Cotton W.R., Pielke R.A. Human Impacts on Weather and Climate
Подождите немного. Документ загружается.

Concluding remarks 149
For some incomprehensible reason, funding of inadvertent weather modification
has apparently been tied to funding of advertent weather modification. To some
extent, funding for inadvertent weather modification fell through the cracks of the
funding agencies. It does not fit into the mission-oriented agencies that have sup-
ported planned weather modification, so little support came from those agencies.
It does fit within the mission of the Environmental Protection Agency (EPA), but
the research program there, with respect to inadvertent local and regional weather
and climate changes, was seriously weakened under the Reagan administration.
Because inadvertent modification and planned weather modification were placed
within the same program in the National Science Foundation, cuts in the planned
weather modification program also cut the already meager program in inadvertent
weather modification. Again, the lack of a lead agency or a funded, coordinated
program in weather modification appears to be limiting progress in furthering
our quantitative understanding of deliberate and inadvertent human impacts on
weather and climate on the local and regional scale.
Part III
Human impacts on global climate
8
Overview of global climate forcings and feedbacks
8.1 Overview
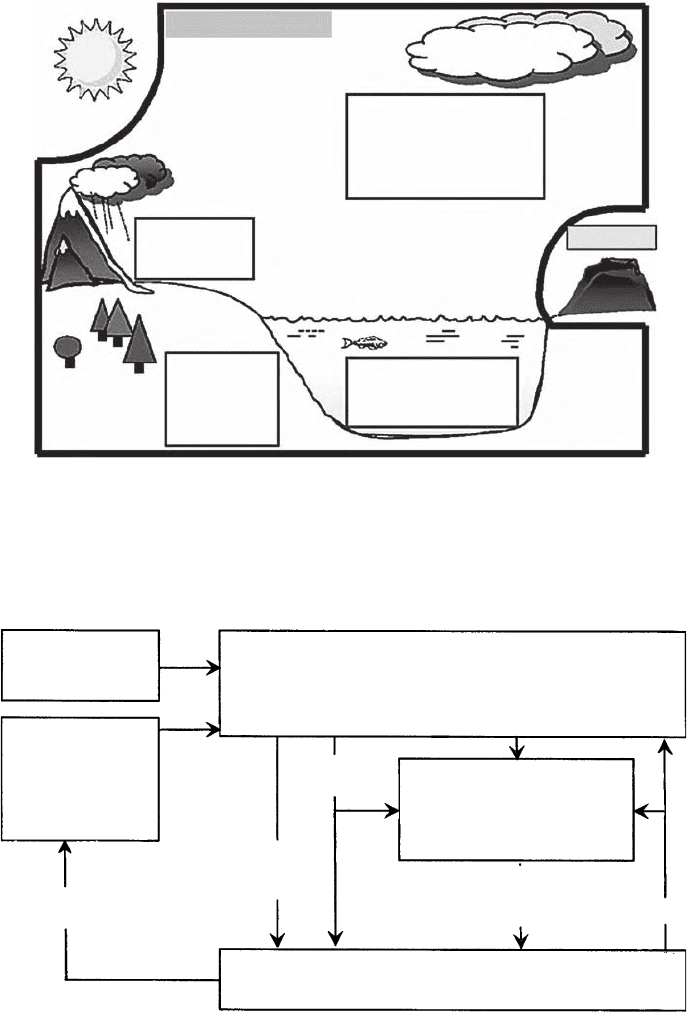
The Earth’s global climate system consists of the atmosphere, oceans, land, and
continental glaciers as illustrated in Fig. 8.1. In this framework, variables such as
salinity, soil moisture, and flora are integral to the functioning of this dynamic
system. All of these variables are climate variables. Several of these climate
forcings were discussed in a regional context in Chapters 5 through 7. Here
we present a global perspective. This definition of climate is broader than the
definition of climate as long-term weather statistics (Pielke, 1998).
A climate forcing is defined as “an energy imbalance imposed on the climate
system either externally or by human activities” (National Research Council,
2005). Climate forcing can be separated into radiative and non-radiative forc-
ing following the definitions provided in National Research Council (2005). A
radiative forcing is reported in the climate change scientific literature as a change
in energy flux at the tropopause, calculated in units of watts per square meter.
A non-radiative forcing is a climate forcing that creates an energy imbalance that
does not immediately involve radiation. An example is the increasing latent heat
flux resulting from agricultural irrigation. A direct forcing is a climate forcing that
directly affects the radiative budget of the Earth’s climate system. For example,
this perturbation may be due to a change in concentration of the radiatively active
gases, a change in solar radiation reaching the Earth, or changes in surface albedo.
A climate feedback is an amplification or dampening of the climate response to
a specific forcing due to changes in the atmosphere, oceans, land, or continental
glaciers. These definitions are summarized in Appendix C as given in National
Research Council (2005).
Figure 8.2 schematically illustrates the relation of climate forcings to the climate
response. Also included in this figure are identified forcings, which are discussed
in this chapter.
153
154 Overview of global climate forcings and feedbacks
The climate system
Atmosphere
Cryosphere
Land
Sun
Oceans
– Temperature
– Currents
– Salinity
– Marine
biota
– Temperature
– Humidity, clouds, and winds
– Precipitation
– Atmospheric trace gas and
aerosol distribution
– Snow cover
– Ice cover
– Temperature
– Soil moisture
– Fauna and
flora
Volcanoes
Figure 8.1 The climate system, consisting of the atmosphere, oceans, and land.
Critical state variables for each sphere of the climate system are listed in the
boxes. For the purposes of this report, the Sun, volcanic emissions, and human-
caused emissions of greenhouse gases or changes to the land surface are consid-
ered external to the climate system. From National Research Council (2005).
NATURAL
PROCESSES
Sun, orbit, volcanoes
HUMAN
ACTIVITIES
FORCING AGENTS
CHANGE IN CLIMATE
SYSTEM COMPONENTS
CLIMATE RESPONSE
Temperature, precipitation, vegetation, etc.
• Emissions of greenhouse gases and precursors, aerosols and
precursors, and biogeochemically active gases
• Solar irradiance and insolation changes
• Land-cover changes
• Fuel usage
• Industrial practices
• Agricultural
practices
• Atmospheric lapse rate
• Atmospheric composition
• Evapotranspiration flux
Societal
impacts
Direct
radiative
forcings
Indirect
radiative
forcings
Feedback
s
Non-radiative
forcings
Figure 8.2 Conceptual framework for the climate forcings that impact the
physical components of the climate system under present-day conditions. From
National Research Council (2005).
Atmospheric radiation 155
8.2 Atmospheric radiation
The principal source of energy driving the atmospheric/ocean system comes from
the Sun. In order to understand the potential for human impact on global climate,
we must have at least a general understanding of how the Sun’s energy is dis-
tributed through the atmosphere–Earth–ocean system. The Sun emits most of its
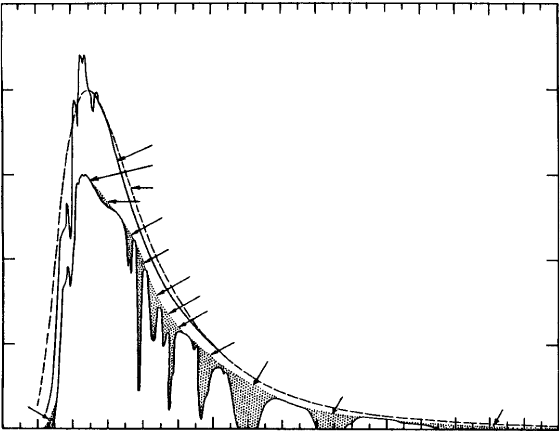
radiant energy over wavelengths ranging from 0.2 to 18 m, with a peak in inten-
sity at 0 470 m. As shown in Fig. 8.3, the spectrum of energy emitted by the Sun
closely approximates the energy spectrum emitted by a perfect absorber–emitter
substance (or what we call a blackbody) having a temperature of 5900 K. On
the other hand the spectrum of energy emitted by the Earth emits radiant energy
with a peak in spectral density at wavelengths between 10 and 15 m. Thus the
combination of radiation energy emitted by the Sun as received by the Earth at
its orbital distance and emitted by the Earth span from less than 02 m to greater
than 50 m. The spectrum of energy emitted by the Sun and the Earth, however,
exhibit very little overlap. We therefore refer to the energy emitted by the Sun as
shortwave radiation and the energy emitted by the Earth and its atmosphere as
longwave radiation.
Solar irradiation curve outside atmosphere
Solar irradiation curve at sea level
Curve for blackbody at 5900 K
O
3
O
3
H
2
O
H
2
O
H
2
O
H
2
O
H
2
O
O
2
, H
2
O
H
2
O, CO
2
H
2
O, CO
2
H
2
O, CO
2
0.25
0.20
0.15
0.10
0.05
0
0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2
WAVELENGTH (μm)
H
λ
(W m
–2
Å
–1
)
Figure 8.3 Spectral distribution curves related to the Sun; shaded areas indicate
absorption at sea level, due to the atmospheric constituents shown. From Gast
et al. (1965). © McGraw-Hill; Reprinted by permission.
156 Overview of global climate forcings and feedbacks
As atmospheric radiation passes through the atmosphere it interacts with the
gases and particulates. Some of the radiant energy is absorbed, some scattered,
and some unaffected. The amount of energy absorbed and scattered varies with
the wavelength of the radiant energy, the type of gases, and the size and chemical
composition of the particles. The absorbed energy is converted to heat in the
atmosphere and that energy is reradiated at a wavelength and intensity that corre-
sponds to the temperature and composition of the absorbing gases or particulates.
The amount of energy that is scattered, however, is simply redirected in space (or
reflected) with no change in intensity. The amount of energy scattered and the
directions in which it is scattered depends on the wavelength of the radiation as
well as the types of gases and size and composition of particles.
8.2.1 Absorption and scattering by gases
As shown in Fig. 8.3, the energy spectrum incident at the top of the atmosphere is
significantly attenuated by the time it reaches the Earth’s surface at sea level. It is
not attenuated uniformly across the spectrum, however, but instead major energy
losses occur over rather narrow bands, called absorption bands. In a cloud-free
clean atmosphere the primary absorbers of shortwave radiation are ozone and
water vapor. Airborne particles, or aerosols, in typical non-polluted air contribute
less to absorption. At wavelengths less than 03 m, oxygen and nitrogen absorb
nearly all the incoming solar radiation in the upper atmosphere. Between 0.3 and
08 m, however, little gaseous absorption occurs. Only weak absorption by ozone
takes place over this spectral range. At wavelengths less than 08 m Rayleigh
scattering by oxygen and nitrogen molecules also depletes the radiant energy that
reaches the surface. At longer wavelengths absorption in various water vapor
bands is quite pronounced while weak absorption by carbon dioxide and ozone
also occurs.
Absorption of longwave radiation occurs in a series of bands. The principal nat-
ural absorbers of longwave radiation are water vapor, carbon dioxide, and ozone.
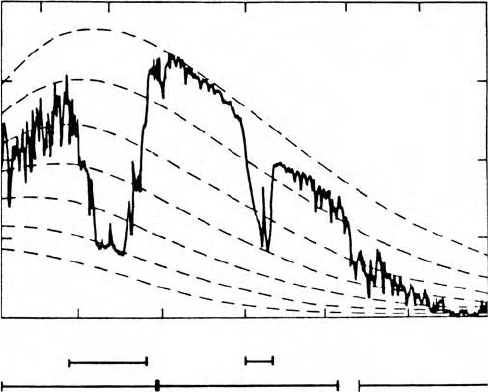
Figure 8.4 illustrates the infrared spectrum obtained by a scanning interferometer
looking downward from a satellite over a desert. Strong absorption by carbon
dioxide at a wavelength band centered at 147 m is shown by the emission of
radiance at a temperature of 220 K which is representative of stratospheric tem-
peratures. The stratosphere contributes mainly to the peak of this band. Water
vapor absorption bands at 1.4, 1.9, 2.7, and 63 m, and greater than 20 m,
cause emissions corresponding to midtropospheric temperatures. Little absorption
is evident in the region called the atmospheric window between 8 and 14 m.
Here the satellite observed radiance corresponds to the surface temperature of
the desert except for a slight depression in magnitude due to departures of the
Atmospheric radiation 157
Wavelength (μm)
20
15 10 8 7
0.010
0.0050
0
0.015
400 600 1000 1200 1400 1600800
CO
2
band
O
3
band
Wavenumber (cm
–1
)
H
2
O Rotation band Atmospheric window H
2
O 6.3 μm band
320 K
280 K
240 K
200 K
Radiance (mW cm
–2
sr
–1
cm
–1
)
Figure 8.4 Atmospheric spectrum obtained with a scanning interferometer on
board the Nimbus 4 satellite. The interferometer viewed the Earth vertically as
the satellite was passing over the North African desert. After Hänel et al. (1972).
© [1972] American Geophysical Union. Reproduced with permission from the
American Geophysical Union.
emittance of sand from its block body value of unity. A distinct ozone absorption
band is evident at about 96 m in the middle of the window. Although not very
evident in the figure, weak continuous absorption also occurs across the window
believed to be due to the presence of clusters or dimers of water vapor molecules.
In summary, the principal natural gaseous absorbers of terrestrial radiation are
carbon dioxide and water vapor. These are the so-called principal “greenhouse”
gases. The basic greenhouse concept was probably first proposed by Fourier
(1827). Later, Arrhenius (1896) calculated the effects of varying carbon dioxide
concentrations on surface temperatures and even included the impact of water
vapor absorption in his calculations. He estimated that a 2.5- to 3-fold increase in
carbon dioxide would increase temperatures in Arctic regions by 8–9
C. His intent
was to examine the impact of greenhouse gases on ice ages and glaciation and
was not particularly concerned about human sources. As early as 1938, Callendar
(1938) became concerned about the impacts of anthropogenic emissions of carbon
dioxide on global temperatures. Therefore, the basic concept that anthropogenic
sources of carbon dioxide and other greenhouse gases can warm the atmosphere
is not new, but reports suggesting that global average surface temperatures are
158 Overview of global climate forcings and feedbacks
rising has caused a great deal of concern about potential human impact on climate,
and resulted in major research activities in global climate change.
In the absence of so-called greenhouse gases, the average surface temperature
of the Earth would be over 30
C cooler than it is today. This is because the
greenhouse gases absorb upwelling terrestrial radiation and radiate the absorbed
gases, both upward and downward, causing a net gain in energy (reduced loss)
at the Earth’s surface. Without those greenhouse gases, a larger fraction of the
upwelling terrestrial radiation would escape through the atmospheric window to
space. The major greenhouse gas is water vapor which varies naturally in space
and time due the Earth’s hydrological cycle (e.g., see Randall and Tjemkes, 1991).
The second most important greenhouse gas is carbon dioxide. In contrast to water
vapor, carbon dioxide is rather uniformly distributed throughout the troposphere,
although the radiative forcing associated with it is more heterogeneous as a result
of spatial (e.g., latitudinal) and temporal variations in tropospheric temperature
and water vapor concentrations, and in surface emissions and absorption.
We discuss the greenhouse effect further in Subsection 8.2.4. Other strong
absorbers of terrestrial radiation are methane, nitrous oxide, and chlorofluoro-
carbons (CFCs). Currently the concentrations of these gases are so small that
their contributions to infrared absorption cannot be easily detected by satellite.
The concentrations of CFCs, for example, are six orders of magnitude (a million
times) smaller than carbon dioxide. The concern about CFCs is that per molecule
they are 20 000 times more effective at absorbing infrared radiation than carbon
dioxide.
8.2.2 Absorption and scattering by aerosols
The radiative properties of aerosol particles is a complicated function of their
chemistry, shape, and size spectra. Moreover, if the aerosol particles are hygro-
scopic, their radiative properties change with the relative humidity of the air.
At relative humidities greater than 70%, the hygroscopic particles (called haze)
take on water vapor molecules and swell in size, thus changing their radiative
properties not only because of size effects but also because of changes in their
complex indices of refraction as the water-solution–particle mixture changes in
relative amounts.
Dry aerosol particles in high concentrations such as over major polluted urban
areas and over deserts can cause substantial absorption and scattering of solar
radiation. In some polluted boundary layers, aerosol absorption has been estimated
to result in heating rates on the order of a few tenths to several degrees per
hour (Braslau and Dave, 1975; Welch and Zdunkowski, 1976). In the Saharan
dust layer, aerosol absorption has been calculated to produce a heating of 1–2
C
