Chung Y.-W. Practical guide to surface science and spectroscopy
Подождите немного. Документ загружается.


27
2.4 ENERGIES AND SHAPES OF AUGER PEAKS
2.4 ENERGIES AND SHAPES OF AUGER PEAKS
Consider the case of a free atom with discrete energy levels. As dis-
cussed previously, the Auger electron energy E
WXY
due to an Auger
WXY transition is given by
E
WXY
⫽ E
W
⫺ E
X
⫺ E
Y
⫺
(2.1)
where is the work function and E
W
is the binding energy of the
W-level for the neutral atom. But E
X
and E
Y
are not the X and Y shell
binding energies for the neutral atom. When the W-level electron is
removed, the resulting species is a positive ion, so that electrons in the
X and Y shells are moving in a potential of a ⫹e ion. Because of
screening effects, the actual charge experienced by these electrons may
be less than ⫹e. One may approximate E
X
and E
Y
as
E
i
⫽
E
i
(Z) ⫹ E
i
(Z ⫹ 1)
2
(2.2)
where Z is the nuclear charge of the atom and i ⫽ XorY.
E
XAMPLE.
Calculate the kinetic energies of all KLL Auger peaks
from a clean polycrystalline aluminum surface, with information pro-
vided below:
Binding energy of 1s (K) electrons of Al ⫽ 1559.6 eV
Binding energy of 2s (L
1
) electrons of Al ⫽ 117.7 eV
Binding energy of 2p(L
23
) electrons of Al ⫽ 73.1 eV
Binding energy of 2s(L
1
) electrons of Si ⫽ 148.7 eV
Binding energy of 2p(L
23
) electrons of Si ⫽ 99.2 eV
Work function of polycrystalline Al ⫽ 4.2 eV
S
OLUTION.
There are three possible KLL transitions, viz., KL
1
L
1
,
KL
1
L
23
, and KL
23
L
23
. Let us first calculate all the required energy
levels:
E
K
⫽ 1559.6 eV
E
L1
⫽ 0.5(117.7 ⫹ 148.7 ⫽ 133.2 eV
E
L23
⫽ 0.5(73.1 ⫹ 99.2) ⫽ 86.15 eV.
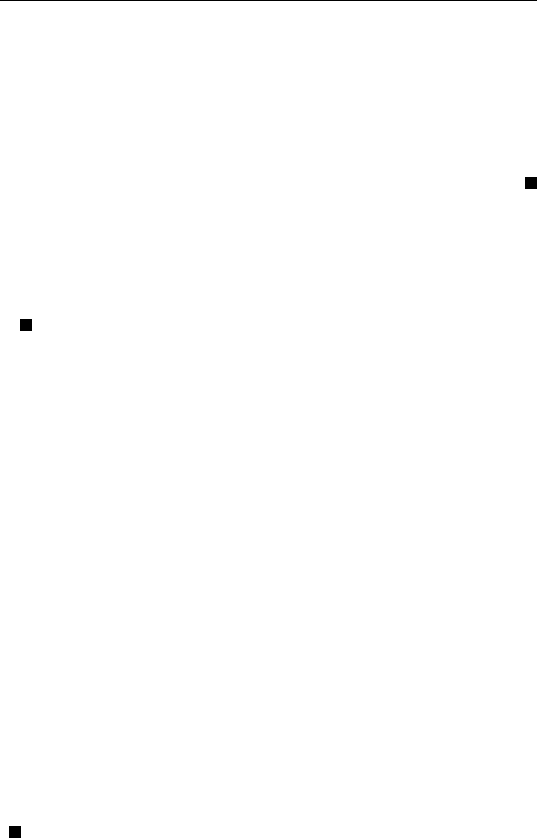
28
CHAPTER2/AUGER ELECTRON SPECTROSCOPY
Therefore,
E(KL
1
L
1
) ⫽ 1559.6 ⫺ 2 ⫻ 133.2 ⫺ 4.2 ⫽ 1289 eV
E(KL
1
L
23
) ⫽ 1559.6 ⫺ 133.2 ⫺ 86.15 ⫺ 4.2 ⫽ 1336.05 eV
E(KL
23
L
23
) ⫽ 1559.6 ⫺ 2 ⫻ 86.15 ⫺ 4.2 ⫽ 1383.1 eV.
Q
UESTION FOR
D
ISCUSSION.
Using a highly monoenergetic inci-
dent beam of electrons, the width of a certain neon KLL Auger transition
is determined to be 3 eV. If the incident beam energy is allowed to
have an energy spread of 10 eV, how will this affect the Ne Auger peak
width?
In some cases, electrons from the valence band are involved, either
as core–core–valence (CCV) or core–valence–valence (CVV) Auger
transitions. Most valence bands exhibit certain structures, that is, the
density of occupied electron states varies across the whole band. Most
valence bands have widths on the order of 5–15 eV. Therefore, in CCV
and CVV transitions, one would expect (1) the Auger peak width ⬇
the width of the valence band, and (2) the Auger peak shape is character-
istic of the valence band structure from which it is derived.
E
XAMPLE.
Show that the energy width of a CCV Auger peak is
equal to that of the valence band.
S
OLUTION.
The Auger kinetic energy for a CCV transition is given
by
E
CCV
⫽ E
C1
⫺ E
C2
⫺ E
V
⫺
.
The energy width ⌬E
CCV
⫽兩⌬E
V
兩, since all core levels are assumed
to be sharp, and the work function
is a constant. Therefore, the
energy width of a CCV Auger transition is equal to that of the valence
band.
2.5 CHEMICAL STATE EFFECTS
When an atom is placed under different chemical environments, two
possible changes may result in the Auger spectrum:
(a) Shift of the Auger peak position. This is caused by shifts of
electron energy levels due to electron transfer to or away from the
atom.
29
2.6 INTENSITY OF AUGER ELECTRON EMISSION
(b) Change of the Auger peak shape. This can be caused by the
change in the valence band structure. Also, as the Auger electron exits
from the solid, it may undergo energy loss (or gain) collisions resulting
in satellite features. Change of chemical environments can affect energy
loss (or gain) mechanisms and thus the overall shape of the Auger
peak. These changes can be useful in studying chemical state of atoms
on surfaces.
2.6 INTENSITY OF AUGER ELECTRON EMISSION
Three intrinsic factors affect Auger electron intensity:
(a) Ionization cross-section. Auger transitions of a given type can
be initiated only after a given core electron is ionized (K-shell electron
in the case of a KLL Auger transition). This requires the incident
electrons to have a minimum (threshold) energy E
th
equal to or greater
than the binding energy of this core electron. Above E
th
, the incident
electrons can produce ionizations at a rate directly proportional to the
incident electron flux ⌽ and the number of atoms per unit volume n,
that is,
dn
dt
⫽ n
⌽
, (2.3)
where dn/dt is the rate of ionization per unit volume, and is known
as the ionization cross-section. A schematic plot of versus incident
electron energy is shown in Fig. 2.3. Typically, maximum is reached
FIGURE 2.3 Variation of ionization cross-section with incident electron energy.

30
CHAPTER2/AUGER ELECTRON SPECTROSCOPY
when the incident electron energy is 3 to 5 times the threshold ionization
energy.
The shape of this curve can be explained as follows. Clearly,
ionization will only occur when E ⬎ E
th
. When E increases, increases.
But when E ⬎⬎ E
th
, electrons are moving very fast and spend a
proportionally shorter time near individual atoms, giving rise to smaller
number of ionizations per unit volume and hence smaller . Since
Auger transitions can only be initiated by first ionizing the atom,
one would expect the intensity of any given Auger peak to follow a
dependence similar to that shown in Fig. 2.3. Therefore, in typical
Auger studies where we are interested in Auger transitions due to inner
shells of ionization energies less than 1500 eV, the primary electron
energy is usually restricted to 5–10 keV. On occasions where high
spatial resolution is required, incident electron energies up to 30 keV
are used. In general, one would want to use the lowest possible electron
beam energy consistent with one’s requirement for optimum signal and
spatial resolution. For a given beam current, the amount of energy
deposited on the surface per unit time is proportional to the beam
voltage. Too much power may cause surface damage or accelerate
surface contamination.
(b) Auger yield. After an inner shell electron is knocked off, a
higher shell electron falls down to fill the vacancy. Following this,
there are two possibilities: Auger electron or X-ray emission. Defining
P
A
as the probability of Auger electron emission and P
X
as that of
X-ray emission, we have
P
A
⫹ P
X
⫽ 1. (2.4)
For instance, if one measures P
A
and P
X
due to K-shell vacancies, one
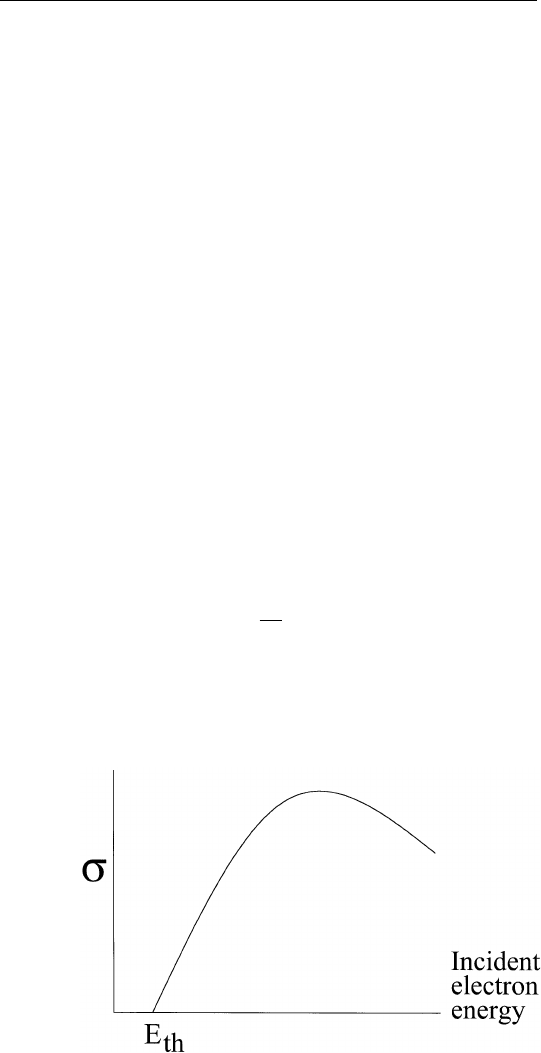
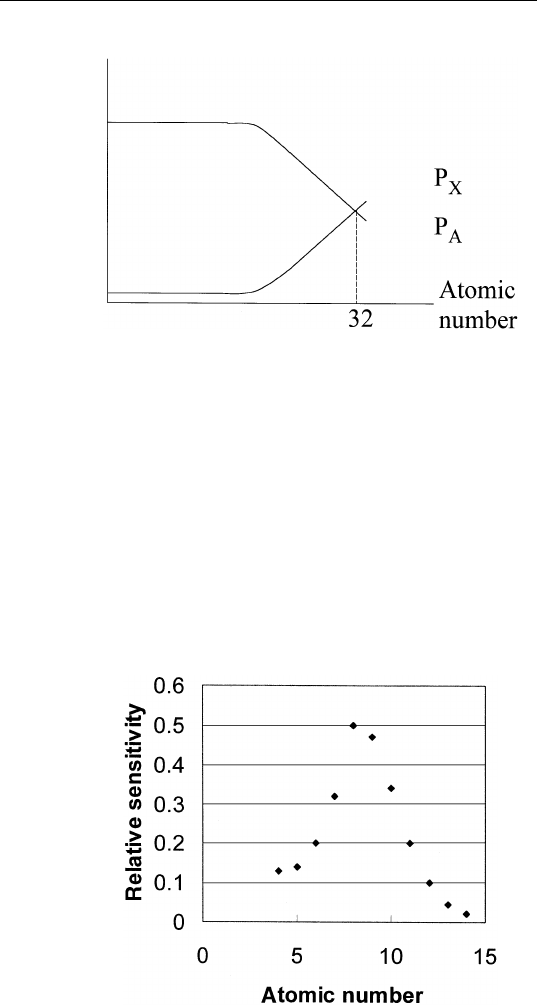
obtains results shown in Fig. 2.4. Note that P
X
starts to overtake P
A
for elements with atomic number greater than 32. This does not mean
that Auger electron spectroscopy is only useful for low atomic number
elements. For the purpose of surface analysis, we are interested in
Auger electron energies less than 1500 eV. For example, for elements
with atomic number from 19 (K) to 70 (Yb), we are interested in Auger
transitions derived from the filling of M-shell vacancies. In this energy
range, the Auger yield always dominates over the corresponding
X-ray yield. As a result, the relative Auger yields do not vary by more
than one order of magnitude for all elements in the periodic table that
give rise to Auger transitions of energies from 50 to 1500 eV. A plot
31
2.6 INTENSITY OF AUGER ELECTRON EMISSION
FIGURE 2.4 Auger and X-ray yields due to K-shell vacancies versus atomic
number.
of the relative Auger sensitivity versus atomic number at an incident
electron energy of 3 keV is shown in Fig. 2.5.
(c) Backscattering. When electrons enter a solid surface, some of
them undergo collisions in the surface region resulting in Auger electron
emission. The majority will penetrate through the surface without gener-
ating any Auger electrons. However, some of these electrons may
undergo single or multiple large-angle scattering bringing them back
to the surface region (backscattering). These electrons can initiate Auger
FIGURE 2.5 Relative KLL Auger sensitivities for elements at 3 keV primary
energy obtained from a cylindrical mirror analyzer.

32
CHAPTER2/AUGER ELECTRON SPECTROSCOPY
transitions if they have sufficient energy. This contribution to the Auger
signal depends on the energy of the incident electrons and the backscat-
tering probability, which is a function of the atomic number of the
specimen. The backscattering contribution is larger for heavier ele-
ments. For incident electrons of energy ⬇ 10 keV, electron backscatter-
ing can occur from a depth ⬇ 0.1 to 1 m (Fig. 2.6), depending on
the atomic number.
Backscattering can lead to some interesting phenomena. Since back-
scattering is a function of atomic number, a light element in a heavy
element matrix will give a larger Auger signal than a light element in
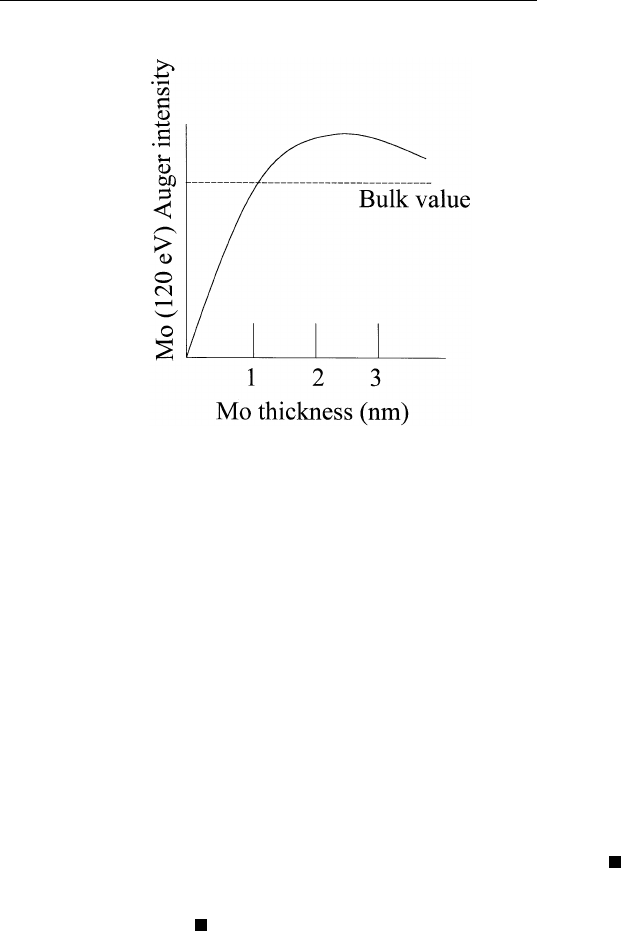
a light matrix of the same concentration. For example, when one depos-
its a thin film of molybdenum onto a tungsten substrate and monitors
the molybdenum Auger peak at 120 eV, one obtains results shown in
Fig. 2.7. At small molybdenum thickness, the Auger signal increases
linearly with thickness, as expected. However, with further increase in
film thickness, the Auger signal reaches a maximum and then decreases
slowly to the steady state value of the bulk. The Auger signal at the
maximum is about 20% greater than that obtained from bulk molybde-
num. This is due to tungsten (Z ⫽ 74) having a larger backscattering
power than molybdenum (Z ⫽ 42). The backscattering contribution
from tungsten gives rise to the extra molybdenum Auger intensity at
120 eV.
FIGURE 2.6 Contribution to Auger electron emission from primary and backscat-
tered electrons.
33
2.8 SCANNING AUGER MICROPROBE
FIGURE 2.7 Auguer intensity due to molybdenum as a function of Mo thickness
on tungsten.
2.7 PROFILE ANALYSIS
Sometimes, one is interested in the depth distribution of elements. This
can be accomplished by removal of surface atoms via inert gas (usually
argon) ion sputtering and simultaneous analysis of the surface composi-
tion using Auger electron spectroscopy. However, the sputtering rates
of different components of the solid can be different. Surface composi-
tion can be affected by sputtering so that the concentration profile
obtained by sequential sputtering and Auger analysis has to be interpre-
ted carefully.
Q
UESTION FOR
D
ISCUSSION.
Even when preferential sputtering is
absent (i.e., all elements are sputtered at the same rate), the sputter
Auger profile does not reveal the true composition profile. Why?
Q
UESTION FOR
D
ISCUSSION.
Argon is the preferred inert gas for
sputtering. Why?
2.8 SCANNING AUGER MICROPROBE
High spatial resolution Auger analysis can be done by using a small
probing electron beam for exciting Auger transitions. The analysis can

34
CHAPTER2/AUGER ELECTRON SPECTROSCOPY
be done in a spot mode. Alternatively, one can scan the electron beam
across the sample surface and tune the electron analyzer to detect Auger
electrons from any selected element. This generates a picture of the
spatial distribution of any element on the surface (Auger element map-
ping). When the same electronics system is equipped with a secondary
electron detector or absorbed current amplifier, the scanning Auger
microprobe (SAM) functions as a scanning electron microscope. In
this way, one can correlate information obtained from Auger electron
spectroscopy and surface topography. Today’s state-of-art SAMs have
electron beam sizes ⬇ 50 nm and are very useful in solving a wide
range of materials problems requiring elemental analysis at the submi-
cron scale.
There are three major considerations in the use of SAM:
(a) Electron beam damage. When one focuses an electron beam
into a small spot, the energy flux can be very high and can result in
local heating. The consequence can range from no effect to accelerated
specimen contamination or destruction of the surface.
(b) Backscattering artifacts. Because of the diffusion of the pri-
mary electron beam in solids, the Auger signal can sometimes arise
from a position different from the original impact point of the primary
electron beam. This can limit spatial resolution.
(c) Topography artifacts. Normally, an electron analyzer does not
collect electrons emitting in all directions. Therefore, shadowing can
result in some cases. Also, near edges (e.g., corners of grain boundary
faces), enhanced Auger signal will result even with a homogeneous
solid. One should be careful not to interpret this as due to higher
concentration of the corresponding element.
Both backscattering and topography artifacts can be partially cor-
rected by normalizing the Auger peak intensity to that of the secondary
electron background at the same energy. The assumption is that back-
scattering and the topography affect the intensity of Auger electrons
and secondary electrons of the same kinetic energy to the same extent.
E
XAMPLE.
In performing Auger point analysis, the following op-
erating conditions are used:
Beam area ⫽ 1 m ⫻ 1 m
Beam voltage ⫽ 10 kV
Beam current ⫽ 1 nA.

35
2.9 QUANTITATIVE ANALYSIS
Calculate the energy flux.
S
OLUTION.
Energy flux ⫽ energy/(area ⫻ time)
⫽ 10
4
⫻ 10
⫺9
/(10
⫺12
)
⫽ 10
7
W/m
2
.
This is about 10
4
times the solar energy flux!
2.9 QUANTITATIVE ANALYSIS
Auger electron spectroscopy is a popular analytical tool because it is
relatively easy to use. Interpretation is usually straightforward. Auger
derivative spectra are compiled for all elements in the periodic table.
Element identification is just a matter of matching peaks.
Quantitative analysis is more difficult. The fundamental goal of
quantitative analysis is to determine the functional relationship between
the measured Auger signal in the N(E)ordN/dE mode and the surface
composition of a given specimen. To explore this, we use a schematic
setup shown in Fig. 2.8 in which the primary electron beam E
p
impinges
on the specimen surface at normal incidence, and the emitted Auger
electrons are collected at an angle from the surface normal. Let us
break the whole Auger process into three discrete steps: ionization,
emission, and collection.
FIGURE 2.8 Auger electron generation from different atomic layers.
36
CHAPTER2/AUGER ELECTRON SPECTROSCOPY
(a) Ionization. At the ith atomic layer from the surface of the solid,
the number of ionization events per unit time dN
i
/dt is given by
dN
i
dt
⫽
1
e
I
p
i
x
i
r (2.5)
where I
p
is the incident electron current,
i
the number of atoms per
unit area in the ith layer, x
i
the atom fraction of the target element in
the ith layer, the ionization cross-section, and r the backscattering
enhancement factor. We define r as the total number of ionizations in
the ith layer divided by the number of ionizations due to the direct
beam alone. We assume no significant attenuation of incident electrons
so that the beam current at the ith layer is still I
p
.
(b) Emission. The probability of emission is equal to P
A
q
i
(E,).
P
A
is the probability of Auger emission after ionization, and q
i
is the
probability that the Auger electron emitted at an angle from the normal
from the ith layer will escape without energy loss and is given by
q
i
(E,
) ⫽ exp
冋
⫺
(i ⫺ 1)d
cos
册
, (2.6)
where d is the spacing between layers and the mean free path of
electrons with energy E. Here, we assume that (i) each layer fully
covers the layer below it, (ii) surface roughness is negligible, and (iii)
q
i
is independent of azimuth angle. For single crystals, we know that
assumption (iii) is not valid. Auger electrons may undergo diffraction,
channel, or attenuate anisotropically in a single crystal. Note also that
the Auger electron may be refracted when exiting the surface because
of the potential difference between the solid and vacuum.
(c) Collection. Of all the Auger electrons emitted, the analyzer
collects a maximum fraction ⍀/4 where ⍀ is the acceptance solid
angle of the analyzer. Because of scattering from grids or walls of the
analyzer, only a fraction T of the electrons entering the analyzer with
the appropriate pass energy will be transmitted through the analyzer
and be detected. T is a function of energy E. The total Auger current
at energy E is then given by
I
E
⫽ I
p
rP
A
⍀
4
T
再
兺
i
x
i
exp
冋
⫺
(i ⫺ 1)d
cos
册冎
. (2.7)
The summation is from i ⫽ 1 (topmost layer) to i ⫽ ∞. Note that
i
,
r, d, and all depend on composition. The variation of these quantities
