger equation
RSPT Rayleigh–Schrödinger perturbation
theory
RT Ramsauer–Townsend
RTE resonant transfer and excitation
RWA rotating wave approximation
S
SA-MCSCF state averaged multiconfiguration
self-consistent field
SACM statistical adiabatic channel model
SBS stimulated Brillouin scattering
SCA semiclassical approximation
SCF self-consistent field
SCIAMACHY scanning imaging absorption
spectrometer for atmospheric
chartography
SD spin-dependent
SD single and double
SDS singly differential cross section
SDTQ single, double, triple, quadruple
SE Schrödinger equation
SEP stimulated emission pump
SEPE simultaneous electron photon
excitation
SEXAFS surface extended X-ray absorption fine
structure
SF space-fixed
SI spin-independent
SIAM Society for Industrial and Applied
Mathematics
SM submillimeter
SM-FIR submillimeter far-infrared
SMS specific mass shift
SOHO solar and heliospheric observatory
SP stationary phase
SPA stationary phase approximations
SQL standard quantum limit
SQUID superconducting quantum interference
detector
SR synchrotron radiation
SRS stimulated Raman scattering
SS strong-short
STIRAP stimulated Raman adiabatic passage
STO Slater type orbital
STP standard temperature and pressure
T
TCDW target continuum distorted wave
TDCS triply differential cross section
TDHF time-dependent Hartree–Fock
TDS thermal desorption spectroscopy
TDSE time dependent Schrödinger equation
TEA transverse-excitation-atmospheric-
pressure
TF toroidal field
TOF time-of-flight
TOP time orbiting potential
TPA two-photon absorption
TSR test storage ring
TuFIR tunable far-infrared
U
UGA unitary group approach
UHF unrestricted Hartree–Fock
UPS ultraviolet photoelectron spectroscopy
UV ultraviolet
UV-VIS ultraviolet-visible
V
VASP Vienna ab-initio simulation package
VCSEL vertical-cavity surface-emitting laser
VECSEL vertical external cavity surface-emitting
laser
VES vibrational energy surfaces
VUV vacuum ultraviolet
W
WCG Wigner–Clebsch–Gordan
WDM warm dense matter
WKB Wentzel, Kramers, Brillouin
WL weak-long
WMAP Wilkinson microwave anisotropy
probe
WPMD wavepacket molecular dynamics
X
XPS X-ray photoelectron spectroscopy
Y
YAG Yttrium Aluminum Garnet
1
Units and Con
1. Units and Constants
The currently accepted values for the physi-
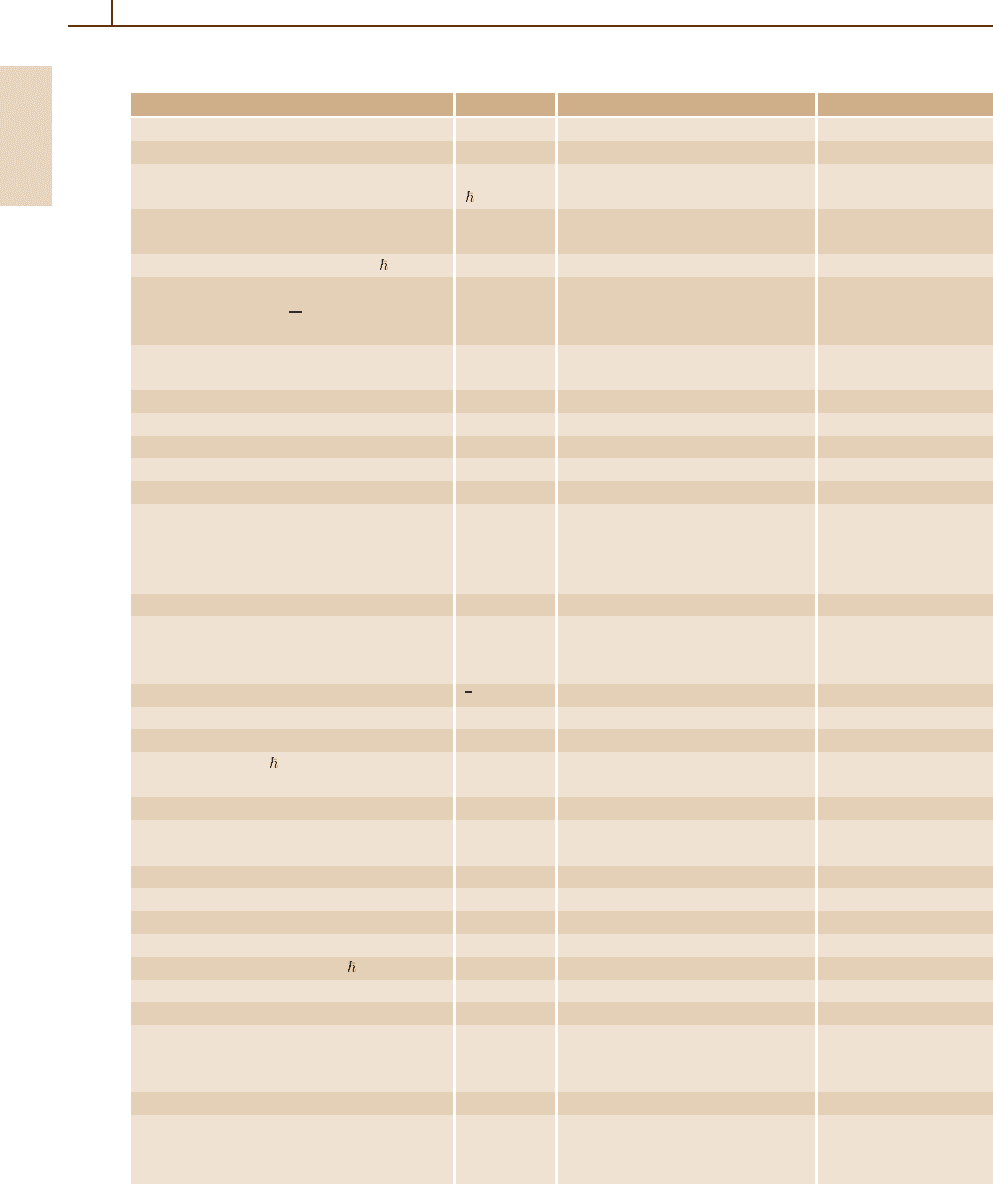
cal constants are listed in Table 1.1,basedon
the 2002 CODATA (Committee on Data for Sci-
ence and Technology) recommendations [1.1].
The quoted values are based on all data avail-
able through 31 December 2002, and replace
the earlier 1998 CODATA set. Because the uncer-
tainties are correlated, the correlation matrix,
given in Table 1.2, must be used in calculating
1.1 Electromagnetic Units .......................... 1
1.2 Atomic Units ....................................... 5
1.3 Mathematical Constants ....................... 5
1.3.1 Series Summation Formula ......... 5
References .................................................. 6
uncertainties for any quantities derived from those
tabulated [1.1].
1.1 Electromagnetic Units
The standard electromagnetic units adopted by most
scientific journals and elementary texts belong to the
système international (SI) or rationalized MKSA (me-
ters, kilograms, seconds, and amperes) units. However,
many authors working with microscopic phenomena
prefer Gaussian units, and theoretical physicists often
use Heaviside–Lorentz (H–L) units. In this Handbook,
SI units are used together with atomic units. The current
section is meant as a reference relating these different
systems.
The relations among different sets of units are not
simple conversions since the same symbol in differ-
ent systems can have different physical dimensions. To
clarify the meanings of the units, we summarize ba-
sic electromagnetic relations for SI, Gaussian, and H–L
systems below.
The Coulomb law for the magnitude F of the force
acting on each of two static charges q and Q separated by
a distance r in a homogeneous medium of permittivity
can be written as
F =
1
4π
qQ
r
2
, (1.1)
where in a vacuum, is
0
=
µ
0
c
2
−1
, SI
(4π)
−1
, Gaussian
1, H–L
,
(1.2)
with the closely related permeability of vacuum given
by
µ
0
=
4π ×10
−7
N/A
2
, SI
4π, Gaussian
1, H–L
.
(1.3)
(We deviate here from Jackson [1.2]whotakes
0
=
µ
0
= 1 in Gaussian units and must introduce additional
constants to relate the units. The physically important
quantities are the relative values
r
≡ /
0
and µ
r
≡
µ/µ
0
, which in traditional Gaussian-unit notation are
written without the r subscript.)
Note that
0
and µ
0
are dimensionless in H–L and
Gaussian units, but not in the SI units. Current or electric
charge is an independent quantity in the MKSA system
but can be expressed in purely mechanical dimensions in
the H–L and Gaussian systems. Thus, in Gaussian units,
1 statcoulomb = 1 dyne
(1/2)
cm, but in SI, even though
theampereisdefined in terms of the attractive force be-
tween thin parallel wires carrying equal currents, there is
no mechanical equivalent for the ampere or the coulomb.
To establish such an equivalence, one can supplement
the SI units by assigning a dimensionless number to
0
or to µ
0
. Gaussian and H–L units arise from two differ-
ent assignments. The result of assigning a number to
0
is analogous to the relation 1 s =
˙
3×10
8
m established
between time and distance units if one sets the speed of
light c =1, a convention often used in conjunction with
H–L units. (Note that for simplicity, the pure number
Introduction
2 Part
Table 1.1 Table of physical constants. Uncertainties are given in parentheses
Quantity Symbol Value Units
Speed of light in vacuum c 2.997 924 58 10
8
ms
−1
Gravitational constant G 6.6742(10) 10
−11
m
3
kg
−1
s
−2
Planck constant h 6.626 0693(11) 10
−34
Js
= h/2π 1.054 571 68(18) 10
−34
Js
Elementary charge e 1.602 176 53(14) 10
−19
C
4.803 204 40(42) 10
−10
esu
Inverse fine structure constant [4π
0
] c/e
2
α
−1
137.035 999 11(46)
Magnetic flux quantum h/2e Φ
0
2.067 833 72(18) 10
−15
Wb
Atomic mass constant
1
12
m
12
C
= 1u m
u
1.660 538 86(28) 10
−27
kg
m
u
c
2
931.494 043(80) MeV
Electron mass m
e
9.109 3826(16) 10
−31
kg
5.485 799 0945(24) 10
−4
u
Muon mass m
µ
0.113 428 9264(30) u
Proton mass m
p
1.007 276 466 88(13) u
Neutron mass m
n
1.008 664 915 60(55) u
Deuteron mass m
d
2.013 553 212 70(35) u
α-particle mass m
α
4.001 506 179 149(56) u
Rydberg constant m
e
cα
2
2h R
∞
1.097 373 156 8525(73) 10
7
m
−1
R
∞
c 3.289 841 960 360(22) 10
15
Hz
R
∞
hc 13.605 692 3(12) eV
2.179 872 09(37) 10
−18
J
Bohr radius α/4πR
∞
a
0
0.529 177 2108(18) 10
−10
m
Hartree energy e
2
/[4π
0
]a
0
= 2R
∞
hc E
h
27.211 3845(23) eV
E
h
/h 6.579 683 920 721(44) 10
15
Hz
E
h
/hc 2.194 746 313 705(15) 10
7
m
−1
Compton wavelength αa
0
λ
C
= λ
C
/2π 3.861 592 678(26) 10
−13
m
Classical electron radius α
2
a
0
r
e
2.817 940 325(28) 10
−15
m
Thomson cross section 8πr
2
e
/3 σ
e
0.665 245 873(13) 10
−28
m
2
Bohr magneton [c]e /2m
e
c µ
B
9.274 009 49(80) 10
−24
JT
−1
5.788 381 804(39) 10
−5
eV T
−1
Electron magnetic moment µ
e
/µ
B
−1.001 159 652 1859(38)
Muon magnetic moment µ
µ
/µ
B
−4.841 970 45(13) 10
−3
Proton magnetic moment µ
p
/µ
B
1.521 032 206(15) 10
−3
Neutron magnetic moment µ
n
/µ
B
−1.041 875 63(25) 10
−3
Deuteron magnetic moment µ
d
/µ
B
0.466 975 4567(50) 10
−3
Electron g factor −2(1 +a
e
) g
e
−2.002 319 304 3718(75)
Muon g factor −2(1 +a
µ
) g
µ
−2.002 331 8396(12)
Proton gyromagnetic ratio 2µ
p
/ γ
p
2.675 222 05(23) 10
8
s
−1
T
−1
Avogadro constant N
A
6.022 1415(10) 10
23
mol
−1
Faraday constant N
A
e F 9.648 533 83(83) 10
4
Cmol
−1
Boltzmann constant R/N
A
k
B
1.380 6505(24) 10
−23
JK
−1
8.617 343(15) 10
−5
eV K
−1
k
B
/E
h
3.166 8153(55) 10
−6
K
−1
Molar gas constant R 8.314 472(15) Jmol
−1
K
−1
Molar volume (ideal gas) RT/P
T = 273.15 K, P =101.325 kPa V
m
0.022 413 996(39) m
3
mol
−1
T = 273.15 K, P =100 kPa V
m
0.022 710 981(40) m
3
mol
−1
Introduction
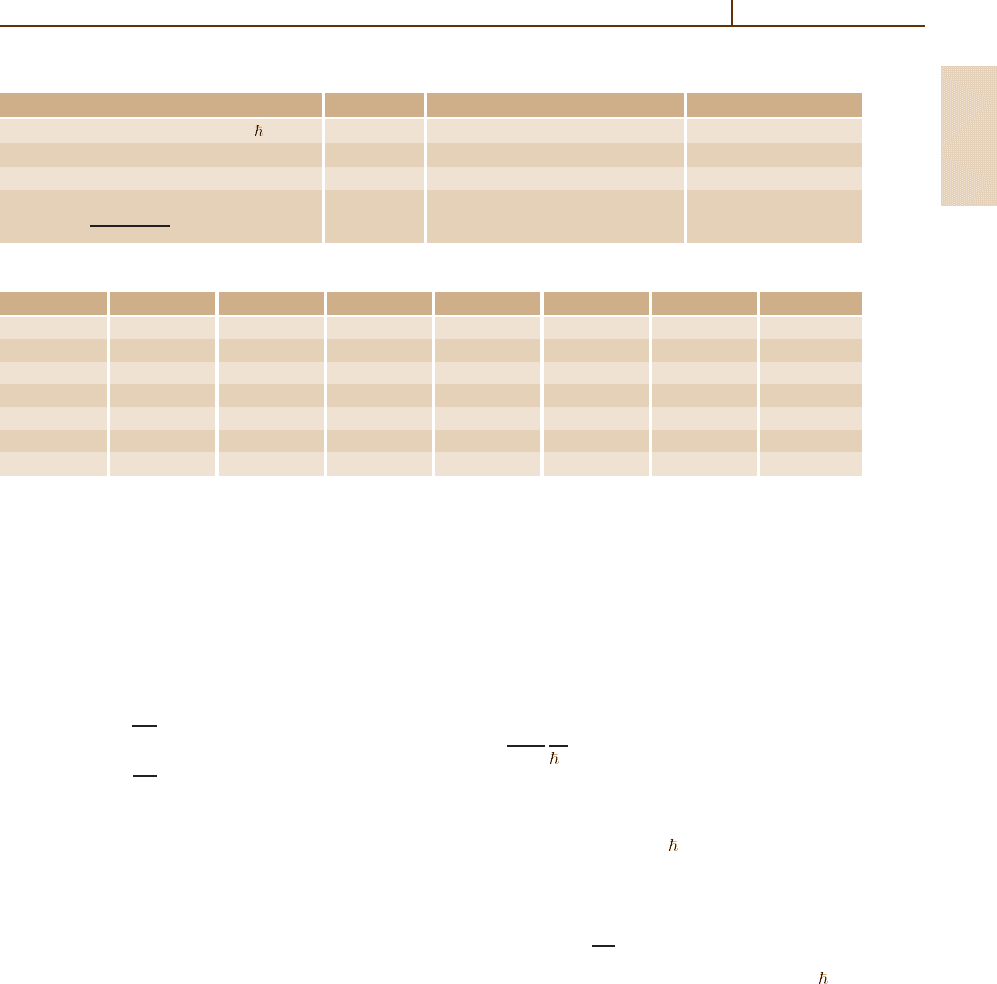
Units and Constants 1.1 Electromagnetic Units 3
Table 1.1 Table of physical constants. Uncertainties are given in parentheses, cont.
Quantity Symbol Value Units
Stefan–Boltzmann constant π
2
k
4
B
/(60
3
c
2
) σ 5.670 400(40) 10
−8
Wm
−2
K
−4
First radiation constant 2πhc
2
c
1
3.741 771 38(64) 10
−16
Wm
2
Second radiation constant hc/k
B
c
2
0.014 387 752(25) mK
Wien displacement law constant b 2.897 7685(51) 10
−3
mK
λ
max
T =
c
2
4.965 114 231...
Table 1.2 The correlation coefficients of a selected group of constants based on the 2002 CODATA [1.1]
α h e m
e
N
A
m
e
/m
p
F
α − − − − − − −
h 0.010 − − − − − −
e 0.029 1.000 − − − − −
m
e
−0.029 0.999 0.998 − − − −
N
A
0.029 −0.999 −0.998 −1.000 − − −
m
e
/m
p
−0.249 −0.002 −0.007 0.007 −0.007 − −
F 0.087 −0.995 −0.993 −0.998 0.998 −0.022 −
2.997 924 58, equal numerically to the defined speed of
light in vacuum in units of 10
8
m/s, is represented by
˙
3.)
Thus, although within the Gaussian system, where the
assignment 4π
0
= 1 is made, it is justified to assert that
1 coulomb equals
˙
3×10
9
statcoulombs, this is not true
in pure SI, where there is no equivalent mechanical unit
for charge.
Maxwell’s macroscopic equations can be written as
λ∇·D = ρ,
λc
∇× H−λ
∂ D
∂t
= j ,
c
∇× E+
∂ B
∂t
= 0 ,
∇·B = 0 ,
(1.4)
with the macroscopic field variables related to the polar-
izations P and M by
λD =
0
E+ P = E
λH =µ
−1
0
B− M = B/µ (1.5)
(the last equalities for D and H hold only for homoge-
neous media) and
λ =
1, SI
0
= µ
−1
0
, Gaussian or H–L ,
(1.6)
c
=
1, SI
c, Gaussian or H–L .
(1.7)
In Gaussian or H–L units, the fields E, B, D, H,
and polarizations (dipole moments per unit volume)
P, M all have the same dimensions, whereas in SI
units the microscopic fields E and B have dimensions
that are generally distinct from each other as well as
from P (or D)andM (or H), respectively. In all three
unit systems, the dimensionless ratio /
0
is called
the dielectric constant (or relative permittivity) of the
medium, and the (dimensionless) fine-structure constant
is
α =
1
4π
0
e
2
c
,
(1.8)
with a numerical value α
−1
= 137.035 999 11 (46).
In atomic units (Sect. 1.3), the factor e
2
/
(
4π
0
)
,
the electron mass m
e
,and , Planck’s constant di-
vided by 2π, are all equal to 1. In Gaussian and
H–L systems, these conditions determine a numerical
value for all electro-mechanical units. Thus in Gaussian
units, the electronic charge is e = 1, whereas in H–L
atomic units e =
√
4π. In the SI system, on the other
hand, the three conditions e
2
/
(
4π
0
)
= m
e
= = 1
determine numerical values for mechanical units but
not for electromagnetic ones. A complete determi-
nation of values requires that
0
also be assigned
a value. The choice most consistent with previous
work is to take e = 1 = 4π
0
. This choice is made
here.
Since a volt is a joule/coulomb and a statvolt is
an erg/statcoulomb, 1 volt corresponds to (but is not
generally equal to, since the physical dimensions may
Introduction
4 Part
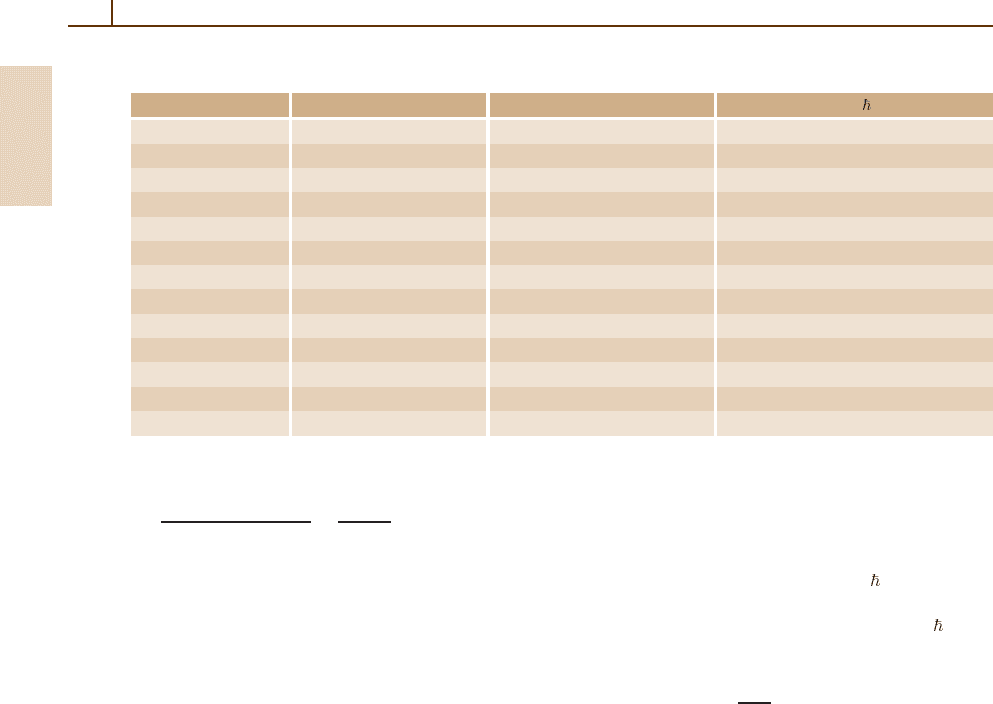
Table 1.3 Conversion factors for various physical quantities
Quantity SI units Gaussian units Natural H–L units: = c =
0
= 1
Length 1m = 10
2
cm = 1m
Mass 1kg = 10
3
g ↔ 2.842 788 82(49) × 10
42
m
−1
Time 1s = 1s ↔
˙
3
×10
8
m
Velocity 1ms
−1
= 10
2
cm s
−1
↔
˙
3
−1
×10
−8
Energy 1J= 1kgm
2
s
−2
= 10
7
erg ↔ 3.163 029 14(54) ×10
25
m
−1
Action 1Js = 10
7
erg s ↔ 0.948 252 28(16) ×10
34
Force 1N= 1Jm
−1
= 10
5
dyne ↔3.163 029 14(54) ×10
25
m
−2
Power 1W= 1Js
−1
= 10
7
erg s
−1
↔ 1.055 072 95(18) ×10
17
m
−2
Intensity 1Wm
−2
= 10
3
erg cm
−2
↔ 1.055 072 95(18) ×10
17
m
−4
Charge 1C= 1As ↔
˙
3
×10
9
statcoul ↔ 1.890 067 14(16) ×10
18
Potential 1V= 1JC
−1
↔ (
˙
3
×10
2
)
−1
statvolt ↔ 1.673 500 94(14) ×10
7
m
−1
Electric field 1Vm
−1
= 1NC
−1
↔ (
˙
3
×10
4
)
−1
statvolt cm
−1
↔ 1.673 500 94(14) ×10
7
m
−2
Magnetic field 1T= 1NA
−1
m
−1
↔ 10
4
gauss ↔ 5.017 029 61(43) ×10
15
m
−2
differ)
10
7
erg
˙
3×10
9
statcoulomb
=
1
˙
3×10
2
statvolt . (1.9)
In Gaussian units, the unit of magnetic field, namely the
Gauss (B)orOersted(H ) has the same physical size
and dimension as the unit of electric field, namely the
statvolt/cm, which in turn corresponds to an SI field
of
˙
3×10
4
V/m. However, the tesla (1 T = 1 weber/m
2
),
the SI unit of magnetic field B (older texts refer to B as
the magnetic induction), has the physical dimensions of
Vs/m
2
. To find the correspondence to Gaussian units,
one must multiply by the speed of light c:
1Tc =
˙
3×10
8
V/m , (1.10)
which corresponds to 10
4
statvolt/cm and hence to
10
4
gauss.
Tables 1.3 and 1.4 related basic mechanical and
electromagnetic quantities in the different unit sys-
tems. Caution is required both because the same
symbol often stands for quantities of different phys-
ical dimensions in different systems of units, and
because factors of 2 π sometimes enter frequencies,
depending on whether the units are cycles/s (Hz)
or radians/s. The double-headed arrows (↔) indicate
a correspondence between quantities whose dimen-
sions are not necessarily equal. Thus for example, the
force on an electron due to a Gaussian electric field
of 1 statvolt/cm is the same as due to an SI elec-
tric field of
˙
3×10
4
V/m. The correspondences between
Gaussian and SI electrostatic quantities become equal-
ities if and only if 4π
0
= 1. Thus they are equalities
within the Gaussian system but not within the less
constrained SI scheme. The SI and Gaussian units of
magnetic field have different dimensions unless both
0
and c are set equal to dimensionless numbers.
Natural H–L units can be considered SI units sup-
plemented by the conditions
0
= c = = 1. They are
listed here in units of meters, although eV are also of-
ten used: 1 eV = 5.067 731 04(43) ×10
6
m
−1
× c.The
correspondences may be considered equalities within
the natural H–L system but not within SI. Note that
the electronic charge in the natural H–L system has
the magnitude e =
√
4πα. More electromagnetic con-
versions can be found in Jackson [1.2]. The data
here are based on the 2002 adjustment by Mohr and
Taylor [1.1].
A few additional energy conversion factors are
1eV= 1.602 176 53 (14) ×10
−19
J
= 2.417 989 40 (21) ×10
14
Hz × h
= 8065.544 45 (69) cm
−1
× hc
= 3.674 932 45 (31) ×10
−2
E
h
= 1.160 4505 (20) ×10
4
K×k
B
= 96.485 3383 (83) kJ mol
−1
The basic unit of temperature, the kelvin, is equiva-
lent to about 0.7cm
−1
, i. e., the value of the Boltzmann
constant k
B
expressed in wavenumber units per kelvin is
0.695 0356(12) cm
−1
K
−1
. Since K is the internationally
accepted symbol for the Kelvin [1.3], this suggests that
the use of the letter K as a symbol for 1 cm
−1
(1 Kayser)
should be discontinued.
Introduction
Units and Constants 1.3 Mathematical Constants 5
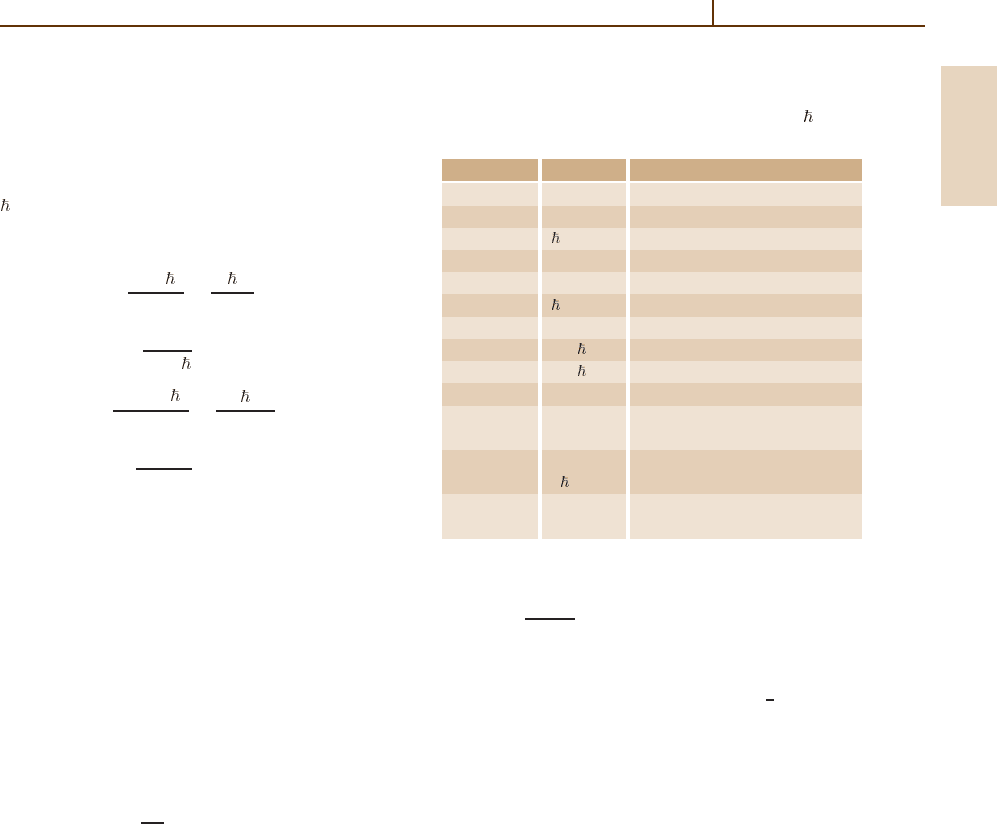
1.2 Atomic Units
Atomic and molecular calculations based on the
Schrödinger equation are most conveniently done in
atomic units (a.u.), and then the final result converted to
the correct SI units as listed in Table 1.4. In atomic units,
= m
e
= e = 4π
0
= 1. The atomic units of length,
velocity, time, and energy are then
length: a
0
=
4π
0
2
m
e
e
2
=
αm
e
c
,
velocity: v
B
=
e
2
4π
0
= αc ,
time: τ
0
=
16π
2
2
0
3
m
e
e
4
=
α
2
m
e
c
2
,
energy: E
h
=
e
2
4π
0
a
0
= α
2
m
e
c
2
,
where, from the definition (1.8), the numerical value
of c is α
−1
= 137.035 999 11(46) a.u. For the lowest
1s state of hydrogen (with infinite nuclear mass), a
0
is
the Bohr radius, v
B
is the Bohr velocity, 2πτ
0
is the
time to complete a Bohr orbit, and E
h
(the Hartree
energy) is twice the ionization energy. To include the
effects of a finite nuclear mass M, one must replace
the electron mass m
e
by the reduced electron mass
µ = m
e
M/(M +m
e
).
Atomic energies are often expressed in units of the
Rydberg (Ry). The Rydberg for an atom having nuclear
mass M is
1Ry= R
M
=
µ
m
e
R
∞
= M(M +m
e
)
−1
R
∞
,
(1.11)
Table 1.4 Physical quantities in atomic units with = e =
m
e
= 4π
0
= 1, and α
−1
= 137.035 999 11(46)
Quantity Unit Value
Length a
0
0.529 177 2108(18) × 10
−10
m
Mass m
e
0.910 938 26(16) × 10
−30
kg
Time /E
h
2.418 884 326 505(16) × 10
−17
s
Velocity v
B
≡ αc 2.187 691 2633(73) × 10
6
ms
−1
Energy E
h
4.359 744 17(75) × 10
−18
J
Action 1.054 571 68(18) × 10
−34
Js
Force E
h
/a
0
0.823 872 25(14) × 10
−7
N
Power E
2
h
/ 0.180 237 811(31) W
Intensity E
2
h
/ a
2
0
64.364 091(11) × 10
18
Wm
−2
Charge e 1.602 176 53(14) × 10
−19
C
Electric E
h
/e 27.211 3845(23) V
potential
Electric E
h
/ea
0
= 0.514 220 642(44) × 10
12
Vm
−1
field α c/ea
2
0
Magnetic E
h
/ea
0
αc 2.350 517 42(20) × 10
5
T
flux density
with
R
∞
=
m
e
cα
2
2h
= 10 973 731.568 525 (73) m
−1
.
(1.12)
The Rydberg constant R
∞
is thus the limiting value of
R
M
for infinite nuclear mass, and hcR
∞
is
1
2
a.u., which
is equivalent to 13.605 6923(12) eV.
The energy equivalent of the electron mass, m
e
c
2
,
is 0.510 998 918(44) MeV. This energy is a natural unit
for relativistic atomic theory. For example, for inner-
shell energies in the heaviest elements, the binding
energy of the 1s electron in hydrogenic Lr (Z = 103)
is 0.338 42 m
e
c
2
.
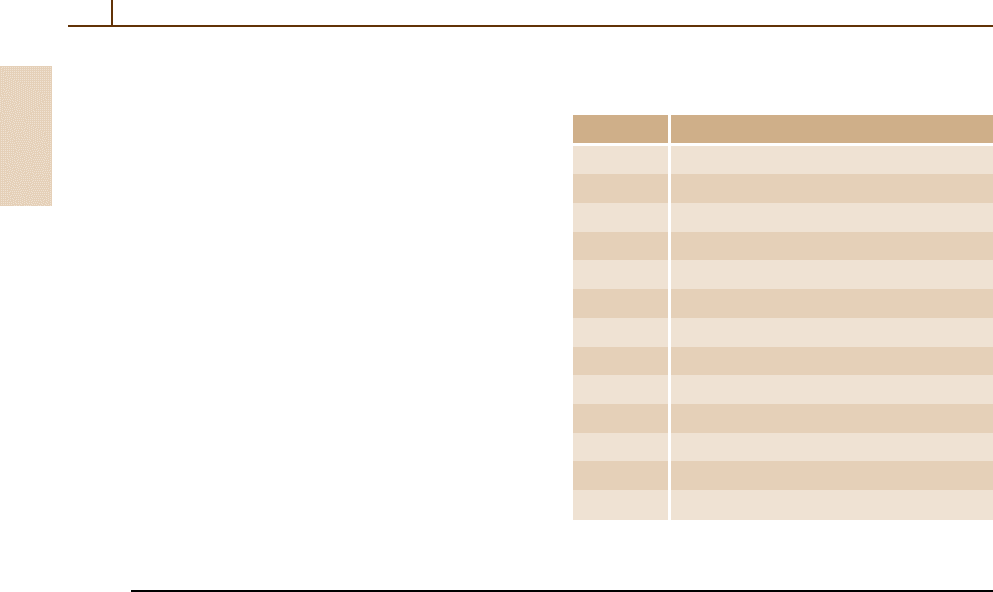
1.3 Mathematical Constants
A selection of the most important mathematical con-
stants is listed in Table 1.5. More extensive tabulations
and formulas can be found in the standard mathematical
works [1.4, 5]
1.3.1 Series Summation Formula
The Riemann zeta function defined by ζ(n) =
∞
i=1
i
−n
(Table 1.5) is particularly useful in summing slowly
convergent series of the form
S =
∞
i=1
T
i
. (1.13)
For example, suppose that the series
T
i
= t
2
i
−2
+t
3
i
−3
+··· (1.14)
for the individual terms in S is rapidly convergent
for i > N,whereN is some suitably large integer.
Introduction
6 Part
Then
S =
N
i=1
T
i
+t
2
ζ
N
(2) +t
3
ζ
N
(3) +···, (1.15)
where ζ
N
(n) = ζ(n) −
N
i=1
i
−n
is the zeta function
with the first N terms subtracted. For N sufficiently
large, only the first fe w t
j
coefficients need be known,
and they can be adequately estimated by solving the
system of equations
T
N
= t
2
N
−2
+t
3
N
−3
+···
+t
k+2
N
−k−2
, (1.16a)
T
N−1
= t
2
(N −1)
−2
+t
3
(N −1)
−3
+···
+t
k+2
(N −1)
−k−2
, (1.16b)
.
.
.
T
N−k
= t
2
(N −k)
−2
+t
3
(N −k)
−3
+···
+t
k+2
(N −k)
−k−2
, (1.16c)
where k +1 ≤ N is the number of terms retained in
(1.14).
Table 1.5 Values o f e, π, Euler’s constant γ , and the Rie-
mann zeta function ζ(n)
Constant Value
e 2.718 281 828 459 045 235 360 287 471 352 66
π 3.141 592 653 589 793 238 462 643 383 279 50
π
1/2
1.772 453 850 905 516 027 298 167 483 341 14
γ 0.577 215 664 901 532 860 606 512 090 082 40
ζ(2) 1.644 934 066 848 226 436 472 415 166 646 02
ζ(3) 1.202 056 903 159 594 285 399 738 161 511 45
ζ(4) 1.082 323 233 711 138 191 516 003 696 541 16
ζ(5) 1.036 927 755 143 369 926 331 365 486 457 03
ζ(6) 1.017 343 061 984 449 139 714 517 929 790 92
ζ(7) 1.008 349 277 381 922 826 839 797 549 849 80
ζ(8) 1.004 077 356 197 944 339 378 685 238 508 65
ζ(9) 1.002 008 392 826 082 214 417 852 769 232 41
ζ(10) 1.000 994 575 127 818 085 337 145 958 900 31
References
1.1 P. J. Mohr, B. N. Taylor: Rev. Mod. Phys. 77,
1 (2005); see also www.physicstoday.org/guide/
fundcon.html; all of the values, as well as the cor-
relation coefficients between any two constants, are
available online in a searchable database provided
by NIST’s fundamental constants data center. The
internet address is http://physics.nist.gov/constants
1.2 J. D. Jackson: Classical Electrodynamics, 3rd edn.
(Wiley, New York 1999)
1.3 B. N. Taylor (Ed.): The International System of Units
(SI), NIST Spec. Publ. 330 (U.S. Government Printing
Office, Washington 2001) p. 7
1.4 M. Abramowitz, I. A. Stegun: Handbook of
Mathematical Functions (Dover, New York
1965)
1.5 I. S. Gradshteyn, I. M. Ryzhik: Table of Inte-
grals, Series, and Products (Academic, New York
1965)
Introduction
