Das Sh.P. Statistical Physics of Liquids at Freezing and Beyond
Подождите немного. Документ загружается.


1.2 Equilibrium properties 11
of the H
B
function is defined as a functional of the one-particle distribution function
f (r, p, t) as
H
B
(t) =
dp f (p, t) ln f (p, t). (1.1.41)
Note that it is consistent to assume that the distribution function is independent of r since
there is no external field present. On taking the time derivative of eqn. (1.1.41), we obtain
dH
B
(t)
dt
=
dp
∂ f (p, t)
∂t
!
1 + ln f (p, t)
"
. (1.1.42)
It is straightforward to establish (Huang, 1987) using the Boltzmann equation that
dH
B
(t)
dt
≤ 0, (1.1.43)
showing that the H function always decreases. This is known as the Boltzmann H theorem.
For f (p, t) equal to the Maxwell–Boltzmann distribution function
φ
MB
( p) =
β
2πm
3/2
exp
−
β p
2
2m
(1.1.44)
the H function H
B
(t) is independent of time and is equal to zero. The function φ
MB
( p) is
normalized and the constant β is determined from the average kinetic energy as
p
2
/(2m)=3/(2β). Hence β is identified with the temperature T of the equilibrium fluid
β = 1/(k
B
T ).
In general the nature of the interaction potential plays an important role in evaluating the
collision integral on the RHS of eqn. (1.1.39). The irreversible kinetic equation obtained
from closing the BBGKY hierarchy is used to compute the basic transport equations for
fluid with dissipative dynamics. The most widely studied system in this regard is a system
of hard spheres. We discuss this further in Section 5.2.2 below.
1.2 Equilibrium properties
By definition for thermodynamic equilibrium, the distribution function f
(N )
depends on
the coordinates r
α
(t) and p
α
(t) but is independent of time t. The equilibrium state of a
fluid corresponds to the stationary solutions of the distribution functions,
∂
∂t
f
(N )
(r
N
, p
N
, t) = 0. (1.2.1)
Therefore from eqn. (1.1.20) it follows that the N-particle equilibrium distribution func-
tion f
(N )
EQ
must satisfy the condition
#
f
(N )
EQ
, H
$
=0. Hence f
(N )
EQ
must be expressed as a
functional of the Hamiltonian H or of function {A
i
} (say), all of which have vanishing
Poisson bracket with the Hamiltonian. These stationary distribution functions determine
the Gibbsian ensembles. In this regard it is particularly useful to consider the following
theorem for the equilibrium state.

12 Statistical physics of liquids
1.2.1 The Gibbs H-theorem
The H function in terms of the N -particle distribution function f
(N )
is defined as
H
G
=
dr
N
dp
N
f
(N )
(r
N
, p
N
) ln f
(N )
(r
N
, p
N
). (1.2.2)
In some cases the ensemble may include variation of the total number of particles N or the
total volume of the system. We denote by Tr an averaging over the whole phase space. If
the different members of the ensemble have only energy as the variable with both the total
number of particles N and the volume V fixed (a canonical ensemble) then Tr stands for
Tr ≡
1
N !
N
%
α=1
dr
α
dp
α
h
3N
, (1.2.3)
where the factor of h
3N
is included to take into account the quantum-mechanical counting
of possible states and the factor of N ! to account for the permutation among the N identical
particles. If the ensemble is one in which, for example, variation of the total number of
particles as well as of the energy is allowed (a grand-canonical ensemble) then we define
Tr ≡
∞
N =0
1
h
3N
N !
dr
N
dp
N
. (1.2.4)
We now define the H function H
G
as
H
G
= Tr
f
(N )
(r
N
, p
N
)ln f
(N )
(r
N
, p
N
)
. (1.2.5)
Let us consider the problem of minimizing the above H function subject to the following
constraints in terms of a specific set of functions A
i
(r
N
, p
N
), i = 1,...,m, given by {
¯
A
i
}
such that
¯
A
i
= Tr
A
i
f
(N )
(r
N
, p
N
)
. (1.2.6)
In equilibrium the time-independent f
(N )
(r
N
, p
N
) describes the probability for the dif-
ferent members of the corresponding ensemble. The condition (1.2.6) implies that the
ensemble averages {
¯
A
i
} characterize the thermodynamic equilibrium state. The H theo-
rem at the N -particle level in this case states that, subject to the constraints (1.2.6),the
normalized distribution function f
(N )
0
corresponding to which the value of H
G
reaches a
minimum is given by
f
(N )
0
(r
N
, p
N
) = W
−1
0
exp
−
i
α
i
A
i
(r
N
, p
N
)
. (1.2.7)
The set of parameters {α
i
} for i = 1,...,m defines the thermodynamic state characterized
by optimum f
(0)
. W
0
is the normalization constant
W
0
= Tr
exp
−
i
α
i
A
i
(r
N
, p
N
)
(1.2.8)

1.2 Equilibrium properties 13
which ensures the condition
Tr f
(N )
0
(r
N
, p
N
) = 1. (1.2.9)
In order to prove the above theorem we first consider an arbitrary choice of the distri-
bution function f
(N )
= f
(N )
0
. Since both the distribution functions represent probabilities,
both of them are real positive numbers and x = f
(N )
/ f
(N )
0
is also a positive number. Since
both the distributions are normalized, we must have
Tr f
(N )
(r
N
, p
N
) = Tr f
(N )
0
(r
N
, p
N
) = 1. (1.2.10)
Now let us consider the difference of the function H
G
as evaluated with the arbitrary
functional f
(N )
from that corresponding to f
(N )
0
:
H
G
[ f ]−H [ f
0
]=Tr
f
0
x ln{xf
0
(r
N
, p
N
, t)}− f
0
ln f
0
, (1.2.11)
where we have dropped the superscript N on f to keep the notation simple. Using the
normalization condition (1.2.10),theRHSofeqn. (1.2.11) reduces to
H
G
[ f ]−H [ f
0
]=Tr
!
f
0
{
x ln x − x + 1
}
+
(
f − f
0
)
ln f
0
"
= I
1
+ I
2
. (1.2.12)
The first integral, I
1
,ontheRHSofeqn. (1.2.12) is always positive since, according to the
Gibbs inequality (see Appendix A1.1), the quantity (x ln x −x +1) is positive definite for
positive x. The second integral, I
2
,ontheRHSofeqn. (1.2.12) vanishes. To demonstrate
this we use the expression (1.2.7) for evaluating ln f
0
,
I
2
=−Tr
( f − f
0
)
&
ln W
0
+
i
α
i
A
i
(r
N
, p
N
)
'
= 0. (1.2.13)
The above result for I
2
follows easily from the normalization condition (1.2.10) and the
constraints (1.2.6) for the ensemble averages of the set of observable {A
i
}. Note that W
0
and the parameters {α
i
} are not dependent on the phase-space variables. We have thus
proved that H
G
for all arbitrary choices of f
(N )
increases from its value corresponding to
the function f
(N )
0
. The latter, as given by the RHS of eqn. (1.2.7), therefore minimizes the
function H
G
. If we identify −k
B
H
G
with the thermodynamic entropy of the equilibrium
state, the other parameters are also readily identified from the relation
−
S
k
B
=−ln W
0
−
i
α
i
¯
A
i
. (1.2.14)
Since for the equilibrium state the entropy is a maximum, we obtain, on taking the deriva-
tive of eqn. (1.2.14) with respect to α
i
, the following result for the thermodynamic average:
¯
A
i
=−
∂
∂α
i
ln W
0
. (1.2.15)
14 Statistical physics of liquids
On taking a second derivative we obtain
∂
¯
A
i
∂α
i
=
∂
2
∂α
2
i
ln W
0
. (1.2.16)
Using the expression (1.2.8) for ln W
0
,theRHSofeqn. (1.2.16) is evaluated as the average
of the mean-square fluctuation of A
i
,
∂
2
∂α
2
i
ln W
0
= Tr
A
2
i
−{Tr[A
i
]}
2
= Tr[{A
i
−
¯
A
i
}
2
]=A
2
i
, (1.2.17)
giving the relative fluctuation of A
i
as
A
2
i
=−
∂
¯
A
i
∂α
i
. (1.2.18)
The result (1.2.18) implies that the relative fluctuation of A
i
is of the order of the square
root of the size S of the system,
#
A
2
i
$
1/2
¯
A
i
∼ O
1
S
. (1.2.19)
The RHS approaches zero in the thermodynamic limit. Different representative statisti-
cal ensembles for the equilibrium state of the system are obtained with corresponding
specific choices of the set of extensive variables {A
i
}. The ensemble-averaged quantities
{
¯
A
i
} remain fixed at their experimentally measured values in the corresponding thermody-
namic state which is characterized by the corresponding set of intensive thermodynamic
variables {α
i
}. The above result (1.2.19) therefore demonstrates the equivalence of the dif-
ferent ensembles since the fluctuation of a specific property A
i
around its average value is
negligible in the thermodynamic limit of a large system, e.g., lim N →∞, lim V →∞
but N/ V remaining finite.
1.2.2 The equilibrium ensembles
Let us consider some of the specific ensembles in the following. For a completely isolated
system we have the micro-canonical ensemble in which the assumption of equal a-priori
probability for each state is applied.
The canonical ensemble
For the canonical ensemble the system is connected to an external heat bath allowing
exchange of energy. The average energy is fixed and is determined by the equilibrium
temperature T of the thermodynamic state. In the present case we have A ≡ H and the cor-
responding parameter is α
i
≡ β. The number of particles N is fixed in this case. Evaluation
of the H function gives
H
G
=
dr
N
dp
N
f
0
ln f
0
=−ln Z
N
−
¯
H, (1.2.20)

1.2 Equilibrium properties 15
where Z
N
≡ W
0
. Upon identifying the average energy
¯
H as the thermodynamic internal
energy U , the above relation (1.2.14) reduces to
−
S
k
B
=−ln Z
N
− βU. (1.2.21)
We identify β = 1/(k
B
T ) and the Helmholtz free energy of the system F as
− k
B
T ln Z
N
= U − TS = F. (1.2.22)
The equilibrium probability distribution is therefore given by
f
(N )
0
r
N
, p
N
=
1
h
3N
N !
exp
[
−β H
]
Z
N
, (1.2.23)
with the partition function Z(N, V , T ) defined as
Z(N, V, T ) ≡ Z
N
=
1
h
3N
N !
dr
N
dp
N
exp
−β H(r
N
, p
N
)
. (1.2.24)
The key relation connecting the thermodynamic property (the Helmholtz free energy F )
with the statistical-mechanical quantity (the canonical partition function Z
N
) is given by
F =−k
B
T ln Z
N
. (1.2.25)
For a noninteracting system (ideal gas) the Hamiltonian H has only a kinetic part and it
is straightforward to obtain the partition function Z
N
by evaluating the Gaussian integrals
involving the p
α
, α = 1,...,N :
Z
N
=
V
N
N !
3
0
, (1.2.26)
with the thermal de Broglie wavelength
0
=h/
√
2πmk
B
T . The free energy for the
noninteracting system is obtained as
F
id
= Nk
B
T
ln
(
n
3
0
)
− 1
. (1.2.27)
By using the relation P =−(∂ F/∂ V )
T
we obtain from eqn. (1.2.27) the ideal-gas equation
of state PV = Nk
B
T . The Gibbs free energy is given by
G = F + PV = Nk
B
T ln
(
n
3
0
)
, (1.2.28)
and the chemical potential for the ideal gas is
μ
id
= k
B
T ln
(
n
3
0
)
. (1.2.29)
The average energy, which is identified as the thermodynamic internal energy U ,is
obtained as
U =
¯
H =−
∂
∂β
ln Z
N
*
*
*
*
V
. (1.2.30)

16 Statistical physics of liquids
The mean-square fluctuation of the internal energy is obtained from the general formula
(1.2.18) as
H
2
=−
∂U
∂β
V,N
= Nk
B
T
2
c
v
, (1.2.31)
where c
v
is the specific heat per particle at constant volume. The relative root-mean-square
fluctuation per particle is of O
1/
√
N
, making it negligible in the thermodynamic limit.
The grand-canonical ensemble
The grand-canonical ensemble describes the system connected to an external bath allowing
exchange of both energy and particles. The ensemble average of the total energy and the
total particle numbers of the system are fixed. In this case we have A ≡{H, N } and the
corresponding α
i
is denoted by {β, −βμ}. Equation (1.2.14) reduces to the form
−
S
k
B
=−ln −
¯
H + βμ
¯
N , (1.2.32)
where ≡ W
0
in this case. The average energy
¯
H and
¯
N , respectively, correspond to the
thermodynamic internal energy U and the average number of particles at temperature T .
If we identify β = 1/(k
B
T ) and μ as the chemical potential, eqn. (1.2.32) reduces to the
thermodynamic relation
− k
B
T ln = U − TS− μ
¯
N = F − G =−, (1.2.33)
where G is the Gibbs free energy of the system and =−PV is the thermodynamic
potential. The equilibrium probability distribution in the grand-canonical ensemble is
therefore given by
f
(N )
0
(r
N
, p
N
) =
1
h
3N
N !
exp
[
−β(H − μN )
]
, (1.2.34)
where the grand-canonical partition function (μ, V, T ) is obtained as
(μ, V, T ) =
∞
N =0
1
N !
dr
N
dp
N
exp
−β(H (r
N
, p
N
) − μN )
. (1.2.35)
The average energy and the average number of particles are respectively obtained as
U =−
∂
∂
β
ln
*
*
*
*
μ,V
,
¯
N =
∂
∂(βμ)
ln
*
*
*
*
T,V
= z
∂
∂z
ln
*
*
*
*
T,V
. (1.2.36)
where z = exp(βμ) is the fugacity. Note that the averaging in the grand-canonical ensem-
ble can be interpreted as a weighted average with respect to the canonical-ensemble
1.2 Equilibrium properties 17
N -particle partition function Z
N
with all possible values of N. Thus we can express the
grand-canonical partition function as
(μ, V, T ) =
∞
N =0
z
N
N !
Z
N
. (1.2.37)
The average number of particles as given by eqn. (1.2.36) is expressed as
¯
N =
∞
N =0
NP(N ), (1.2.38)
where P(N ) is the probability for the N -particle state given by
P(N ) =
z
N
N !
Z
N
. (1.2.39)
The key relation in the case of the grand-canonical ensemble connecting the thermody-
namic property (the thermodynamic potential ≡−PV) with the statistical-mechanical
quantity (the grand-canonical partition function )isgivenby
=−k
B
T ln . (1.2.40)
The mean-square fluctuation of the number of particles is obtained from the general for-
mula (1.2.18) as
N
2
=
∂
¯
N
∂(βμ)
*
*
*
*
T,V
= k
B
T
∂
¯
N
∂μ
T
. (1.2.41)
The relative root-mean-square fluctuation per particle is of O
1/
√
N
, making it negligi-
ble in the thermodynamic limit. The RHS of eqn. (1.2.41) is further simplified by using the
Gibbs–Duhem relation (McQuarie, 2000)
¯
Ndμ = VdP− SdT, (1.2.42)
and hence
∂μ
∂
¯
N
T
=
V
¯
N
∂ P
∂
¯
N
T
=
1
¯
N
∂ P
∂n
T
, (1.2.43)
since n =
¯
N /V . However, (∂ P/∂n)
T
can also be written as
∂ P
∂n
T
=
∂ P
∂V
T
∂V
∂n
T
=−
V
2
¯
N
∂ P
∂V
T
=
1
nκ
T
, (1.2.44)
where
κ
T
=−
1
¯
V
∂
¯
V
∂ P
T
(1.2.45)
18 Statistical physics of liquids
is the isothermal compressibility of the system. Using the result (1.2.44) in eqn. (1.2.41),
we obtain
N
2
−
¯
N
2
¯
N
=
n
β
κ
T
. (1.2.46)
The root-mean-square fluctuation N is therefore of O(1/
√
N ) and negligible in the ther-
modynamic limit – except near the critical point, where the compressibility is diverging
and hence the role of fluctuations becomes important there.
The isobaric–isothermal ensemble
The isobaric ensemble describes the system connected to an external bath allowing both
exchange of energy and variation of the volume V of the system. The number of particles
remains constant in this case. The ensemble average of the total energy and the volume
of the system are fixed. In this case we have A
i
≡{H, V } and the corresponding α
i
is
denoted by {β, βα}. Evaluation of the H function gives
H
G
=
dr
N
dp
N
f
0
ln f
0
=−ln − β
H
− βα
V
, (1.2.47)
where ≡ W
0
. Upon identifying the average energy
H
as the thermodynamic internal
energy U the above relation (1.2.14) reduces to
−
S
k
B
=−ln − β(U + α
¯
V ), (1.2.48)
where
¯
V is the volume in the thermodynamic equilibrium state at temperature T . We iden-
tify β = 1/(k
B
T ) and α as the thermodynamic pressure P so that eqn. (1.2.48) reduces to
the thermodynamic relation
− k
B
T ln = U − TS+ α
¯
V = U − TS+ PV = G. (1.2.49)
The equilibrium probability distribution in the isobaric ensemble is therefore given by
f
(N )
0
r
N
, p
N
=
−1
exp
[
−β{H + PV}
]
, (1.2.50)
where (N, P, T ) is the isobaric partition function. The latter is obtained as a Laplace
transform (see eqn. (1.3.10) for its definition) at “frequency” β P with respect to the
volume V ,
(N, P, T ) =
0
h
3N
N !
∞
0
dV
dr
N
dp
N
exp
[
−β{H + PV}
]
, (1.2.51)
where
0
is a constant with the dimension of volume introduced so as to make the par-
tition function dimensionless. The average volume and the mean square of the volume
fluctuations are, respectively, obtained as
¯
V =−k
B
T
∂
∂ P
ln
*
*
*
*
T,N
, (1.2.52)
1.2 Equilibrium properties 19
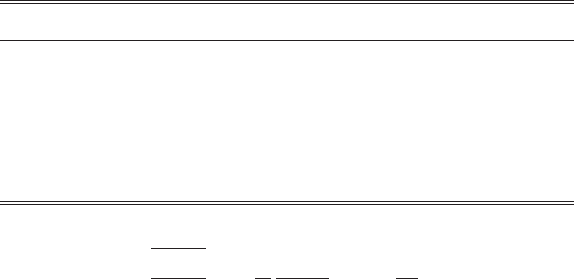
Table 1.1 Different ensembles, the corresponding partition functions,
and how they relate to the appropriate thermodynamic function.
Ensemble type Partition function Thermodynamic function
Helmholtz free energy, F
Canonical Z
N
(N , V, T ) F =−k
B
T ln Z
N
Grand-canonical (μ, V , T ) Thermodynamic potential,
=−PV =−k
B
T ln
Isothermal–isobaric (N , P, T ) Gibbs free energy, G
G =−k
B
T ln
(V )
2
¯
V
=−
1
¯
V
∂
¯
V
∂(βP)
*
*
*
*
T,N
=
κ
T
β
. (1.2.53)
The above results for the different thermodynamic properties corresponding to the canoni-
cal, grand-canonical, and isobaric ensembles are shown in Table 1.1.
1.2.3 The static structure factor
In the present section we discuss the correlation functions of fluctuations of dynamic
variables at different spatial points but at the same time. These represent the static or
the structural properties of the liquid. For a high-density liquid the most basic quantity
characteristic of its structural or thermodynamic properties is the pair correlation function.
The pair correlation function g(r)
For an N -particle system the equilibrium solution to the Liouville equation (1.1.22) is
given by the Gibbsian distributions discussed in the previous section. Let us consider
the reduced probability distributions f
s
eq
(r
s
, p
s
) of s particles as defined in eqn. (1.1.32),
specifically for the equilibrium fluid. We use the short-hand notation r
s
≡{r
1
, r
2
,...,r
s
},
etc. here. The momentum dependence of the distribution function f
s
eq
in this case of a
thermodynamic equilibrium state is Maxwellian,
f
(s)
eq
(r
s
) =
&
N
%
s=1
φ
MB
(p
s
)
'
n
(s)
N
(r
s
), (1.2.54)
where φ
MB
(p
s
) is the normalized Maxwell distribution function as defined in eqn. (1.1.44).
The conditional probability of there being s particles simultaneously at {r
1
,..., r
s
} irre-
spective of their momenta is obtained from the corresponding f
(s)
eq
by integrating out the
momentum variables {p
s
},
n
(s)
N
(r
s
) =
dp
s
f
(s)
eq
(r
s
, p
s
). (1.2.55)
The simplest example of the above distribution function is the s = 2 case, i.e., g
N
(r
1
, r
2
),
which is termed the radial distribution function. It represents the conditional probability

20 Statistical physics of liquids
of a particle being at r
2
while another is at r
1
. In general a distribution function at the
s-particle level is defined from n
(s)
N
(r
s
),
g
(s)
N
(r
1
, r
2
,...,r
s
) =
n
(s)
N
(r
1
, r
2
,...,r
s
)
(n
N
)
s
, (1.2.56)
where we define n
N
= N/V , the number of particles per unit volume in the N -particle
system. In the grand-canonical-ensemble description, we average the reduced distribution
function n
(s)
N
(r
s
) over the total number of particles N in the system to obtain the averaged
quantity
n
(s)
(r
s
) ≡
+
n
(s)
N
(r
s
)
,
=
∞
N =s
P(N )n
N
(r
s
). (1.2.57)
The simplest and most important is the s = 2 case. The thermodynamic quantity g(r
1
, r
2
)
termed the pair correlation function is defined as
n
(2)
(r
1
, r
2
) = n
2
g(r
1
, r
2
), (1.2.58)
where the factor n
2
on the LHS is for normalization of g(r); n is the average number of
particles per unit volume of the liquid, n =
¯
N /V =n
N
. Some useful properties of
this function follow directly from simple physical considerations. Thus, in the equilibrium
state translational invariance holds and for an isotropic system g(r
1
, r
2
) is a function only
of the distance between the two points and can be denoted as g(r
12
). For a small sep-
aration r between two points g(r) vanishes due to the repulsive core of the interaction
potential, whereas, for large r , g(r) → 1 so that the probability of two particles being at
the two points goes to the trivial limit n
2
. The radial distribution function g(r) is the most
fundamental quantity in describing the thermodynamic properties of the equilibrium fluid.
In this respect it is important to note two very basic relations linking the thermodynamic
properties of a liquid to the pair correlation function.
The virial equation
For a liquid in which the interaction between the particles involves only two-body poten-
tials as introduced in the expression (1.1.16) for the Hamiltonian, the thermodynamic
pressure is obtained in terms of the pair correlation function only. To demonstrate this, we
begin from the thermodynamic relation P =−(∂ F/∂ V )
T
with the free energy being given
by F =−k
B
T ln Z
N
. The canonical partition function Z
N
is obtained for the interacting
system as
Z
N
=
1
N !
3N
0
dr
N
exp
[
−βU (r
1
,..., r
1
)
]
, (1.2.59)
where
0
is the thermal de Broglie wavelength as defined with eqn. (1.2.26). The pressure
is obtained as
P =
1
N !
3N
0
1
Z
N
∂
∂V
Z
N
*
*
*
*
*
T
. (1.2.60)
