Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


volumes and many consecutive extractions to achieve total recovery. A
value of K close to zero, on the other hand, means that no extraction
occurs (Harrison et al., 2003).
Liquid–liquid extraction offers several advantages, such as low cost,
high yield, ease of continuous operation, and scale-up. Also, in the case of
aqueous two-phase systems, the process would not cause degradation of
proteins, enzymes, virus, or organelles (Cunha et al., 2003; Harrison et al.,
2003). Efficient extraction techniques can lead to a reduction in volume,
providing not only purification, but also a concentration of the sample.
They are suitable for processing cell suspensions, enabling an integration
of the solid–liquid separation and the primary purification stages and a
consequent reduction in the number of stages in the downstream proces-
sing. However, resolution and purification factors are relatively low, com-
pared with chromatographic techniques. Because of this, liquid–liquid
extractions are often designed in the early stages of a purification process.
Significant improvements in resolution can be achieved with the use of
affinity ligands in one of the phases, although this can increase cost
(Johansson, 1998).
12.4.2 Separation processes based on differences in molar mass
Proteins have high molar mass, the value of which differs from one protein
to another. This allows the use of simple methods to separate proteins
from low molar mass molecules, as well as to separate a specific protein
from others.
Density gradient centrifugation
Just like other techniques, centrifugation can be applied at different stages
in the downstream processing of a given protein. The main application of
centrifugation in biotechnology (as seen in Chapter 11) is in the clarifica-
tion of protein-containing suspensions, usually for the removal of cells
and cell debris, but also after flotation and precipitation operations.
However, density gradient centrifugation or zone centrifugation widens
the range of possible applications of centrifugation by allowing the
separation of small bioparticles such as organelles and viruses, as well as
proteins. In the most widely used method, a continuous density gradient
of sucrose is prepared with a device that adds a concentrated solution of
sucrose to water, in decreasing proportions along a tube. In this way, the
medium density is higher at the bottom of the tube, and the macromole-
cules to be fractionated are applied on the top. After centrifugation, the
different proteins form bands or layers, according to their molar mass,
shape, and density. Two consecutive centrifugation steps (the first using
CsCl and the second using sucrose) were employed by Deml et al.
(1999), for purifying HBsAg from cell culture supernatant of genetically
modified DS-2 cells.
304 Animal Cell Technology

Dialysis
Desalting or buffer exchanges are often required between purification
steps. At the laboratory scale, the protein solution is placed in a tube of a
semipermeable polymer membrane immersed in the desired buffer. The
membrane pore size determines the minimum molar mass of the com-
pounds that are retained. Small molecules with a molar mass below the
membrane cut-off will flow freely across the membrane until the osmotic
pressure equilibrium is reached. Complete buffer exchange requires several
changes of the dialysis liquid. The process should be carried out at a
temperature around 48C, to avoid loss of activity.
In industry, dialysis is not widely used because it is slow and labor-
intensive. Here, desalting and/or buffer exchange is usually performed by
diafiltration or molecular exclusion chromatography, which are discussed
later.
Microfiltration
The principle of microfiltration is the application of hydrostatic pressure
on a microporous filter membrane, so that the pressure difference forces
solutes, water molecules, and particles smaller than the membrane pore
size to flow across the pores, retaining and concentrating the larger
particles in the suspension.
Microfiltration membranes usually have a nominal pore diameter in the
range of 0.1–10 m. However, the membrane specification is not an
absolute parameter. The membranes usually present a pore size distribu-
tion around the nominal value and the shape of the bioparticles can
determine whether they are retained or pass through the membrane. The
membranes are manufactured from polymers, such as Teflon
1
, polyester,
PVC (polyvinyl chloride), Nylon
1
, polypropylene, polyethersulfone, and
cellulose, or from inorganic materials, such as ceramic and sinterized
stainless steel.
In microfiltration, the permeate flux increases inversely with the suspen-
sion viscosity and proportionally to the applied pressure, provided that
there is no membrane fouling (Belford, 1988; Ho and Zydney, 2000). To
accelerate the process, it is possible to decrease the solution viscosity by
increasing the temperature, although not so much as to denature the
protein.
Microfiltration is widely used for the removal of cells and fragments
from suspension. It is also used as a method of sterilization of solutions,
and has the advantage of high efficiency, simplicity, compactness, and
reliability.
Ultrafiltration
In ultrafiltration, water and other low molar mass molecules are forced
through a semi-permeable membrane by the application of high pressures
(1–7 bar) or of a centrifugal field. This technique involves membranes
with pore diameters in the range of 1.0–20 nm, which are most commonly
characterized and selected based on their nominal molar mass cut-off
Produc t purification processes 305

(MWCO), usually defined as the molar mass at which the membrane
rejects 90% of solute molecules. However, as in microfiltration, the
molecular shape can affect permeability through the membrane pores. For
example, a membrane with a nominal cut-off of 100 kDa, which does not
allow globular molecules with a molar mass of 100 kDa to flow through,
may allow fibrous molecules with higher molar masses to flow across the
pores. As in microfiltration, the membrane pore size is not uniform, with a
normal distribution around an average value.
A common problem of this technique is the gradual decrease in
permeate flux associated with membrane clogging or fouling, caused by
adsorption or physical deposition of particles and/or macromolecules on
membrane pores. Fouling can be minimized by prior clarification (particu-
late removal) of the feed solution, by the selection of operational condi-
tions that minimize interactions between membranes and macromolecules,
by the use of tangential flow, or by performing intermittent back-flushing
operations.
Diafiltration
Diafiltration consists of the application of microfiltration or, more com-
monly, ultrafiltration membranes for solvent exchange. In diafiltration
systems, schematically shown in Figure 12.2 , the permeate is collected
continuously at the same rate at which fresh solvent is added, so that
undesirable solutes are removed in the permeate stream and the retentate
volume is kept constant. Although diafiltration is being increasingly used,
especially on a large scale, a disadvantage is the generation of large
volumes of liquid effluents. For instance, in a system with a 100 m
2
membrane operating at a permeate flux of 30 L m
–2
h
–1
, a volume of
9210 L of water is required to remove 99.99% of the salt present in a
protein solution (Harrison et al., 2003).
Permeate
Fresh
buffer
Feed
tank
Diafiltration
module
Figure 12.2
General scheme of a diafiltration system.
306 Animal Cell Technology
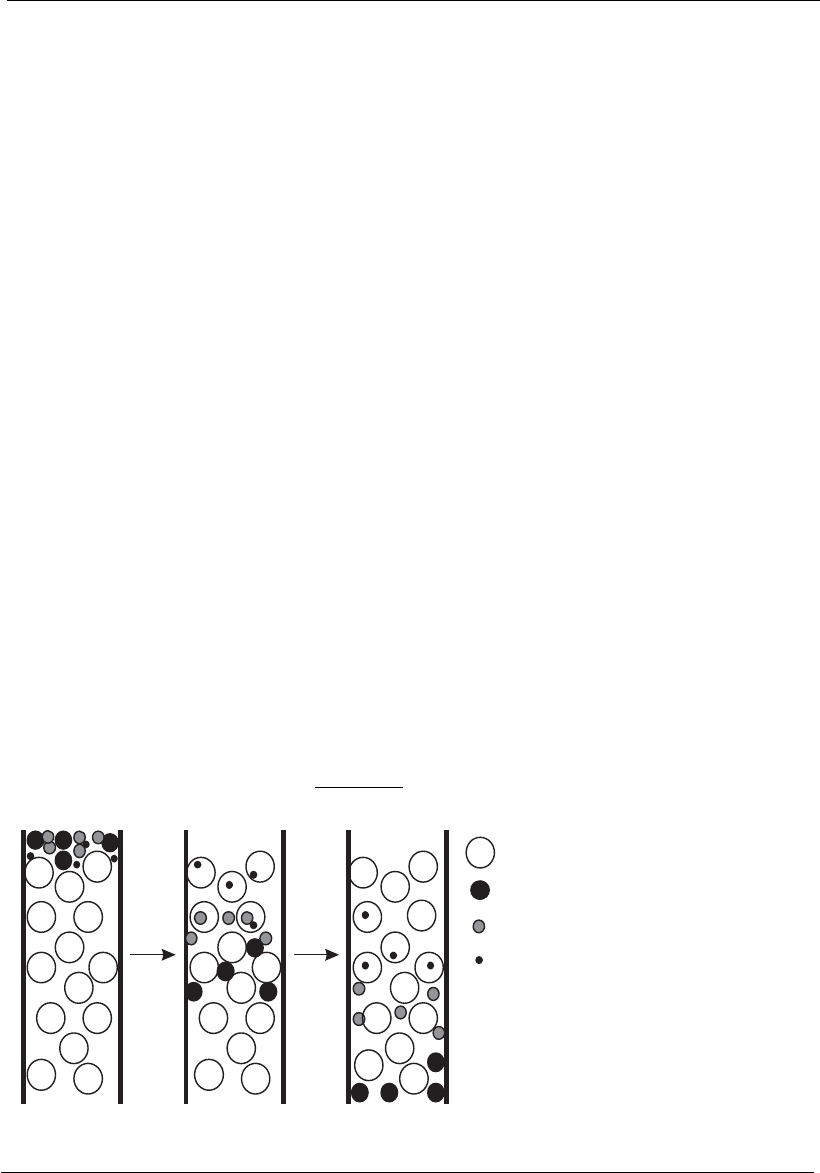
Molecular exclusion chromatography
Molecular exclusion chromatography, also known as gel filtration, size
exclusion chromatography, gel permeation chromatography, or simply gel
chromatography, is another separation method based on differences in
molecular size.
In this method, molecules partition between a solvent (aqueous buffer)
and a stationary phase of defined porosity. A protein mixture dissolved in
a suitable buffer flows through a column packed with spherical porous
particles made of an inert material (usually a polymer or a gel). The
column is equilibrated with a pre-selected buffer appropriate for sample
elution. The flow occurs by gravity or aided by a pump.
In a sample containing a mixture of molecules, the smallest permeate the
matrix pores and follow a slow trajectory along the column axis and are
collected later forming the later chromatogram peaks. The largest mole-
cules are excluded from the pores, migrating through the interstitial space
and are, therefore, eluted earlier. The intermediate sized molecules may
partially penetrate the pores, allowing intermediate elution times.
Therefore, the molecules are eluted in the inverse order of their
molecular size, as indicated in Figures 12.3 and 12.4, and the differences in
the elution times of different proteins are related to the fraction of pores
accessible to the solutes. Equations can be obtained that relate the fraction
of pores of different dimensions, the gel structure, and the molecular size
of solutes with so-called distribution coefficients.
The distribution coefficient K
d
(Equation 2) is defined as the volume
fraction of pores, in a stationary phase, which is effectively permeated by a
solute of a given size. V
o
is the interstitial volume of the porous medium,
measured by the elution volume of a high molar mass solute that is totally
excluded from the matrix pores. V
e
is the elution volume of the product of
interest. V
S
represents the total solvent volume within the pores, available
for small solutes.
K
d
¼
(V
e
V
o
)
V
S
(2)
Stationary phase
Molecules larger than the pores
Molecules with intermediate size
Molecules smaller than the pores
Figure 12.3
Schematic illustration of the separation principle involved in molecular exclusion chromatography.
Produc t purification processes 307
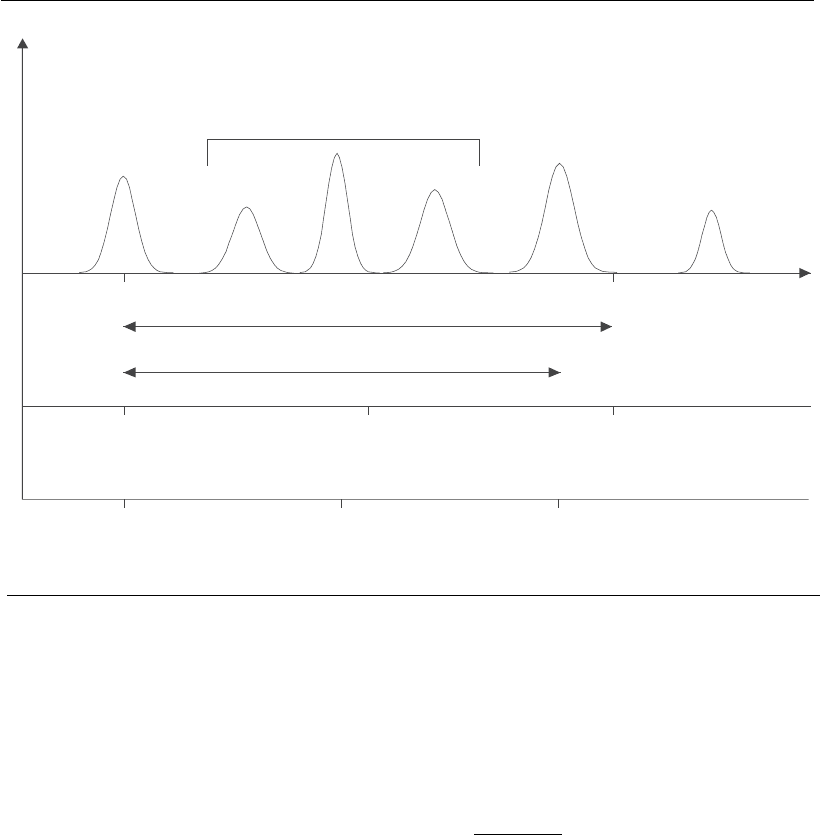
Since V
S
is difficult to measure, it is common to use an alternative
distribution coefficient, called K
av
(Equation 3). In this approach, V
S
is
replaced by the difference between the total column volume (V
t
) and the
interstitial volume (V
o
) of the column packed with the chromatographic
matrix (Ladisch, 2001; Moraes and Rosa, 2005).
K
av
¼
(V
e
V
o
)
(V
t
V
o
)
(3)
For a given matrix, K
d
or K
av
can be plotted against the log of the molar
mass of different solutes. For a given molar mass range, a linear relation-
ship will be observed. Therefore, by applying to the column molecules of
known molar mass, and similar shape and density, it is possible to
determine the molar masses of solutes present in a sample (Kumpalume
and Ghose, 2003; Moraes and Rosa, 2005).
Ideally, the column matrix should be inert with regard to the molecules
to be separated. If electrostatic or hydrophobic interactions occur between
proteins and the stationary phase, it is recommended, respectively, to
increase or decrease the ionic strength of the liquid phase, otherwise partial
adsorption of the protein may cause a delay in elution from the column.
This would cause a longer elution time than that expected based on the
molar mass.
V
0
V
t
V-V
t0
V
S
K
av
K
d
0
0
0.5
0.5
1.0
1.0
High
molar
mass
Solute
that interacts
with the
resin
Low
molar
mass
Intermediate
molar masses
Figure 12.4
Elution profile of a mixture containing solutes of different molar masses and the variables used to
describe solute and matrix behavior (adapted from Ladisch, 2001).
308 Animal Cell Technology

The most commonly used matrices for molecular exclusion chromato-
graphy are cross-linked polymer gels, such as agarose, dextran, and
polyacrylamide. Since dextran and agarose are biodegradable, they should
be stored in the presence of antimicrobial agents.
The selection of a gel matrix should take into account pH and tempera-
ture stability, which should be compatible with the characteristics of the
target protein. The selection of the most suitable gel should also take into
account the main goal to be achieved. If the process is intended to separate
proteins from low molar mass solutes (, 5 kDa), a small pore size matrix
is recommended, so that proteins are completely excluded from the porous
medium. Such a strategy is used for desalting samples.
Matrices composed of small particles have high resolution, since the
molecular diffusion in the interstitial region is small and, therefore, peak
broadening is low. However, these systems are associated with low flow
rates, since small particles result in larger pressure drops along the column.
Because the application of high pressure can lead to particle deformation
and, consequently, to bed compaction, particles that are large and rigid,
capable of withstanding high flow rates, are commonly preferred for large-
scale processes, even if they are associated with lower resolution.
In molecular exclusion chromatography, resolution is directly related to
the sample loading volume, since the higher the sample volume, the higher
the volume in which the protein is eluted. Usually, sample volume should
be 0.5–5% of the total bed volume. For volumes below 0.5% the sample
becomes too diluted, while volumes above 5% work well only for
molecules with large differences in size. For desalting, due to the pro-
nounced difference in the molecular size of the components, sample
volumes can reach up to 30% of the column capacity without a significant
decrease in resolution.
With regard to the total protein concentration in the injected sample, it
should be ensured that the ratio between the viscosity of the sample and
the eluent is not higher than 2. High viscosities can cause problems, such
as the occurrence of regions with distorted and irregular flow patterns.
Hence, protein concentration in the loaded sample should ideally be in the
range of 10–20 mg mL
–1
, although in some cases it can reach 70 mg mL
–1
.
Therefore, to achieve high resolutions in molecular exclusion chromato-
graphy, it is common to use columns with large height-to-diameter ratios
(in the range of 20–40), although at an industrial scale this can become
difficult.
12.4.3 Separation processes based on differences in electrical
charge
Separation of proteins based on differences in their electrical charge
depends on their acid-base properties, which are mostly determined by the
number and type of ionizable side groups in the peptide chain. Since
proteins are different from each other with respect to their composition
and amino acid sequence, they also have distinct acid-base properties.
Information on these properties allows a prediction of the behavior of a
given protein when exposed to an electrical field.
Product purification processes 309

The acid-base properties of proteins are exploited in two methods,
electrophoresis and ion exchange chromatography. These are widely used
in the analysis and separation of protein mixtures.
Electrophoresis
Protein molecules are electrically charged at pH values different from their
isoelectric point (pI). As a consequence, they can migrate when exposed to
an electrical field, at a rate dependent upon their electrical charge densities.
In the separation of proteins by electrophoresis, the sample is submitted
to an electrical field, causing the electrically charged proteins to move in
the direction of the applied current. If the experiment is carried out in
solution (free electrophoresis) and the proteins have different charge
densities, they will move with different velocities, allowing their separa-
tion. In practice, the process is normally carried out in a gel matrix, instead
of in solution, and the gel can act not only as an inert support for the
electrophoresis buffer, but also, if desired, as an active material that
interacts with the proteins.
A very popular electrophoretic technique is SDS-PAGE (sodium dode-
cyl sulfate-polyacrylamide gel electrophoresis), used for separating protein
molecules based on their molar masses, providing high resolution and also
allowing the determination of the protein molar masses. In this method,
the sample is pre-treated with the detergent SDS, which causes the
dissociation of protein subunits and the complete unfolding of the poly-
peptide chain, which interacts with SDS molecules forming elongated rod-
shaped complexes. This provides identical charge/size ratios to all pro-
teins, so that the migration velocity of the denatured proteins becomes
dependent only on the molar mass.
Another version of electrophoresis, that is also very efficient for protein
separation, is isoelectric focusing or electrofocusing. In this technique, a
protein mixture is submitted to an electrical field in a gel support, such as
polyacrylamide or agarose, with a gradient of increasing pH established
from anode to cathode. Since the loaded proteins will be positively
charged at pH values below their pI, and negatively charged at pH values
above their pI, a protein will migrate until it finds a location within the gel
where the pH is the same as its pI (La
˚
a
˚
s, 1998).
Electrophoretic techniques have a number of practical advantages, such
as high resolution, equipment of relative simplicity and low cost, feasi-
bility for multiple samples, high sensitivity, easy detection of specific
molecules, and availability of standards giving reasonably well defined
bands. However, as a consequence of its low capacity, electrophoresis is
mostly used for analytical purposes rather than as a preparative technique.
More details on electrophoretic techniques are provided in Chapter 13.
Ion exchange chromatography
Among the chromatographic methods, ion exchange is the most com-
monly used in protein purification, due to its simplicity for scale-up, wide
applicability, and low cost compared with other chromatographic meth-
ods. Ion exchange of proteins involves their adsorption onto the charged
310 Animal Cell Technology

groups of a solid support, followed by their elution and concentration in
an aqueous buffer of high ionic strength.
For the effective use of ion exchange for protein purification, the
stationary phase should be able to bind to positively or negatively charged
proteins. There are two types of ion exchangers: anion exchangers (posi-
tively charged matrix) and cation exchangers (negatively charged matrix).
Low molar mass counter-ions are associated with the proteins, as well as
with the stationary phase. For protein binding onto the stationary phase,
the counter-ions should be dissociated. The most widely used counter-
ions are Na
þ
and H
þ
in cation exchangers, and Cl
–
and OH
–
in anion
exchangers.
The counter-ions can be arranged according to their intensity of inter-
action with the other ionic groups at the same concentration. Conse-
quently, a chloride would displace hydroxide ions as a counter-ion in ion
exchange. The counter-ions are not permanently bound to an ionic group,
but are in an equilibrium state, where continuous replacements take place.
So, ionic groups can become free to bind a protein. The higher the
counter-ion concentration, the lower will be the probability of the ionic
groups being available for protein binding. Sometimes, before the ion
exchange process, counter-ions should be replaced by others more suitable
for the specific application.
The support can be cellulose, agarose, dextran, silica, polyacrylate,
polyvinyl, or polystyrene, among other resins. The most suitable matrix
for a given application is often selected based on data provided by the
manufacturers.
The selection of the ionic functional groups of the support is based on
the strength of these groups, as listed in Table 12.2. The most widely used
functional groups are S, C, DEAE, and Q. The functional groups S and Q
are a strong acid and a strong base, respectively. Their pK values are
around 1 and 14, respectively, and thus, they are completely ionized at
practically any pH. The functional groups C and DEAE are a weak acid
Table 12.2 Chemical groups used in ion exchange for protein purification
Formula Group
Strong anion exchangers
–CH
2
N
þ
(CH
3
)
3
Trimethylaminomethyl (TAM)
–C
2
H
4
N
þ
(C
2
H
5
)
3
Triethylaminoethyl (TEAE)
–CH
2
N
þ
(CH
3
)
3
Quaternary amine (Q)
Weak anion exchangers
–C
2
H
4
N
þ
H
3
Aminoethyl (AE)
–C
2
H
4
N
þ
H(C
2
H
5
)
2
Diethylaminoethyl (DEAE)
Strong cation exchangers
–SO
3
–
Sulfonate (S)
–CH
2
SO
3
–
Sulfomethyl (SM)
–C
3
H
6
SO
3
–
Sulfopropyl (SP)
Weak cation exchangers
–COO
–
Carboxy (C)
–CH
2
COO
–
Carboxymethyl (CM)
From Ladisch (2001), Karlsson et al. (1998).
Produc t purification processes 311

and a weak base, respectively, with pK values around 4 and 10. For the
weak groups, the prevailing pH affects the ionization, and the pK is used
as an indication of the adequate operational pH range. Therefore, to ensure
a suitable ionization, the cation exchanger C is used at a pH above 6, and
the anion exchanger DEAE at a pH below 9.
Besides influencing the adsorbent ionizing groups, the pH also affects
protein charge and stability. In practice, if the protein is more stable at a
pH below its pI, a cation exchanger is used, and conversely, if it is more
stable at a pH above its pI, an anion exchanger is used.
Some adsorbents present a remarkably high density of ionic groups on
the surface, allowing multiple adsorption points, which require high salt
concentrations to promote elution, which may cause denaturation.
The aqueous buffers used in ion exchange should contribute to the ion/
counter-ion dissociation. The buffer minimizes pH fluctuations, avoiding
protein denaturation. The selection of the most suitable buffer depends on
the type of the ion exchanger, on product stability, and also on the
optimum pH for maximum adsorption. An important requirement is that
the buffer should not interact with the adsorbent and this is the reason
why the selected buffer, when charged, usually has the same charge as the
ion exchanger. Depending on the chromatographic step, adsorption or
elution, the pH should be adjusted to promote protein adsorption or its
displacement from the matrix.
During adsorption, the pH should be adjusted to one unit above or
below the protein pI, since larger differences result in a greater net charge
of the protein, and consequently, multiple adsorption points, requiring
severe conditions for elution. An ideal pH is suitable for adsorption at a
level which allows the elution to be performed only by a small pH change.
The buffer ionic strength determines the degree to which the ionic
groups of both the protein and stationary phase are blocked. During
adsorption, the highest ionic strength that still enables adsorption of the
desired protein is used, whereas during elution, the lowest ionic strength
that promotes its desorption is recommended. If the ionic strength is
excessively low during adsorption, the protein will adsorb very strongly,
making elution difficult. Keeping the ionic strength as high as possible
during adsorption minimizes the adsorption of contaminants, and keeping
it low during the elution minimizes the desorption of contaminants. Such
a strategy simplifies the elution step.
Once the optimum adsorption/desorption conditions are established,
other issues should be considered, such as the need for matrix pretreatment
and the operational mode for adsorption and elution steps. Pretreatment
of the ion exchanger can involve, for example, the removal of fine particles,
swelling, washing, or counter-ion replacement.
Adsorption can be carried out in tanks and in a batch system, as well as
in chromatographic columns. Batch adsorption can be carried out in the
early purification step, allowing the processing of large product volumes,
despite having a low efficiency. Column adsorption, on the other hand,
presents limitations with regard to the flow rates, but gives better resolu-
tion. There are two distinct methods of elution. The first method is that
protein adsorbed on the static ion exchange matrix is completely eluted by
a small volume of a strong eluent. This method is useful for the concentra-
312 Animal Cell Technology

tion of a protein present in a large sample volume. The second method
involves a dynamic ion exchange in which protein separation relies on
relative migration velocities along the column. In this case, there are three
distinct modes of elution:
(i) isocratic, employed for weakly bound proteins, resulting in large
elution volumes;
(ii) in steps, based on discontinuous and sequential changes of pH and/or
salt concentration;
(iii) gradient, based on continuous changes in the eluent composition (pH
and/or ionic strength).
The protein concentration in the eluted fractions collected from a
chromatography column is frequently determined by measuring the absor-
bance in the UV region (usually at 280 nm).
An advantage in ion exchange chromatography is that the matrix can be
regenerated by the removal of bound contaminants and by the reconstitu-
tion of the counter-ions, providing the matrix in the condition required
for a new protein adsorption cycle. If the support matrix is stored wet, it is
susceptible to microbiological degradation, and the use of antimicrobial
agents during storage is recommended.
12.4.4 Separation processes based on differences in hydrophobicity
The surfaces of proteins are mostly hydrophilic. Although the majority of
the hydrophobic residues tend to be buried in the interior of the protein,
some hydrophobic regions are also found on the surface (Voet and Voet,
1995; Ladisch, 2001). The level of surface hydrophobicity differs from one
protein to another, mainly as a consequence of the amino acid composition
and sequence. Difference in surface hydrophobicity is the property
exploited in hydrophobic interaction chromatography (HIC) and reverse
phase chromatography (RPC).
In both chromatographic methods, the adsorbent contains hydrophobic
groups. However, in RPC adsorbent hydrophobicity is much higher than
that in HIC (Eriksson, 1998). Therefore, in HIC the adsorbent is a weak
hydrophobic phase, whereas in RPC it is a dense hydrophobic phase.
Hydrophobic interaction chromatography
The adsorbents used in hydrophobic interaction chromatography are
usually polymer resins, such as reticulated agarose, derivatized with
aliphatic groups such as butyl and octyl, or with aromatic groups, such as
phenyl.
The tendency for protein binding to hydrophobic groups is often
reduced in low salt concentrations. Therefore, to increase the hydrophobic
interaction intensity, high salt concentrations are used during equilibration
and sample application. Due to their effect on strengthening the hydro-
phobic interactions, the most effective salts are those used in salting-out
precipitation, such as ammonium sulfate.
Produc t purific ation processes 313
