Douce A.P. Thermodynamics of the Earth and Planets
Подождите немного. Документ загружается.

118 Energy sources in planetary bodies
(Scott, 2007). The values of C
0
i
for the extinct radionuclides are simply the product of these
initial isotopic abundances by the corresponding elemental abundances.
We now seek an order of magnitude estimate of the contribution of radioactive decay to
present-day terrestrial heat output. This requires knowledge of the Earth’s bulk chemical
composition, on which there are considerable uncertainties. Estimates of the bulk composi-
tion of the Earth are based upon the compositions of chondritic meteorites, complemented
by a number of inferences and reasonable assumptions. We know, for example, that the Earth
is depleted in volatile elements relative to chondritic meteorites, including in potassium,
which is a moderately volatile element. Starting from the present-day bulk earth elemental
abundances given by Kargel and Lewis (1993) we use equations (2.98) and (2.97) and the
data in Table 2.2 to calculate the temporal evolution of radioactive heat production in the
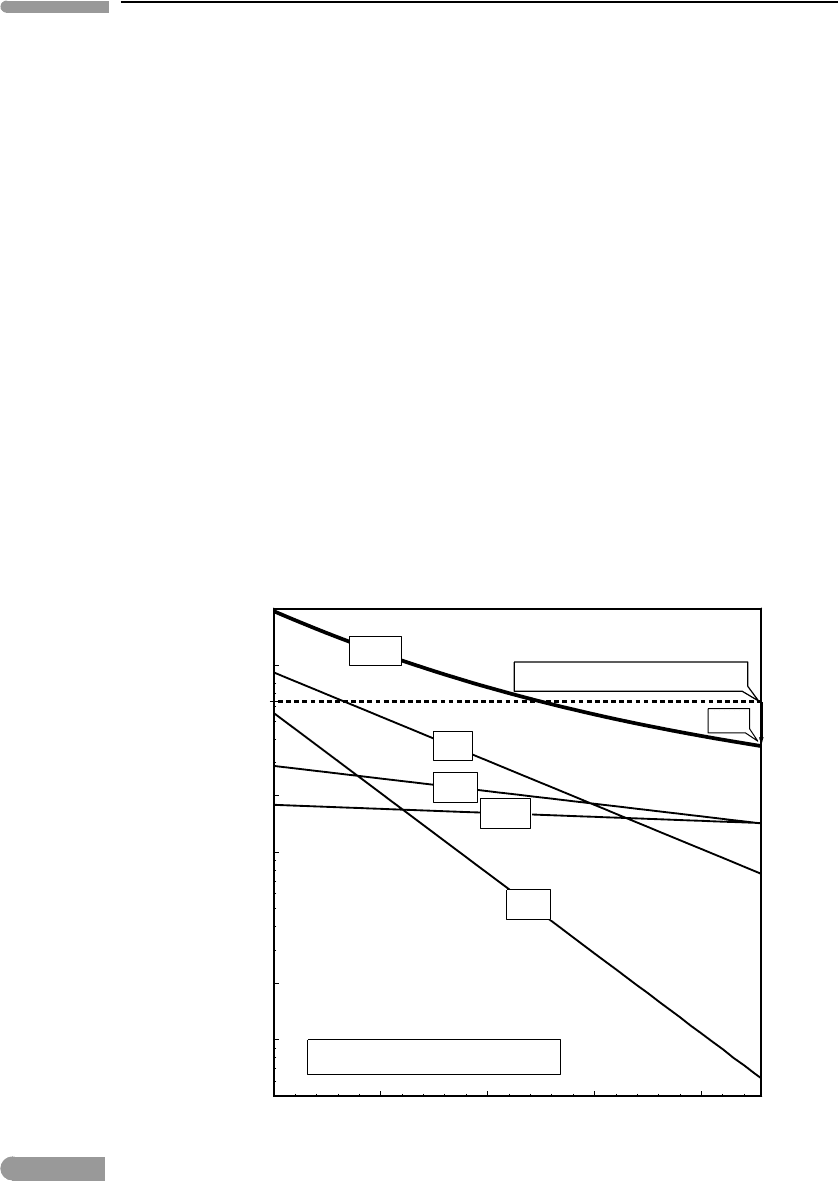
Earth. The results are shown in Fig. 2.21. We can see that at the present time radioactive
heating accounts for about one half of the observed terrestrial heat output. The balance
represents secular cooling, i.e., slow loss of internal energy that was stored during an earlier
epoch. The source of some of this energy may have also been radioactive decay, which
generated considerably more heat in the distant geologic past than today, largely as a result
of significantly higher abundances of
40
K and
235
U, with half lives of ∼1.3 and 0.7 billion
years, respectively (Fig. 2.21). Much of the Earth’s secular cooling, however, may still
reflect core formation. As we saw in Section 2.6, differentiation of the Earth dissipated
enough gravitational potential energy to melt the whole planet several times over. Given
that result (Worked Example 2.4) and the temporal evolution of radioactive heat production
0
10
–13
10
–12
10
–11
Time after formation of the Solar System (Ga)
Radioactive heat production – Wkg
–1
Bulk Earth radioactive heating
Present terrestrial heat output
40
K
235
U
238
U
232
Th
Total
57%
1
2
3
4
Fig. 2.21
Temporal evolution of bulk Earth radioactive heating per unit mass. Broken line shows the present-day terrestrial heat
output. Present radioactive heating accounts for about half of this value.
119 2.9 Radioactive heating
shown in Fig. 2.19, it is hard to see how one can avoid the conclusion that terrestrial heat
output during the Archean and Early Proterozoic must have been much higher than today’s.
A refined estimate of the history of terrestrial heat flow requires that we also take into
account the fact that there are strong geochemical controls on the distribution of radioactive
elements, as well as the effects of heat transfer, which we will do in Chapter 3.
A wide range of thermal regimes is known to have affected different early Solar System
objects. For instance, some carbonaceous chondrites may have undergone aqueous alter-
ation at liquid water temperatures, whereas basaltic achondrites and iron meteorites indicate
that some asteroid-sized bodies underwent essentially complete melting. Electromagnetic
induction heating is a possible energy source for thermal processing of early Solar System
objects (Section 2.8), but it is not the only one. Decay of short-lived radionuclides may
have fueled an early but very short phase of extreme heating of planetesimals. Consider
as an example an object with the composition of CI chondrites (this is the group of chon-
dritic meteorites that most closely match the Sun’s composition, except for loss of volatile
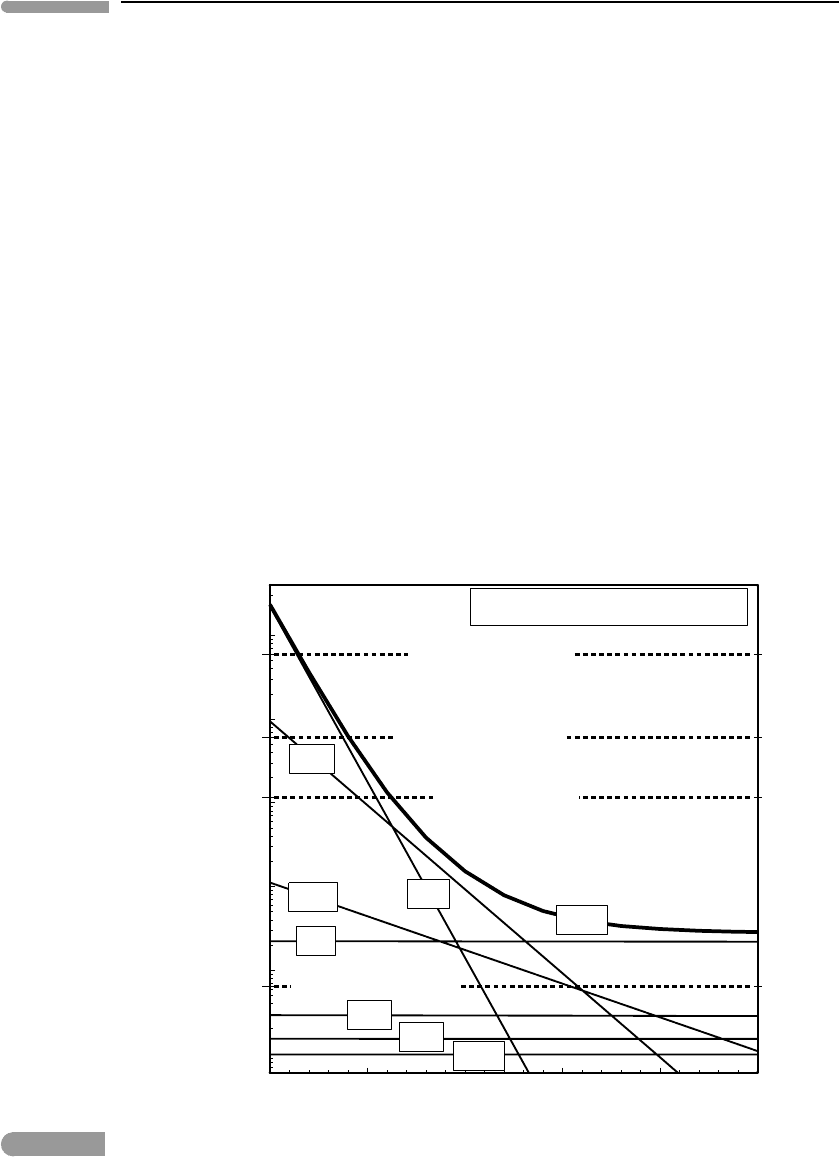
elements). Figure 2.22 shows radioactive heat production in such an object during the first
25 million years after formation of the Solar System, using data from Table 2.2 and chon-
dritic bulk composition from Palme and Beer (1993). Decay of
26
Al dominates during the
first ∼3 million years, and during that time it generates heat at rates 1–2 orders of magnitude
greater than Io’s present-day extreme heat output. Decay of
60
Fe is the chief heat source
between ∼6 and ∼13 million years, after which time the long-lived isotopes take over. The
0510 15 20 2
5
10
–12
10
–11
10
–10
10
–9
10
–8
10
–7
Time after formation of the Solar System (10
6
yr)
Radioactive heat production – Wkg
–1
CI chondrite radioactive heating
Earth’s present heat output
40
K
235
U
238
U
232
Th
26
Al
60
Fe
53
Mn
Total
Chondrite melting
in1 m. yr.
Chondrite melting
in 10 m. yr.
lo’s present
heat output
Fig. 2.22
Radioactive heating per unit mass in CI chondrites during the initial 25 million years of the Solar System. The broken
horizontal lines labeled “Chondrite melting” are average heating rates required to melt chondritic material in 1 and 10
million years.

120 Energy sources in planetary bodies
most intriguing observation to come out of Fig. 2.22 is that heat output from decay of
26
Al
during the first million years may have been several times higher than the average rate of
supply of heat that would have been required to completely melt the chondritic object in 1
million years. This comparison is of course incomplete, as it ignores the effects of heat loss
(Chapter 3), but for a large enough body heat loss over a time scale of 10
6
years, before it
melts and starts convecting, is negligible. It is hard to see how objects that accreted while
26
Al was still abundant could have escaped wholesale melting. Why, then, are there chon-
dritic meteorites that presumably come from parent bodies that never underwent melting
temperatures, and in many cases may have been barely heated? The most likely explanation
is that chondritic parent bodies accreted late enough that most short-lived isotopes were well
on their way to becoming extinct, whereas differentiated parent bodies may have been the
first ones to accrete (Bizzarro et al., 2004; Scott, 2007). A question that in my view has not
been satisfactorily answered is how short-lived radioisotope heating explains the thermal
gradient across the asteroid belt (see Section 2.8) and whether electromagnetic induction
heating also played a role in the origin of this gradient.
Exercises for Chapter 2
2.1 Derive equations (2.20), (2.21) and (2.22). Note that in both the linear and exponential
accretion models the accretion rate does not vanish when the planet reaches its final
mass, whereas in the sinusoidal model accretion rate is forced to be zero at the final
planetary mass. Comment on the relative merits of the three models, and discuss which
may be a better representation of the way in which terrestrial planets accrete.
2.2 Use the Maple worksheet accretion to study the effects of emissivity, nebular
temperature, total mass and accretion time on the initial thermal structure of terrestrial
planets. Search the literature for possible thermal gradients in the solar nebula, and
discuss the extent to which the initial thermal structures of Mercury and Mars could
have differed, assuming that they accreted at their present day heliocentric distances.
2.3 Use accretion to study accretion of icy bodies. Explore the effects of lower den-
sity and lower emissivity of ice compared to rock, and of possibly lower nebular
temperatures.
2.4 Derive the equation for escape velocity, V
e
(see Section 2.4.1), from the definition that
V
e
is the speed at which the kinetic energy of a body equals its gravitational potential
energy. Note that V
e
depends on the distance to the attractor’s center of mass, but the
value is always well defined in the neighborhood of a planet’s surface.
2.5 Show that for a contracting self-gravitating body at hydrostatic equilibrium the change
in gravitational binding energy equals PdV work on the planet.
2.6 The virial theorem describes equilibrium conditions during contraction. In order for
contraction of a self-gravitating cloud of gas to start, however, it must be out of
equilibrium. Its specific gravitational binding energy (e.g. given by equation (2.10))
must be greater than its specific internal energy (Section 1.14). Derive an equation
that yields the minimum density that a gas cloud must have in order for contraction
to start, as a function of its radius and its temperature, assuming that it consists of
monatomic ideal gas. Assume that the Sun formed from a cloud of gas with a diameter
of 10 light years, at an initial temperature of 50 K. What were the density and the

121 Exercises for Chapter 2
mass of the cloud? How does the mass of the cloud compare to the mass of the Sun?
Comment on your results.
2.7 Derive a relationship between the linear contraction rate of a proto-star (dR/dt ) and its
surface temperature, assuming that it contracts at equilibrium and that it is composed
of monatomic ideal gas. Before the discovery of nuclear fusion, one of the explanations
proposed to account for solar radiation was Kelvin–Helmholtz contraction. Estimate
the necessary contraction rate, assuming that the Sun radiates at 6000 K. Comment
on your results.
2.8 Demonstrate that the gravitational acceleration at a point inside a solid sphere is
derived only from the mass contained inside the radius of the point (see Box 2.1).
2.9 Prove to yourself that I did not make any mistakes in the tedious derivation of equation
(2.61). This is good Maple practice.
2.10 Assume that for Mercury µ =5 ×10
10
Pa, Q
d
=100 and h = 1. Calculate the likely
magnitude of present-day tidal heating in Mercury from Solar-induced tides. Is this
a plausible energy source for Mercury’s magnetic field? Calculate the possible tidal
heating of Mercury before tidal despinning, assuming that it had a 10-hour day. Com-
pare this value with present-day measured planetary heat flows (e.g. Earth, Moon,
Io). All data needed to solve this problem not listed here can be found in Lodders and
Fegley (1998).
2.11 Assume that for Tritonµ =10
9
Pa, Q
d
=100 and h =1. Calculate the likely magnitude
of present-day tidal heating in Triton (all data needed to solve this problem not listed
here can be found in Lodders & Fegley, 1998). Comment on your results, in view of
Triton’s young and active surface features.
2.12 Discuss how the quasi-static accretion model may be affected if short-lived isotopes
such as
26
Al,
60
Fe and
53
Mn are present in the accreted material (it is fairly straightfor-
ward to modify accretion in order to include this effect). Research the literature
to find out what is known about the presence of short-lived isotopes during accretion
of the Earth.

3
Energy transfer processes in planetary
bodies
We have developed a comprehensive physical description of the processes and pathways
by which planetary bodies acquire internal energy. Our next task is to examine how this
internal energy drives planetary processes. The hallmark of an active planetary body is that
it has surface features, other than impact craters, that have ages that are negligible compared
to the age of the Solar System. This is true for any epoch of the Solar System. For instance,
the youngest features on the Moon, the immense basaltic plains that we call lunar maria, are
about 3 billion years old. This means that the Moon is dead today, but it was active when
the age of the Solar System was of the order of 1.5 billion years
Active planetary processes are associated to heat flow, but the causal connection is
not always the same. Consider the ascent of magmas. This is a process that transfers
mass and heat from the planet’s interior towards its surface, and that is made possible
by melting, which entails conversion of thermal energy to chemical energy. Ascent of
magma and construction of volcanoes, however, are not driven by thermal energy but
by gravitational energy. Magmas rise to the surface of a planet if they are buoyant, and
magmas are buoyant if melting causes a decrease in density. The essence of the process
can be captured by considering a parcel of magma of unit volume and density ρ
m
, ris-
ing from a depth at which the planetary radius is r to the planet’s surface at radius R
> r, and exchanging places with an equal volume of country rock of density ρ
c
>ρ
m
(Fig. 3.1). The process takes place inside a solid sphere (Box 2.1). We neglect energy
dissipation by friction. The change in gravitational potential energy of the magma is
given by:
U
g,m
=−
GMρ
m
2R
3
r
2
−R
2
(3.1)
and the change in gravitational potential energy of the country rock by:
U
g,c
=−
GMρ
c
2R
3
R
2
−r
2
. (3.2)
The change in gravitational potential energy of the magma ascent process is, therefore:
U
g
=U
g,m
+U
g,c
=
GM
2R
3
(
ρ
c
−ρ
m
)
r
2
−R
2
< 0. (3.3)
Ascent of magma corresponds to a decrease in gravitational potential energy and, in fact,
it is physically and mathematically indistinguishable from planetary differentiation – the
distinction is one of scale only. But the First Law of Thermodynamics requires that we
122
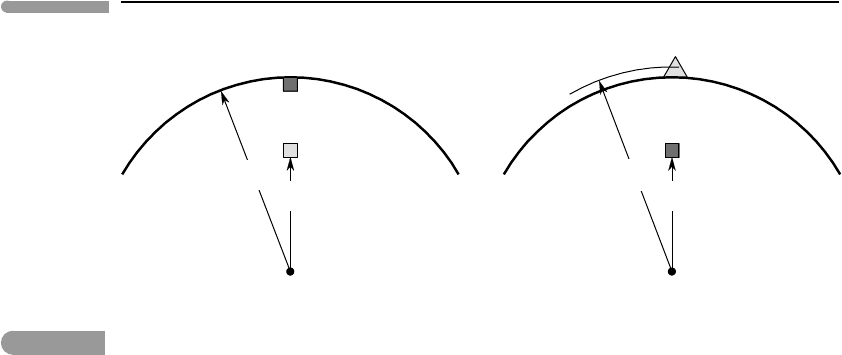
123 Energy transfer processes in planetary bodies
R
r
ρ
m
ρ
c
ρ
m
ρ
c
R
v
r
(a) (b)
Fig. 3.1
Energy aspects of magma ascent. In (a) a batch of magma of density ρ
m
initially located at radius r exchanges places
with an equal volume of country rock of density ρ
c
, initially located at the geoid, radius =R. If the magma ascends
no further then there is an “excess” in gravitational potential energy which must be dissipated as heat. In (b) the
magma is allowed to rise until its gain in potential energy balances that lost by the country rock, building a volcano
with center of mass at radius R
v
.
account for the gravitational potential energy that is “lost” in equation (3.3). In order to
do so, we must realize that the process depicted in Fig. 3.1a is not the whole story. If
the magma stops at or below the planet’s geoid (say, it forms a shallow intrusion) then
the potential energy that is “missing” in equation (3.3) is dissipated as heat, just as in the
case of planetary differentiation. If the magma is able to rise above the geoid then the
potential energy released by ascent of magma from its source to the geoid becomes the
gravitational potential energy of the magma (i.e. of a volcano) relative to the geoid (Fig.
3.1b). If we neglect frictional losses, the potential energy lost by the sinking country rock
must be balanced by the potential energy gained by the magma in rising from its source to
the volcano’s center of mass (radius R
v
in Fig. 3.1b).
Volcanism is not possible in the absence of planetary internal energy, but there is no net
conversion of thermal energy to mechanical energy in the process. The thermal energy that
is converted to chemical energy during melting is released as heat during crystallization
and is eventually radiated to space. There is net transfer of mass in a gravitational field, so
that the source of energy for magma ascent is gravity.
Consider now processes such as plate tectonics, atmospheric circulation or planetary
dynamos, which are all manifestations of convection. In contrast to volcanism, in all of
these processes there is conversion of thermal energy to mechanical energy, but there may
not be mass transfer in a gravitational field. This is so because convection is a cyclic process
in which there is no accumulation of mass anywhere on the cycle, at least on time scales
that are long relative to the time of one convective overturn. Convection is not possible in
the absence of a gravitational field, but, since there is no net mass transfer, gravity cannot
be the energy source for convection. Convection is driven by conversion of thermal energy
(either a planet’s internal heat or solar energy) to mechanical energy. There is a remarkable
symmetry here with magma ascent, which is not possible in the absence of internal energy,
but that is not driven by internal energy. The key to this symmetry is whether or not there
is net transfer of mass down a gravitational field. The study of planetary sciences requires
a good physical and mathematical understanding of heat-transfer processes and this is the
goal of this chapter.

124 Energy transfer processes in planetary bodies
3.1 Transport processes
The First Law of Thermodynamics is the mathematical expression of the principle of con-
servation of energy. Two other quantities that are conserved are mass and momentum. Each
of these three conserved quantities is associated with another quantity, which we may call
a potential, such that if there is a potential gradient the conserved quantity will tend to
flow down the potential gradient. This is one possible way of stating the Second Law of
Thermodynamics, as we shall see in greater detail in Chapters 4 and 12. For example, a
temperature gradient drives the flow of internal energy. A gradient in concentration (or,
more accurately, in chemical potential, Chapter 5) causes matter to flow, for example in
an initially inhomogeneous solution or across an osmotic barrier. Transfer of momentum
is more complicated because momentum is a vector quantity, in contrast to energy and
mass that are scalar quantities, but the principles are the same. The mathematical law that
describes the flow of these three conserved quantities is one and the same and can be written
in its most simple form as follows:
f =−c
dΦ
dx
. (3.4)
In this equation f is the flux of the conserved quantity, i.e. the amount of energy, mass or
momentum that is transported per unit of time and per unit of area perpendicular to the
direction of transport. The potential that drives the flow is Φ, so that dΦ/dx is the potential
gradient, and the negative sign expresses the fact that the conserved quantity is transported
down the potential gradient. The parameter c is a material property which for now we will
assume to be a constant, although in general it is a function of temperature and it may also be
a function of dΦ/dx. This latter point can introduce immense computational complications.
We also note that, in its complete mathematical formulation, f is a vector, dΦ/dx is a one-
form, and c is another geometric object called a second-order tensor. In order not to get
bogged down in these mathematical complications we restrict our discussion to transport
in one spatial dimension, in which case all three geometric objects can be thought of as
scalars, as shown in equation (3.4) (in fact, a scalar is a zeroth-order tensor). But one must
always keep in mind that flow and flux (flow per unit area) are vectors.
Consider energy conservation first, which is schematized in the drawings at the top
of Fig. 3.2. Temperature is the potential that drives heat flow. If there is a difference in
temperature across a parcel of matter such that T
1
>T
2
, then internal energy flows from
the high temperature region towards the low temperature one. The magnitude of the heat
flux, q, is given by:
q =−k
dT
dx
, (3.5)
where k is a material property called thermal conductivity. If we express q in units of
Js
−1
m
−2
then the units of k are J s
−1
m
−1
K
−1
. Because energy is conserved, in the
absence of energy exchanges with the environment the system evolves to a state at a time t
1,
later than t
0,
in which its internal energy, and therefore its temperature T
3
, are uniform and
such that T
1
>T
3
>T
2
(see Fig. 3.2). Equation (3.5) is also known as Fourier’s law of heat
conduction, in honor of the French scientist Jean Baptiste Joseph Fourier (1768–1830) who
first gave it its mathematical form, and who made many other fundamental contributions
to physics and mathematics. In Fourier’s time it was not realized that equation (3.5)is
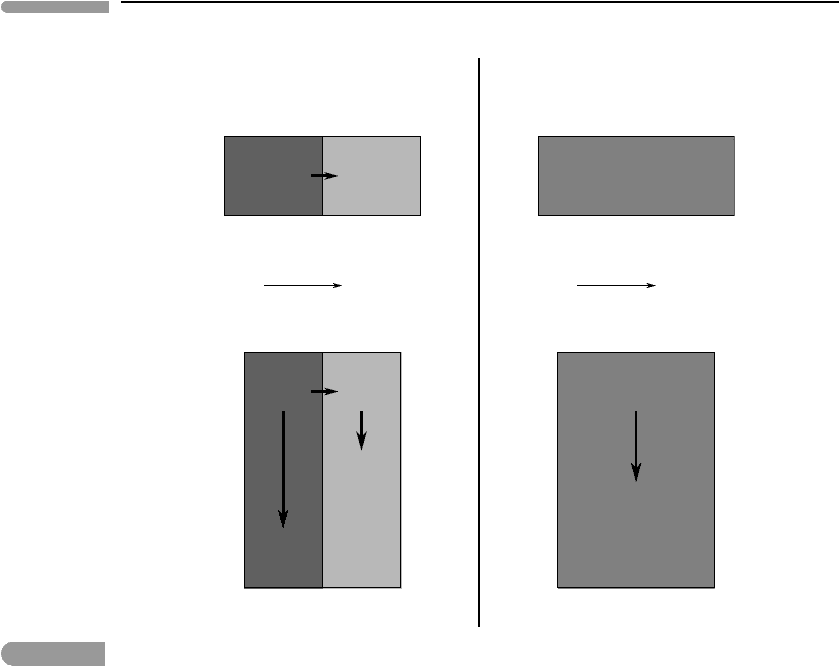
125 3.1 Transport processes
T
1
T
2
T
3
u
1
u
2
u
3
Q
p
x x
t
0
t
1
> t
0
Fig. 3.2
Analogy between diffusive transfer of heat down a temperature gradient dT/dx (top panels, where T
1
> T
3
> T
2
) and
transfer of momentum down a velocity gradient du/dx (bottom panels, with u
1
> u
3
> u
2
). Time flows from t
0
to a
later time t
1
.
actually a special case of (3.4). Now it is better to think of equation (3.5) as one of several
constitutive relations, which describe the physical behavior of a material. The behavior that
is described by equation (3.5) is how the material transports heat by diffusion, which is
the macroscopic expression of propagation of kinetic energy among microscopic particles
without bulk macroscopic displacement of matter. Equation (3.5) tells us the rate at which
diffusive heat transfer takes place.
Diffusion of momentum is analogous to diffusion of heat. To see this, consider the draw-
ings at the bottom of Fig. 3.2, which represent flow of a fluid in which there is a velocity
gradient. The fluid flows downwards, as shown by the velocity vectors ¯u
1
> ¯u
2
, but the
velocity gradient is in the x direction, which is the direction in which we apply equation
3.4. An important point of this thought experiment is that the boundary of the fluid region
drawn in Fig. 3.2 does not represent a physical boundary, but rather a region within an arbi-
trarily large volume of fluid. In the absence of external forces the momentum inside this
region is conserved, just as internal energy is conserved inside a region which undergoes
neither heat nor work exchanges with its environment. The result is that at a time t
1
, later
than t
0
, the velocity will be distributed uniformly across the fluid region, and will have a
magnitude ¯u
3
, such that ¯u
1
> ¯u
3
> ¯u
2
, see Fig. 3.2. Momentum has been transported from
left to right, driven by the velocity gradient du/dx. One can visualize this process in terms of

126 Energy transfer processes in planetary bodies
infinitesimally thin layers of fluid, which initially move with progressively higher velocities
towards the left. Because of the velocity difference there is a drag force between the layers.
This force transfers momentum from a layer to its neighbor to the right, that moves more
slowly, so that the former layer slows down and the latter speeds up. Momentum is thus
transported down the velocity gradient.
Momentum flux is the rate of transport of momentum per unit of area. As we can easily
see, momentum flux has dimension of stress:
[
momentum
]
×
[
time
]
−1
×
[
distance
]
−2
=
[
M
]
×
[
L
]
×
[
T
]
−1
×
[
T
]
−1
×
[
L
]
−2
=
[
M
]
×
[
L
]
×
[
T
]
−2
×
[
L
]
−2
=
[
force
]
×
[
area
]
−1
. (3.6)
This dimensional result agrees with our physical image in which the process that transports
momentum is friction between layers of different velocity. More precisely, the flux of
momentum in a fluid is the shear stress, τ . Equation (3.4) for momentum transport then
becomes:
τ =−µ
du
dx
, (3.7)
where µ is called the viscosity (also, dynamic viscosity) of the fluid and the potential
gradient that drives flux of momentum is the velocity gradient taken perpendicularly to the
direction of fluid flow (Fig. 3.2). This is another constitutive relationship for a material.
Just as the thermal conductivity specifies heat flux, viscosity specifies momentum flux or,
in other words, shear stress. For a given velocity gradient, as viscosity increases so does
shear stress and, therefore, the magnitude of the drag force between layers. Momentum is
then homogenized at a faster rate. This agrees with our intuitive concept of viscosity. For
example, if we stir water or motor oil at the same rotational speeds and then stop stirring,
the motor oil will come to rest sooner, because momentum flux = shear stress is higher in
the more viscous oil than in water. Note that mechanical energy is not conserved during
viscous flow, as viscous drag forces engendered by shear stress are dissipative (Chapter 1).
But momentum is a different quantity and it is conserved. In our example, stirring causes
the fluid to heat up by dissipation of mechanical energy. When we stop stirring, momentum
is transported across the fluid via the vessel to the Earth.
In this chapter we will use constitutive relations (3.5) (Fourier’s law) and (3.7) to study
energy transport processes in planetary bodies. The equivalent relation for mass transport
will be discussed in Chapter 12.
3.2 Heat transport by diffusion
3.2.1 Overview of heat transfer processes
Heat diffusion (also called conduction) is a process in which internal energy flows as a result
of the propagation of kinetic energy among microscopic particles (e.g. molecules, atoms,
ions, or electrons), without bulk macroscopic displacement of matter. Internal energy is

127 3.2 Heat transport by diffusion
transported as neighboring particles interact with one another and exchange kinetic energy.
All microscopic kinetic energy modes: translation, rotation and vibration (Section 1.14),
may be involved in the process. Heat advection occurs when there is a net macroscopic
displacement of matter, which exchanges places with other parcels of matter at a different
temperature, so that internal energy is carried by the flow of matter.Although not customary
in the engineering literature, in planetary sciences it is convenient to make a distinction
between advection, as defined above, and convection, which is a process in which heat
is advected as a result of a cyclical motion of matter driven by a temperature gradient
that engenders buoyancy differences. Engineers call this process natural convection, and
distinguish it from forced convection, in which heat advection occurs as a result of motion
of fluid propelled by machineries, such as fans or pumps. As an example of the difference
between advection and convection, consider heat transfer in the Earth’s mantle and heat
transfer by magma ascending along a volcanic feeder conduit. The former is heat advection
resulting from mantle convection, whereas the latter is heat advection without convection
(to an engineer, magma ascent would be an example of forced convection). The processes
of diffusion and advection can only occur within matter. Radiation, in contrast, is a process
in which part of a body’s internal energy is transformed to electromagnetic energy that can
travel through matter only if it is transparent to that specific range of wavelengths, but that
travels unimpeded in the absence of matter. When electromagnetic radiation interacts with
an opaque body it is absorbed and converted to internal energy. Radiation is not an important
heat transfer process in planetary interiors, but it is important in planetary atmospheres and
is the only process by which planetary bodies receive energy from the Sun. We discuss
radiation in Chapter 13.
3.2.2 The diffusion equation
Heat diffusion is part of every heat transfer process. Beyond the obvious case, that it is the
only form of heat transfer that is possible in a rigid body, heat diffusion takes place between
layers of fluid moving at different velocities and at the boundary between a moving fluid
and its rigid environment. Heat diffusion is also responsible for carrying internal energy to
the interior of a rigid body whose surface receives energy by radiation. We seek to construct
an equation that describes heat diffusion in general, allowing for the possibility that both
temperature and temperature gradient vary as a function of space and time. We will also
include the effects of heat sources (e.g. radioactive, gravitational or electromagnetic heat
generation; dissipation of mechanical energy by friction, etc.) and heat sinks (e.g. melting
and metamorphic devolatilization reactions).
We begin by considering an infinitesimal volume element inside a material in which there
is heat flow (Fig. 3.3). We assume that the material is rigid and at rest relative to a coordinate
system fixed to the observer. Our goal is to write the equation for energy conservation in
the infinitesimal volume element. Because the process is assumed to take place at constant
pressure, any net gain or loss of heat corresponds to a change in the enthalpy content of the
volume element, and is reflected in a temperature change given by c
p
dT , where c
p
is the
specific heat capacity. The lengths of the sides of the volume element are δx, δy and δz,so
that the heat flow across the element’s left face (amount of heat flowing per unit of time
across a surface with area δy δ z) is given by (see equation (3.5)):
−kδyδz
∂T
∂x
x
. (3.8)
