Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


(iv) a promoter, usually a viral promoter, which allows the transcription
of the gene of interest in eukaryotic cells.
After the gene of interest is cloned into the plasmid, it is transferred to a
host bacterium, usually Escherichia coli, by the process of bacterial
transformation, to produce plasmids on a large scale and to obtain a
sufficient amount of DNA for therapeutic use.
Carrier systems for plasmid DNA
Plasmid DNA may be administered to several animal species, including
humans, by several routes and schedules. In addition to intramuscular
injection, it may be administered orally, intranasally (as an airspray), or by
an intradermal route, by bombing gold microparticles covered with the
genetic material (Lima et al., 2003a). Although plasmid intramuscular
injection is a simple and widely used technique, there are some problems,
such as the presence of enzymes (nucleases) able to degrade the plasmid
DNA, making it ineffective. Another limitation is the size of the DNA
molecule and its superficial molecular charge, which limits its penetration
into the target cell. A prerequisite for use of DNA as a vaccine or gene
therapy is that the nucleic acid is released effectively in the target cell. An
estimation is that only one in every 1000 plasmid molecules administered
is able to reach the nucleus and express the message for the desired protein
synthesis, meaning that a treatment usually requires the administration of
high plasmid DNA doses, of possibly several hundred micrograms up to
milligrams (Friedman, 1997).
Several investigators have shown that intramuscular injection requires
up to 100 times higher the amount of plasmid to generate an expression
equivalent to one produced by DNA carrying systems, as discussed in the
next few paragraphs. In contrast, with adsorption or encapsulation techni-
ques in non-viable systems, the plasmid release may occur preferentially in
the intracellular environment, avoiding functional plasmid degradation.
Among the strategies used, we will mention the use of biobalistics,
liposomes, lipoplexes, and polyplexes, in addition to the use of biodegrad-
able polymeric microparticles.
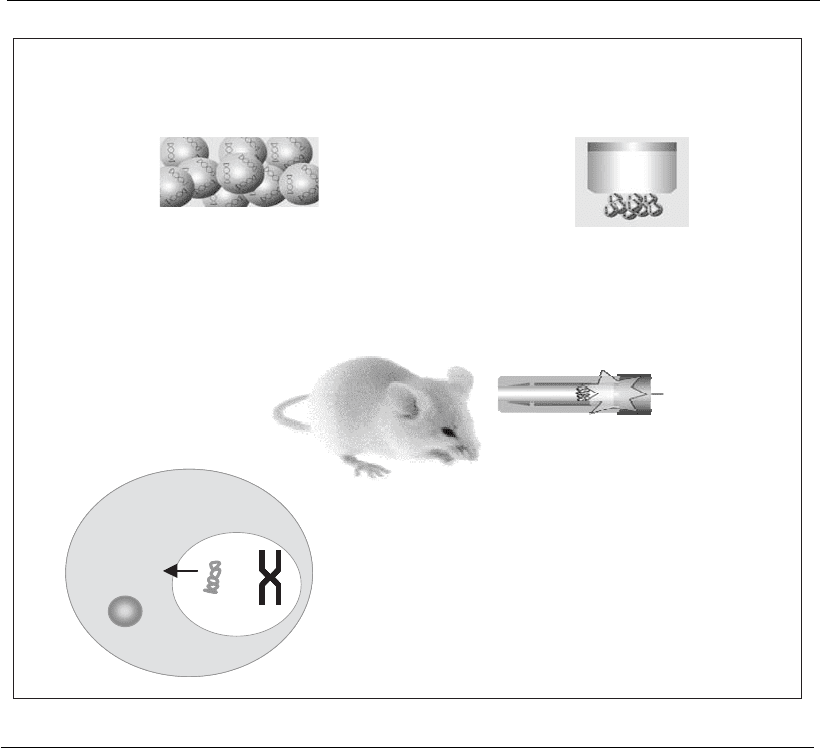
Bioballistics or ‘‘gene gun’’ consists of transfecting individual cells,
using DNA adsorbed onto gold particles (0.6–2 m in diameter). For the
transfection, the particles are placed in a device known as a gene gun
(Figure 21.3), which, by means of an acceleration process using helium gas
discharge under high pressure, is projected at an individual’s skin, enabling
the particles to reach the epidermis (Haynes et al., 1996). Transfection
using a gene gun requires a 100 times lower the amount of plasmid to
generate an expression corresponding to one produced by intramuscular
administration (Barry and Johnston, 1997), because bioballistics enables
the release of the plasmid inside the cells, avoiding its degradation. This
technique of introducing a vector by bioballistics, although effective for
several transfection procedures, requires a specialized device for its use.
However, promising clinical results have motivated companies to invest in
improving this technology.
494 Animal Cell Technology
An alternative method for introducing a plasmid DNA vector is to use
liposomes. As they are composed of aqueous vesicles surrounded by a
phospholipid bilayer, liposomes allow encapsulation and transportation of
many substances, both hydrophilic and lipophilic, along with the plasmid.
Liposomes also allow molecules such as antibodies, proteins, and sugars to
be incorporated into their surface, to target them to specific sites. Due to
the structural versatility shown by these systems, the chances of effective
transfection may be increased by changes in the lipid composition, which
may alter the superficial charge or the vesicle size (Bramwell and Perrie,
2005). Cationic liposomes have wide applications in gene transfection.
Lipoplexes and polyplexes are DNA–cationic molecular complexes,
formed, respectively, by DNA interaction with lipids or polymers. The
main property of these complexes is to allow easier passage of DNA
through the cell plasma membrane, by means of two mechanisms: DNA
charge neutralization and plasmid condensation, which reduces its size.
Such complexes are formed by an excess of positive charges to neutralize
DNA phosphate groups, resulting in transfecting particles with a net
DNA is adsorbed onto gold particles Particles are placed in a cartridge
The cartridge is coupled to the “gene gun”, and the particles are
accelerated against the animal’s skin
On hitting the cell, the DNA is released from
the particle and gets to the nucleus, where it
controls the protein synthesis
Cytoplasm
Nucleus
Chromosome
Plasmid
Gold particle
Figure 21.3
Use of bioballistics as a plasmid DNA carrier.
Gene therapy 495
positive charge. The effectiveness of this approach is associated with
adsorptive endocytosis between the positive particle and the cell’s negative
surface. Once inside the cell, the complexes containing the DNA are
released from the endocytotic vesicle and spread through the cytosol
leading to the nucleus. However, this electrostatic adsorption may also
result in low bioavailability in vivo, and the absence of cellular specificity,
probably due to interactions with the cell surface proteoglycans and to
polyanionic glycans present in the extracellular matrix (Mislick and
Baldeschwieler, 1996). Thus, the use of this method may be limited by
several factors: its variable plasma binding when the complexes are admin-
istered systemically; its association to macromolecules present in the
extracellular matrix; the low efficacy of membrane penetration through
endocytosis; and its passage from cytoplasm to nucleus.
Some charged polymers, such as polyethyleneimine (PEI) have a prop-
erty known as proton sponge. When at an acidic pH, as in an endolysoso-
mal compartment, PEI expands, which may cause the organelle it is in to
break, resulting in DNA release into the cytosol. Thus, some complexes,
in addition to allowing easier DNA penetration into the cell, may also
make their traffic to the nucleus easier, and contribute to reduced plasmid
degradation.
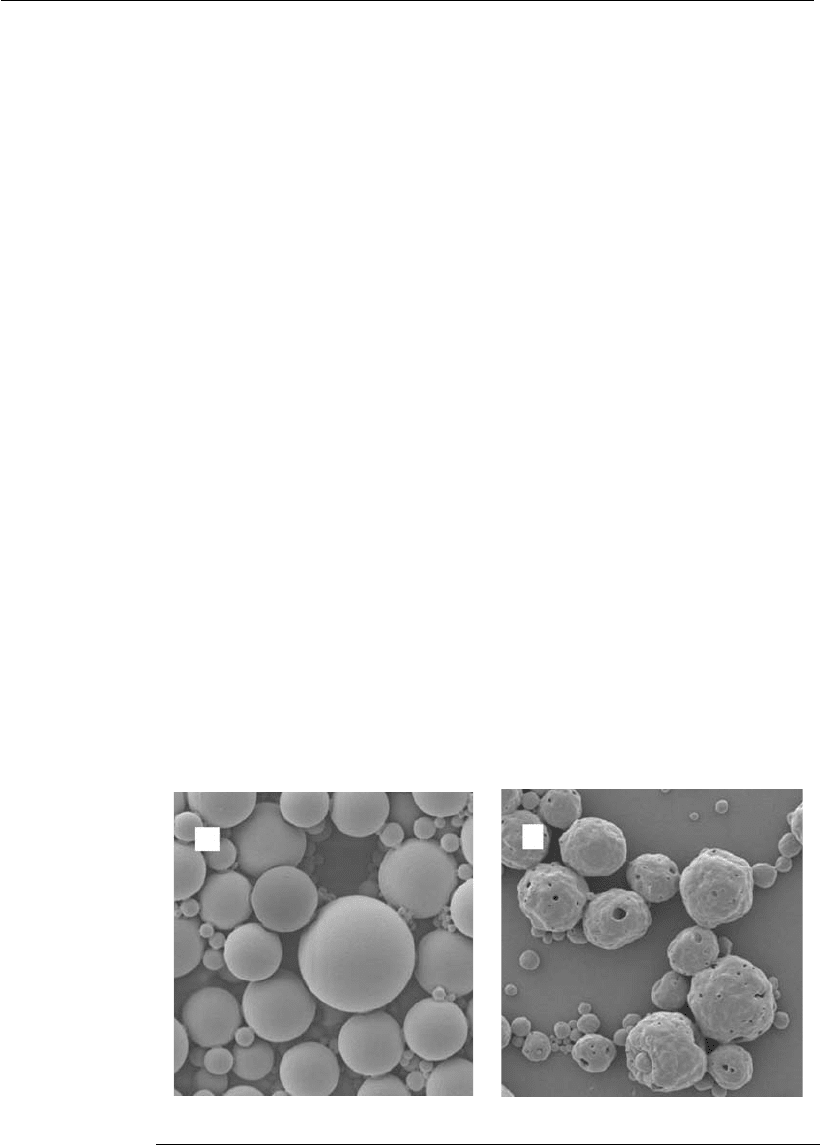
Recently, biodegradable polymeric spheres have been developed as an
interesting strategy to be used in the transfection process (Lima et al,
2003b). These microspheres are composed of lactic and glycolic acid
polyesters, and have the advantage of being biodegradable, with no adverse
effects at the site of administration, if used by the parenteral route. In the
organism, these polymers are hydrolyzed, and once degraded, they release
lactic and glycolic acid, which are innocuous substrates for the organism
(Figure 21.4). The potential of these systems as carriers is associated with:
(i) the protection of encapsulated plasmid, allowing a reduction in the
amount to be used;
A
B
Figure 21.4
Electron scanning micrograph showing intact polymeric microspheres (A) or
matrix degradation (B).
496 Animal Cell Technology
(ii) interaction with mononuclear phagocytic cells, since particles with a
diameter under 10 m are easily phagocytosed by macrophages,
which could contribute to triggering the immune response, making
them good vehicles to carry DNA;
(iii) the possibility of administering the plasmid by other routes, such as
oral, nasal, and pulmonary;
(iv) easy administration;
(v) stability, once the spheres are stored as lyophilized powder, which
may be reconstituted immediately before administration.
21.4 Principles of gene therapy
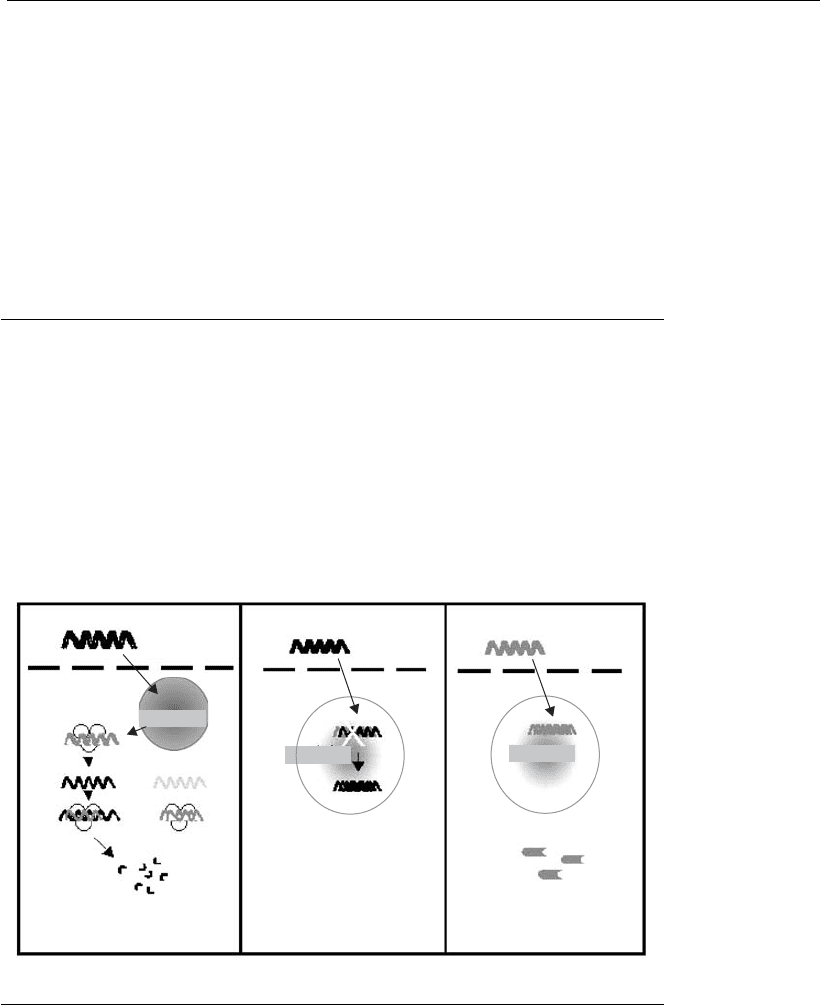
As shown in Figure 21.5, gene therapy is based on three basic principles:
(i) replacement or correction of a gene for the purpose of generating
products appropriate to cell or organ function; (ii) the introduction of a
heterologous gene into the target cell, leading it to produce something that
is not innate; (iii) inactivation of a gene causing a cell dysfunction.
21.4.1 Replacement or correction of a mutant gene
The replacement of an abnormal gene with a normal copy of the same gene
may restore the cell capacity to produce functional proteins, contributing
to cell homeostasis (Figure 21.5B). This type of gene therapy is one of the
Nucleus
Nucleus
Nucleus
Heterologous
protein
1
2
4
3
ABC
Figure 21.5
Basic principles of gene therapy. (A) Gene inactivation by interference RNA (iRNA).
The DNA encoding the iRNA is int roduced in the cell. The iRNA forms the silencing
complex with proteins present in the cytoplasm (1). The mRNA generated by the
changed gene is homologous to the iRNA and connects to the silencing complex
(2),beingdestroyedbyit(3).ThemRNAgeneratedbynormalgenesisnot
affected by the iRNA (4). (B) Gene replacement. The defective gene is replaced
with a normal copy of the same gene. (C) Introduction of a heterologous gene.
The gene encoding a protein from another organism is introduced into the target
cell, allowing it to produce the heterologous protein.
Gene therapy 497

most widely used, and may be applied to different types of diseases.
Usually, viral vectors are used in this kind of therapy, since in most cases
long-lasting transgene expression is required.
21.4.2 Introduction of a heterologous gene
This technique is especially used in DNA vaccine protocols or therapies
for infectious diseases, where the objective is to trigger an immune
response against a specific antigen. In this case, the gene encoding an
antigenic protein of a pathogen is identified and inserted into an expression
vector, which is then transferred to the target cell to make it start
producing the heterologous protein in question (Figure 21.5c). In this case,
the plasmid DNA-based synthetic vectors are the most commonly used,
due to safety reasons and because these are generally prophylactic inter-
ventions.
21.4.3 Gene inactivation
When the expression of the product of an abnormal gene leads to cell
imbalance, and the gene expression is not fundamental for the cell or for
the individual, one of the therapeutic alternatives is its inactivation. The
inactivation of a gene may be performed at the DNA, messenger RNA, or
protein level. In several organisms, the introduction of double-stranded
RNA has proved to be a powerful tool to suppress gene expression, in a
process known as interference RNA (iRNA), and has been largely used in
gene therapy research. The use of iRNA is a process in which the double-
stranded RNA induces degradation of the homologous messenger RNA
(Figure 21.5A). However, in most mammalian cells, this causes a strong
cytotoxic response, which may be controlled by using synthetic iRNA
able to mediate a highly specific suppression of the target gene. Although
effective, the silencing caused by the synthetic iRNA is transient, limiting
its application. To mitigate this problem, plasmid and viral vectors have
been created, to produce the iRNA inside the cells.
21.5 Gene therapy and clinical studies
From 1989 up to the present, over 500 clinical studies of gene therapy have
already been approved and are being conducted worldwide, as shown in
Figure 21.6. About 70% of these studies are intended for cancer treatment,
and most (97%) are conducted in Phase I and II in terminal patients.
About 3% are conducted in Phases II/III or III. So far, only a single
product (Gendicine
1
) was approved in 2003 for marketing, and is cur-
rently in assessment Phase IV. Although it is not part of that statistic, in
Brazil there is only one Phase I study, started in 2004 and currently in
progress, to assess the effectiveness of plasmid DNA encoding the thermal
shock protein hsp65 of Mycobacterium leprae for head and neck epidermal
cancer treatment (non-published data). Table 21.2 shows the number of
approved clinical studies and the study phases.
498 Animal Cell Technology

Year
Number of clinical trials in gene therapy
1989
NE
1990
1991
1992
1993
1994
1995
1996
1997
1998
1999
2000
2001
2002
2003
2004
2005
02550
75
100 125
Figure 21.6
Number of clinical trials in g ene t herapy approv ed worldwide between 1989 and
2005 (www.wiley.co.uk/genmed/clinical). NE, not established.
Table 21.2 Gene therapy protocols classified by clinical study phase
Phase Gene therapy clinical studies
Number %
Phase I 714 62.4
Phase I/II 234 20.4
Phase II 161 14.1
Phase II/III 12 1
Phase III 24 2.1
Total 1145 100
Adapted from Journal of Gene Medicine 2006 (www.wiley.co.uk/genmed/clinical).
Table 21.3 Gene therapy clinical trials classified by the type of disease
Indications Gene therapy clinical trials
Number %
Cancer 762 66.6
Marking genes 52 4.5
Healthy volunteers 19 1.7
Infectious diseases 75 6.6
Monogenic diseases 100 8.7
Others 37 3.2
Vascular diseases 100 8.7
Total 1145 100
Adapted from Journal of Gene Medicine 2006 (www.wiley.co.uk/genmed/clinical).
Gene therapy 499

Table 21.3 shows the clinical studies that have been conducted world-
wide, with their different applications, showing that therapies intended for
monogenic disease treatment are the second most assessed group, after
therapies for tumor treatment. The most used vectors in gene therapy
clinical studies are viral vectors (68%), and among those, retroviruses and
adenoviruses are the viruses of choice. Synthetic vectors were used in 25%
of the studies performed, and about 16% correspond to the use of naked
plasmid DNA (Table 21.4).
Although many gene therapy protocols have been approved for clinical
trial in humans, gene therapy safety has been questioned, due to two
adverse events. The first event involved the death, in 1999, of a young man
who underwent experimental therapy in the United States. This patient
had a rare genetic disorder known as ornithine transcarbamylase (OTC)
deficiency, which affects the individual’s capacity to eliminate ammonia, a
toxic product resulting from protein metabolism. The second event oc-
curred in 2002, when it was announced that children treated for severe
combined immunodeficiency (SCID) syndrome had signs of leukemia
after receiving the treatment (Hacein-Bey-Abina et al., 2003). This syn-
drome, also known as ‘‘bubble boy disease,’’ is caused by a single mutated
Table 21.4 Vectors used in gene therapy clinical trials
Vector Gene therapy clinical trials
Number %
Adeno-associated viruses 38 3.32
Adenovirus 287 25.07
Gene gun 5 0.44
Flavivirus 5 0.44
Herpes simplex virus 38 3.32
Lentivirus 5 0.44
Lipofection 95 8.30
Listeria monocytogenes 1 0.09
Measles virus 2 0.17
Naked plasmid DNA 192 16.77
Naked plasmid DNA + adenovirus 1 0.09
Newcastle disease virus 1 0.09
Poliovirus 1 0.09
Recombinant Poxvirus 1 0.09
Poxvirus 59 5.15
Retrovirus 276 24.10
Transference RNA 14 1.22
Saccharomyces cerevisiae 2 0.17
Salmonella typhimurium 2 0.17
Semliki Forest virus 1 0.09
Simian 40 virus 1 0.09
Vaccinia virus 51 4.45
Adenovirus + retrovirus 3 0.26
Poxvirus + vaccinia virus 21 1.83
Not commented 43 3.76
Total 1145 100
Adapted from Journal of Gene Medicine 2006 (www.wiley.co.uk/genmed/clinical).
500 Animal Cell Technology

gene, and forces the individual to live under sterile conditions, since he/she
does not have an effective defense against infections. Tumor cells were
found in the blood of the patients following the gene therapy, containing
an intact copy of the retroviral vector that had integrated near or in the
LMO2 gene. Although the exact mechanism by which the LMO2 gene
activation may have been responsible for the origin of leukemic cells is not
clear, discussions led to debates about the safety of using viral gene
vectors. These questions are reflected in the sharp fall in the number of
new clinical trial protocols approved recently, as shown in Figure 21.6.
Other causes for failure of gene therapy in clinical trials up to now
include: short-lasting therapeutic effect; triggering of host’s immune
response mainly against viral vectors; problems with viral vectors, such as
treatment of patients with SCID, which caused the development of
complications, leading to death; multigenic disorders, such as heart condi-
tions, Alzheimer’s disease, arthritis, and diabetes.
21.5.1 The first gene therapy product
Despite all these questions related to the safety of gene therapy, efforts are
still being made to eliminate the existing problems. These efforts reached
their highest point for the approval of the first product for gene therapy,
which is based on an adenoviral vector. It is Gendicine
1
, a medication
produced by the Chinese company, Shenzhen Sibiono GeneTech. The
medication is intended for head and neck carcinoma treatment, and was
approved for marketing in 2003 by China’s regulatory agency (China’s
State Food and Drug Administration – SFDA). Recombinant adenovirus,
in the form of 90 nm particles, contain the tumor-suppressing gene p53.
p53 gene is mutated in about 50–70% of human tumors. The mutant genes
are not necessarily inactive, but may have oncogenic functions that
contribute to tumor genesis. Proteins originating from the mutant gene are
also associated with over-regulation of a multidrug-resistant gene, which
results in resistance to several chemotherapeutics. The exogenous intro-
duction of gene p53 and subsequent expression of protein p53 leads to
tumor control and elimination. A synergistic effect may also be obtained
when the gene therapy is associated with radiotherapy and chemotherapy.
Gendicine
1
is produced in SBN cells, a cell lineage subcloned from
human embryonic renal cells, lineage 239 (HEK-293). As these are
adherent cells, culture was at first assessed in roller bottles, but this did not
prove satisfactory for production. As an alternative, a parallel plate reactor
(CellCube
1
) was used. In this kind of system, the production obtained
was low (about 4.9 3 10
9
viral particles/cm
2
). Subsequently, the develop-
ment of a perfusion culture process in packed-bed bioreactors using disks
(Fibra-Cel
TM
Disks) as support provided a 15-fold higher production,
compared with the CellCube
1
. In addition, a batch culture using sus-
pended cells and fetal bovine serum-free medium is being developed. The
most important point in the production process was the preparation of
reference and working cell banks. After production of adenoviral vectors
in bioreactors, they are submitted to clarification and ultrafiltration, and
finally purified in automated systems. After the whole downstream
process, the recovery rate of viral particles is about 65%. The purified and
Gene therapy 501

formulated product is dispensed in sterile glass bottles and stored frozen,
up to the time of use.
21.6 Perspectives
Gene therapy is still new, and its scientific base will be established with
further preclinical and clinical tests. Although many patients are currently
being evaluated, several questions are posed about vector safety. The
advent of non-viable vectors with biodegradable substances and construc-
tion of mimetic systems will be of great value for solving the problems
associated with the viral vectors. Nanotechnology tools, as well as new cell
visualization and characterization tools, will allow the advent of safer
transfection techniques. As there are several disciplines involved, advances
in gene therapy depend on different technologies, which may be obtained
only by multidisciplinary research.
The collection of toxicological data is important to allow the clarifica-
tion of the level of risk of different classes of viral vectors. The approval of
the first medication intended for gene therapy opens new perspectives to
obtain such essential data at a critical time for discussion about the safety
of these vectors. Such data are very valuable for regulating agencies. It is
important to emphasize that investigators and businessmen interested in
this area are responsible for ensuring that the patients’ health and well-
being are the paramount objectives, and strict adherence to currently
available rules and guidelines is required. Additionally, the increasing
availability of new data will form the basis of future guidelines for
production, marketing, clinical trials, and safety for these products.
Detailed knowledge of the human genome, which has been achieved in
the last few years, provide a rapid development tool and targets for gene
therapy. A great impact is expected on human healthcare with this new
knowledge, creating high expectations concerning the genesis of new
products intended for the treatment of infectious diseases and tumors,
which do not have alternative treatments.
On the other hand, from the technological point of view, several barriers
have already been overcome, allowing production of viral or plasmid
DNA vectors at a cost that enables clinical application. Safe manufacturing
processes are already available, and the production capacity for these
systems is easily accessible worldwide.
Thus, it is possible to conclude that this therapy, still in its early phases,
will cause a great impact on human health in the next few decades.
Furthermore, the scale-up of biomanufacture of products for clinical use
from technologies for animal cell cultures will be an important step in the
application of gene therapy,
References
Anderson WF (1998), Human gene therapy, Nature 392(6679 Suppl):25–30.
Barry MA, Johnston SA (1997), Biological features of genetic immunization,
Vaccine 15:788–791.
Bramwell VW, Perrie Y (2005), Particulate delivery systems for vaccines,
Crit. Rev. Ther. Drug. Carrier Syst. 22:151–214.
502 Animal Cell Technology

Brown MD, Schatzlein AG, Uchegbu IF (2001), Gene delivery with synthetic (non-
viral) carriers, Int. J. Pharm. 229:1–21.
Brunnell A, Morgan R (1998), Gene therapy for infectious diseases, Clin. Microbiol.
Rev. 11:42–56.
Cristiano, RJ (1998), Viral and non-viral vectors for cancer gene therapy, Anticancer
Res. 18:3241–3245.
Ferber D (2001), Gene therapy. Safer and virus free?, Science 294:1638–1642.
Friedman T (1997), Overcoming the obstacles to gene therapy, Sci. Am. 97:80–85.
Hacein-Bey-Abina S, von Kalle C, Schimidt M, Le Deist F, Wulffraat N, McIntyre E,
Radford I, Villeval JL, Fraser C, Cavazzana-Calvo M, Fischer A (2003), A serious
adverse event after successful gene-therapy for X-linked severe combined
immunodeficiency, N. Engl. J. Med. 348:255–256.
Haynes JR, McCabe DE, Swain WF, Widera G, Fuller JT (1996), Particle-mediated
nucleic acid immunization, J. Biotechnol. 44:37–42.
Hendrie PC, Russell DW (2005), Gene targeting with viral vectors, Mol. Ther. 12:
9–17.
Journal of Gene Medicine (2005). Available at: www.wiley.co.uk/genmed/clinical
(accessed May 2006).
Lima KM, dos Santos SA, Santos RR, Brandao IT, Rodrigues JM Jr, Silva CL (2003a),
Efficacy of DNA-hsp65 vaccination for tuberculosis varies with method of DNA
introduction in vivo, Vaccine 22:49–56.
Lima KM, dos Santos SA, Lima VM, Coelho-Castelo AA, Rodrigues JM Jr, Silva CL
(2003b), Single dose of a vaccine based on DNA encoding mycobacterial hsp65
protein plus TDM-loaded PLGA microspheres protects mice against a virulent
strain of Mycobacterium tuberculosis, Gene Ther. 10:678–685.
Mislick KA, Baldeschwieler JD (1996), Evidence for the role of proteoglycans in
cation-mediated gene transfer, Proc. Natl Acad. Sci. U S A 93:12349–12354.
Morizono K, Chen IS (2005), Targeted gene delivery by intravenous injection of
retroviral vectors, Cell Cycle 4:231–237.
Nardi NB, Ventura AM (2004), Terapia geˆnica, In: Mir L (Ed.), Genoˆ mica, Editora
Atheneu, Sa
˜
o Paulo, pp. 625–542.
Niidome T, Huang L (2002), Gene therapy progress and prospects: non-viral vectors,
Gene Ther. 9:1647–1652.
Quinonez R, Sutton RE (2002), Lentiviral vectors for gene delivery into cells, DNA
Cell Biol. 21:937–951.
Romano G, Michell P, Pacilio C, Giordano A (2000), Latest development in gene
transfer technology: achievements, perspectives and controversies over therapeutic
applications, Stem Cells 18:19–39.
Zaiss AK, Muruve DA (2005), Immune responses to adeno-associated virus vectors,
Curr. Gene Ther. 5:323–331.
Gene therapy 503
