Cohen I.M., Kundu P.K. Fluid Mechanics
Подождите немного. Документ загружается.


12 Introduction
Example 1.1. With reference to Figure 1.7, show that the rise of a liquid in a narrow
tube of radius R is given by
h =
2σ sin α
ρgR
,
where σ is the surface tension and α is the “contact” angle.
Solution. Since the free surface is concave upward and exposed to the atmo-
sphere, the pressure just below the interface at point E is below atmospheric. The
pressure then increases linearly along EF. At F the pressure again equals the atmo-
spheric pressure, since F is at the same level as G where the pressure is atmospheric.
The pressure forces on faces AB and CD therefore balance each other. Vertical equi-
librium of the element ABCD then requires that the weight of the element balances
the vertical component of the surface tension force, so that
σ(2πR)sin α = ρgh(πR
2
),
which gives the required result.
8. Classical Thermodynamics
Classical thermodynamics is the study of equilibrium states of matter, in which the
properties are assumed uniform in space and time. The reader is assumed to be familiar
with the basic concepts of this subject. Here we give a review of the main ideas and
the most commonly used relations in this book.
A thermodynamic system is a quantity of matter separated from the surroundings
by a flexible boundary through which the system exchanges heat and work, but no
mass. A system in the equilibrium state is free of currents, such as those generated
by stirring a fluid or by sudden heating. After a change has taken place, the currents
die out and the system returns to equilibrium conditions, when the properties of the
system (such as pressure and temperature) can once again be defined.
This definition, however, is not possible in fluid flows, and the question arises as
to whether the relations derived in classical thermodynamics are applicable to fluids
in constant motion. Experiments show that the results of classical thermodynamics
do hold in most fluid flows if the changes along the motion are slow compared to a
relaxation time. The relaxation time is defined as the time taken by the material to
adjust to a new state, and the material undergoes this adjustment through molecular
collisions. The relaxation time is very small under ordinary conditions, since only
a few molecular collisions are needed for the adjustment. The relations of classical
thermodynamics are therefore applicable to most fluid flows.
The basic laws of classical thermodynamics are empirical, and cannot be proved.
Another way of viewing this is to say that these principles are so basic that they
cannot be derived from anything more basic. They essentially establish certain basic
definitions, upon which the subject is built. The first law of thermodynamics can be
regarded as a principle that defines the internal energy of a system, and the second
law can be regarded as the principle that defines the entropy of a system.

8. Classical Thermodynamics 13
First Law of Thermodynamics
The first law of thermodynamics states that the energy of a system is conserved. It
states that
Q + W = e, (1.10)
where Q is the heat added to the system, W is the work done on the system, and e
is the increase of internal energy of the system. All quantities in equation (1.10) may
be regarded as those referring to unit mass of the system. (In thermodynamics texts it
is customary to denote quantities per unit mass by lowercase letters, and those for the
entire system by uppercase letters. This will not be done here.) The internal energy
(also called “thermal energy”) is a manifestation of the random molecular motion of
the constituents. In fluid flows, the kinetic energy of the macroscopic motion has to be
included in the term e in equation (1.10) in order that the principle of conservation of
energy is satisfied. For developing the relations of classical thermodynamics, however,
we shall only include the “thermal energy” in the term e.
It is important to realize the difference between heat and internal energy. Heat and
work are forms of energy in transition, which appear at the boundary of the system
and are not contained within the matter. In contrast, the internal energy resides within
the matter. If two equilibrium states 1 and 2 of a system are known, then Q and W
depend on the process or path followed by the system in going from state 1 to state 2.
The change e = e
2
− e
1
, in contrast, does not depend on the path. In short, e is a
thermodynamic property and is a function of the thermodynamic state of the system.
Thermodynamic properties are called state functions, in contrast to heat and work,
which are path functions.
Frictionless quasi-static processes, carried out at an extremely slow rate so that
the system is at all times in equilibrium with the surroundings, are called reversible
processes. The most common type of reversible work in fluid flows is by the expansion
or contraction of the boundaries of the fluid element. Let v = 1/ρ be the specific
volume, that is, the volume per unit mass. Then the work done by the body per unit
mass in an infinitesimal reversible process is −pdv, where dv is the increase of v.
The first law (equation (1.10)) for a reversible process then becomes
de = dQ − pdv, (1.11)
provided that Q is also reversible.
Note that irreversible forms of work, such as that done by turning a paddle wheel,
are excluded from equation (1.11).
Equations of State
In simple systems composed of a single component only, the specification of two
independent properties completely determines the state of the system. We can write
relations such as
p = p(v, T ) (thermal equation of state),
e = e(p, T ) (caloric equation of state).
(1.12)
14 Introduction
Such relations are called equations of state. For more complicated systems composed
of more than one component, the specification of two properties is not enough to
completely determine the state. For example, for sea water containing dissolved salt,
the density is a function of the three variables, salinity, temperature, and pressure.
Specific Heats
Before we define the specific heats of a substance, we define a thermodynamic prop-
erty called enthalpy as
h ≡ e + pv. (1.13)
This property will be quite useful in our study of compressible fluid flows.
For single-component systems, the specific heats at constant pressure and con-
stant volume are defined as
C
p
≡
∂h
∂T
p
, (1.14)
C
v
≡
∂e
∂T
v
. (1.15)
Here, equation (1.14) means that we regard h as a function of p and T , and find the
partial derivative of h with respect to T , keeping p constant. Equation (1.15) has an
analogous interpretation. It is important to note that the specific heats as defined are
thermodynamic properties, because they are defined in terms of other properties of
the system. That is, we can determine C
p
and C
v
when two other properties of the
system (say, p and T ) are given.
For certain processes common in fluid flows, the heat exchange can be related
to the specific heats. Consider a reversible process in which the work done is given
by pdv, so that the first law of thermodynamics has the form of equation (1.11).
Dividing by the change of temperature, it follows that the heat transferred per unit
mass per unit temperature change in a constant volume process is
dQ
dT
v
=
∂e
∂T
v
= C
v
.
This shows that C
v
dT represents the heat transfer per unit mass in a reversible
constant volume process, in which the only type of work done is of the pdv type.
It is misleading to define C
v
= (dQ/dT )
v
without any restrictions imposed, as the
temperature of a constant-volume system can increase without heat transfer, say, by
turning a paddle wheel.
In a similar manner, the heat transferred at constant pressure during a reversible
process is given by
dQ
dT
p
=
∂h
∂T
p
= C
p
.

8. Classical Thermodynamics 15
Second Law of Thermodynamics
The second law of thermodynamics imposes restriction on the direction in which
real processes can proceed. Its implications are discussed in Chapter 4. Some conse-
quences of this law are the following:
(i) There must exist a thermodynamic property S, known as entropy, whose
change between states 1 and 2 is given by
S
2
− S
1
=
2
1
dQ
rev
T
, (1.16)
where the integral is taken along any reversible process between the two states.
(ii) For an arbitrary process between 1 and 2, the entropy change is
S
2
− S
1
2
1
dQ
T
(Clausius-Duhem),
which states that the entropy of an isolated system (dQ = 0) can only increase.
Such increases are caused by frictional and mixing phenomena.
(iii) Molecular transport coefficients such as viscosity µ and thermal conductivity
k must be positive. Otherwise, spontaneous “unmixing” would occur and lead
to a decrease of entropy of an isolated system.
TdSRelations
Two common relations are useful in calculating the entropy changes during a process.
For a reversible process, the entropy change is given by
TdS = dQ. (1.17)
On substituting into (1.11), we obtain
TdS = de + pdv
TdS = dh − vdp
(Gibbs), (1.18)
where the second form is obtained by using dh = d(e + pv) = de + pdv +
vdp. It is interesting that the “TdSrelations” in equations (1.18) are also valid for
irreversible (frictional) processes, although the relations (1.11) and (1.17), from which
equations (1.18) is derived, are true for reversible processes only. This is because
equations (1.18) are relations between thermodynamic state functions alone and are
therefore true for any process. The association of TdSwith heat and −pdv with
work does not hold for irreversible processes. Consider paddle wheel work done at
constant volume so that de = TdSis the element of work done.
16 Introduction
Speed of Sound
In a compressible medium, infinitesimal changes in density or pressure propagate
through the medium at a finite speed. In Chapter 16, we shall prove that the square
of this speed is given by
c
2
=
∂p
∂ρ
s
, (1.19)
where the subscript “s” signifies that the derivative is taken at constant entropy. As
sound is composed of small density perturbations, it also propagates at speed c.For
incompressible fluids ρ is independent of p, and therefore c =∞.
Thermal Expansion Coefficient
In a system whose density is a function of temperature, we define the thermal expan-
sion coefficient
α ≡−
1
ρ
∂ρ
∂T
p
, (1.20)
where the subscript “p” signifies that the partial derivative is taken at constant pressure.
The expansion coefficient will appear frequently in our studies of nonisothermal
systems.
9. Perfect Gas
A relation defining one state function of a gas in terms of two others is called an
equation of state. A perfect gas is defined as one that obeys the thermal equation of
state
p = ρRT,
(1.21)
where p is the pressure, ρ is the density, T is the absolute temperature, and R is the
gas constant. The value of the gas constant depends on the molecular mass m of the
gas according to
R =
R
u
m
, (1.22)
where
R
u
= 8314.36 J kmol
−1
K
−1
is the universal gas constant. For example, the molecular mass for dry air is
m = 28.966 kg/kmol, for which equation (1.22) gives
R = 287 J kg
−1
K
−1
for dry air.
9. Perfect Gas 17
Equation (1.21) can be derived from the kinetic theory of gases if the attractive
forces between the molecules are negligible. At ordinary temperatures and pressures
most gases can be taken as perfect.
The gas constant is related to the specific heats of the gas through the relation
R = C
p
− C
v
, (1.23)
where C
p
is the specific heat at constant pressure and C
v
is the specific heat at constant
volume. In general, C
p
and C
v
of a gas, including those of a perfect gas, increase with
temperature. The ratio of specific heats of a gas
γ ≡
C
p
C
v
, (1.24)
is an important quantity. For air at ordinary temperatures, γ = 1.4 and C p =
1005 J kg
−1
K
−1
.
It can be shown that assertion (1.21) is equivalent to
e = e(T )
h = h(T )
and conversely, so that the internal energy and enthalpy of a perfect gas can only be
functions of temperature alone. See Exercise 7.
A process is called adiabatic if it takes place without the addition of heat. A
process is called isentropic if it is adiabatic and frictionless, for then the entropy of
the fluid does not change. From equation (1.18) it is easy to show that the isentropic
flow of a perfect gas with constant specific heats obeys the relation
p
ρ
γ
= const. (isentropic) (1.25)
Using the equation of state p = ρRT , it follows that the temperature and density
change during an isentropic process from state 1 to state 2 according to
T
1
T
2
=
p
1
p
2
(γ −1)/γ
and
ρ
1
ρ
2
=
p
1
p
2
1/γ
(isentropic) (1.26)
See Exercise 8. For a perfect gas, simple expressions can be found for several
useful thermodynamic properties such as the speed of sound and the thermal expansion
coefficient. Using the equation of state p = ρRT , the speed of sound (1.19) becomes
c =
γRT, (1.27)
where equation (1.25) has been used. This shows that the speed of sound increases
as the square root of the temperature. Likewise, the use of p = ρRT shows that the

18 Introduction
thermal expansion coefficient (1.20) is
α =
1
T
,
(1.28)
10. Static Equilibrium of a Compressible Medium
In an incompressible fluid in which the density is not a function of pressure, there is
a simple criterion for determining the stability of the medium in the static state. The
criterion is that the medium is stable if the density decreases upward, for then a particle
displaced upward would find itself at a level where the density of the surrounding
fluid is lower, and so the particle would be forced back toward its original level. In
the opposite case in which the density increases upward, a displaced particle would
continue to move farther away from its original position, resulting in instability. The
medium is in neutral equilibrium if the density is uniform.
For a compressible medium the preceding criterion for determining the stability
does not hold. We shall now show that in this case it is not the density but the entropy
that is constant with height in the neutral state. For simplicity we shall consider the
case of an atmosphere that obeys the equation of state for a perfect gas. The pressure
decreases with height according to
dp
dz
=−ρg.
A particle displaced upward would expand adiabatically because of the decrease of
the pressure with height. Its original density ρ
0
and original temperature T
0
would
therefore decrease to ρ and T according to the isentropic relations
T
T
0
=
p
p
0
(γ −1)/γ
and
ρ
ρ
0
=
p
p
0
1/γ
, (1.29)
where γ = C
p
/C
v
, and the subscript 0 denotes the original state at some height z
0
,
where p
0
>p(Figure 1.8). It is clear that the displaced particle would be forced back
toward the original level if the new density is larger than that of the surrounding air
at the new level. Now if the properties of the surrounding air also happen to vary
with height in such a way that the entropy is uniform with height, then the displaced
particle would constantly find itself in a region where the density is the same as that
of itself. Therefore, a neutral atmosphere is one in which p, ρ, and T decrease in
such a way that the entropy is constant with height. A neutrally stable atmosphere is
therefore also called an isentropic or adiabatic atmosphere. It follows that a statically
stable atmosphere is one in which the density decreases with height faster than in an
adiabatic atmosphere.

10. Static Equilibrium of a Compressible Medium 19
Figure 1.8 Adiabatic expansion of a fluid particle displaced upward in a compressible medium.
It is easy to determine the rate of decrease of temperature in an adiabatic atmo-
sphere. Taking the logarithm of equation (1.29), we obtain
ln T
a
− ln T
0
=
γ − 1
γ
[ln p
a
− ln p
0
],
where we are using the subscript “a” to denote an adiabatic atmosphere. A differen-
tiation with respect to z gives
1
T
a
dT
a
dz
=
γ − 1
γ
1
p
a
dp
a
dz
.
Using the perfect gas law p = ρRT , C
p
− C
v
= R, and the hydrostatic rule
dp/dz =−ρg, we obtain
dT
a
dz
≡
a
=−
g
C
p
(1.30)
where ≡ dT /dz is the temperature gradient;
a
=−g/C
p
is called the adiabatic
temperature gradient and is the largest rate at which the temperature can decrease
with height without causing instability. For air at normal temperatures and pressures,
the temperature of a neutral atmosphere decreases with height at the rate of g/C
p
10
◦
C/km. Meteorologists call vertical temperature gradients the “lapse rate,” so that
in their terminology the adiabatic lapse rate is 10
◦
C/km.
Figure 1.9a shows a typical distribution of temperature in the atmosphere. The
lower part has been drawn with a slope nearly equal to the adiabatic temperature gra-
dient because the mixing processes near the ground tend to form a neutral atmosphere,
with its entropy “well mixed” (that is, uniform) with height. Observations show that
the neutral atmosphere is “capped” by a layer in which the temperature increases with
height, signifying a very stable situation. Meteorologists call this an inversion, because
the temperature gradient changes sign here. Much of the atmospheric turbulence and
mixing processes cannot penetrate this very stable layer. Above this inversion layer the
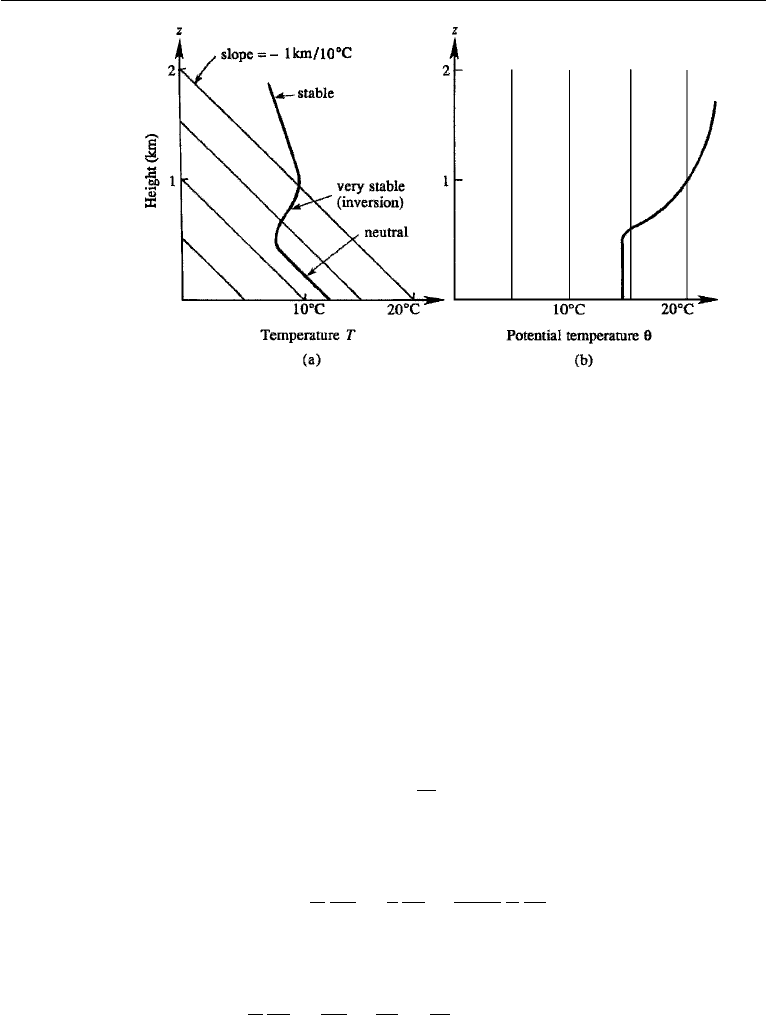
20 Introduction
Figure 1.9 Vertical variation of the (a) actual and (b) potential temperature in the atmosphere. Thin
straight lines represent temperatures for a neutral atmosphere.
temperature decreases again, but less rapidly than near the ground, which corresponds
to stability. It is clear that an isothermal atmosphere (a vertical line in Figure 1.9a) is
quite stable.
Potential Temperature and Density
The foregoing discussion of static stability of a compressible atmosphere can be
expressed in terms of the concept of potential temperature, which is generally denoted
by θ. Suppose the pressure and temperature of a fluid particle at a certain height are
p and T . Now if we take the particle adiabatically to a standard pressure p
s
(say,
the sea level pressure, nearly equal to 100 kPa), then the temperature θ attained by
the particle is called its potential temperature. Using equation (1.26), it follows that the
actual temperature T and the potential temperature θ are related by
T = θ
p
p
s
(γ −1)/γ
. (1.31)
Taking the logarithm and differentiating, we obtain
1
T
dT
dz
=
1
θ
dθ
dz
+
γ − 1
γ
1
p
dp
dz
.
Substituting dp/dz =−ρg and p = ρRT , we obtain
T
θ
dθ
dz
=
dT
dz
+
g
C
p
=
d
dz
(T − T
a
) = −
a
. (1.32)
Now if the temperature decreases at a rate =
a
, then the potential temperature θ
(and therefore the entropy) is uniform with height. It follows that the stability of the
10. Static Equilibrium of a Compressible Medium 21
atmosphere is determined according to
dθ
dz
> 0 (stable),
dθ
dz
= 0 (neutral), (1.33)
dθ
dz
< 0 (unstable).
This is shown in Figure 1.9b. It is the gradient of potential temperature that determines
the stability of a column of gas, not the gradient of the actual temperature. However,
the difference between the two is negligible for laboratory-scale phenomena. For
example, over a height of 10 cm the compressibility effects result in a decrease of
temperature in the air by only 10 cm × (10
◦
C/km) = 10
−3 ◦
C.
Instead of using the potential temperature, one can use the concept of potential
density ρ
θ
, defined as the density attained by a fluid particle if taken isentropically to
a standard pressure p
s
. Using equation (1.26), the actual and potential densities are
related by
ρ = ρ
θ
p
p
s
1/γ
. (1.34)
Multiplying equations (1.31) and (1.34), and using p = ρRT, we obtain
θρ
θ
= p
s
/R = const. Taking the logarithm and differentiating, we obtain
−
1
ρ
θ
dρ
θ
dz
=
1
θ
dθ
dz
. (1.35)
The medium is stable, neutral, or unstable depending upon whether dρ
θ
/dz is nega-
tive, zero, or positive, respectively.
Compressibility effects are also important in the deep ocean. In the ocean the
density depends not only on the temperature and pressure, but also on the salinity,
defined as kilograms of salt per kilogram of water. (The salinity of sea water is ≈3%.)
Here, one defines the potential density as the density attained if a particle is taken to
a reference pressure isentropically and at constant salinity. The potential density thus
defined must decrease with height in stable conditions. Oceanographers automatically
account for the compressibility of sea water by converting their density measurements
at any depth to the sea level pressure, which serves as the reference pressure.
From (1.32), the temperature of a dry neutrally stable atmosphere decreases
upward at a rate dT
a
/dz =−g/C
p
due to the decrease of pressure with height and
the compressibility of the medium. Static stability of the atmosphere is determined
by whether the actual temperature gradient dT /dz is slower or faster than dT
a
/dz.To
determine the static stability of the ocean, it is more convenient to formulate the crite-
rion in terms of density. The plan is to compare the density gradient of the actual static
state with that of a neutrally stable reference state (denoted here by the subscript “a”).
