Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


13.3 Control of the production process
13.3.1 Cultures
Cultures with a finite number of passages
This culture method is defined by a limited number of passages or
population doublings, which should not be surpassed during the produc-
tion process. The maximum number of doublings or passages should be
established for the process.
The procedure and materials used for cell propagation and product
induction should be described in detail (see Chapter 14). For each produc-
tion lot, data should be given about the scope and nature of any microbial
contamination of culture containers immediately before collection. The
sensitivity of the methods used for detection should be described.
Data should be available about the uniformity of the fermentation
conditions and cell propagation, and about the maintenance of the product
yield (cell concentration and viability, nutrient and metabolite concentra-
tions, product concentration, etc.). The criteria as to when to discard a
culture should be established (when they are not included in the unifor-
mity specifications mentioned). The characteristics of the host cell and
vector at the end of production cycles should be observed. If pertinent, the
nucleotide sequence of the insert coding the cloned DNA-derived product
should be determined at least once after the culture is carried out on a
large scale.
Continuous cultures
The number of population doublings or passages in this type of culture is
neither defined nor restricted for production. The manufacturer will define
the criteria adopted for both the cell culture and the end of the production
process (see above – Cultures with a finite number of passages). During
the culture stage, these criteria must be monitored; the frequency and type
of monitoring will depend on the nature of the production system and the
product (Bollati et al., 2005). These should be defined and recorded when
the product is registered.
Information should also be given about the integrity of the gene that is
being expressed and the phenotypic and genotypic characteristics of the
host cell after a long term culture. If pertinent, the nucleotide sequence of
the insert that codes the cloned DNA-derived product should be deter-
mined, at least once after a large scale culture. Although, as mentioned in
the section on the MCB above, when multiple copies of the cloned gene
are inserted in the genome of a continuous cellular line, it may be
inappropriate to sequence the cloned gene.
Data should also be presented showing that the variations in cell density
and product concentration fall within established limits. The acceptance of
culture supernatants for further processing must be clearly linked to the
predefined program adopted, and this will depend upon a clear definition
of the characteristics of a ‘‘product lot.’’ The criteria to discard suspen-
sions and stop cultures, when they do not follow the specifications (see
334 Animal Cell Technology

above), should also be established. Systemic tests should be performed to
investigate the microbial contamination according to a pre-defined har-
vesting strategy.
The maximum length of a continuous culture should be based on
information about the system and product uniformity and stability. In
long continuous cultures, the cell line and product will be repeatedly
evaluated at intervals determined by the information on the stability of the
host–vector system and the product characteristics.
13.3.2 Purification
The methods used in the recovery, extraction, and purification must be
described in detail. Special attention must be paid to the elimination of
viruses, nucleic acids, and undesirable antigenic materials.
In procedures involving affinity chromatography, which may use bio-
logical components such as mAbs, appropriate measures should be taken
to ensure that no contamination arises from its use, such as adventitious
viruses, that could threaten the safety of the final product. The more
commonly used methodologies to determine the levels of contamination
are presented in Section 13.4.8.
The ability of the purification process to eliminate product related or
host cell derived proteins, nucleic acid, carbohydrates, viruses, or other
undesirable impurities, including undesirable media derived and chemical
components, must be thoroughly investigated, as well as the reproduci-
bility of the process.
13.4 Product control
13.4.1 Characterization and specification
The requirements for identity, purity, activity, and stability of the product
are closely related to the processing technology and the physicochemical
and biological characteristics of a specific drug. The planned use of the
product should also be considered.
In general the quality control procedures for products obtained through
biotechnology are very similar to those routinely used with traditional
pharmaceutical products in areas such as raw material testing, documenta-
tion of process control, and aseptic processing. The fundamental difference
is in the type of methods used, so as to determine the product’s identity,
uniformity, and purity. In the quality control of products obtained
through recombinant DNA technology, it is necessary to employ vali-
dated tests for the final and intermediary products to ensure the elimina-
tion of undesirable impurities.
It is essential to characterize the final active substance through chemical,
physical, and biological methods. Special consideration should be given to
the use of a range of analytical techniques to determine a range of
physicochemical properties of the molecule. The methods used must be
validated and should have a known sensitivity.
Contamination of the product generally may come from three sources:
Quality control of biotechnological products 335

(i) host organism: proteins or DNA of the host;
(ii) proteins and impurities from the production process – mainly from
the purification stage;
(iii) impurities related to the active substance, for example, aggregation
state, reduced or oxidized forms.
The purity of a protein preparation obtained through recombinant
DNA technology should be maximized. The allowed levels of contami-
nants for each product should be specified for the manufacturing process.
13.4.2 Protein content
Quantification of total protein is very important because the values of
other product parameters depend on the accurate determination of protein
content, for example the specific activity.
There are various accepted methods to determine the protein content,
namely:
(i) UV absorbance. This is one of the simplest methods and it does not
require a standard. However, the coefficient of molar extinction of
the protein should be known because it is specific for each protein
(Goldfarb et al., 1951).
(ii) Lowry method. This is a colorimetric method and needs to be
compared to a standard, e.g. the bovine serum albumin (Lowry et al.,
1951). The reaction products are spectrophotometrically quantified
between 540 and 560 nm. This technique is linear at the microgram
range and can be used for any protein. Alcohol, sugars, and detergents
interfere in the measurements, making their removal necessary before
any reliable measurement.
(iii) Bradford method. This is a colorimetric method that uses Coomassie
brilliant blue, which forms a conjugate with proteins under acidic
conditions (Bradford, 1976). An unknown needs to be compared to a
standard, e.g. bovine serum albumin. The reaction products are
quantified spectrophotometrically at 595 nm. The technique is linear
at the microgram range and can be used for any protein. There are
various substances/factors that interfere with the measurements (SDS,
Tween, Triton-X100, high ionic strength, alkaline pH) making their
removal necessary before any reliable measurement.
(iv) Kjeldahl method. This is a technique used to determine the amount
of nitrogen present in a protein. It is estimated that 1 mg of nitrogen
equates to 6.5 mg of protein (Kjeldahl, 1883).
13.4.3 Amino acid analysis (identification and/or protein content)
The method consists of the complete hydrolysis of a protein or peptide to
release its component amino acids. These may be separated by reverse
phase high performance liquid chromatography (HPLC), and quantified
with fluorometric detection after derivatization with o-phthaldehyde
(Larsen and West, 1981). The method serves to determine both the amino
acid composition and the total quantity of protein present in the sample.
336 Animal Cell Technology

Disadvantages of this method include the total or partial destruction of
some amino acids (e.g. tryptophan, serine, and threonine), the under-
estimation of the quantity of amino acids that are difficult to hydrolyze
(valine and isoleucine), and the difficulty of quantifying cysteine and
methionine, except by prior oxidation.
The amino acid composition should be determined from the average of
a minimum of three separate hydrolyses per lot.
13.4.4 Protein sequencing (identification)
This technique gives information about the protein’s primary structure,
which may include its amino and/or carboxyl terminal groups (Edman,
1950). For recombinant DNA-derived proteins, this analysis serves to
confirm the amino acid sequence predicted by the DNA sequence. The
analysis can also be useful to determine the protein’s homogeneity.
(i) Amino-terminal: This is a classic chemical technique of sequential
breakdown from the N-terminal end of a protein and can provide
limited, but rapid, sequence information to provide data on homo-
geneity and identity. The method is semi-quantitative, and the data
cannot be used to determine the exact proportions of amino acids
originating from different N-terminals if present in a protein.
(ii) Carboxyl-terminal: This provides sequence information about the
primary structure by degradation from the C-terminal end. The
method is also semi-quantitative.
13.4.5 Peptide mapping
This method consists of specific cleavage of the target protein using an
endoprotease or by a chemical method. The resulting peptides are sepa-
rated by reverse phase HPLC or ionic exchange to obtain a highly specific
profile.
Peptide mapping is a method that enables the determination of protein
identity when compared to a standard. When compared to previous lots of
the same product, it serves to determine the stability of the protein’s
primary sequence, which in turn reflects the genetic stability of the
producer cells. This method is capable of detecting small differences
between proteins in one or more amino acids. The detection will be
dependent upon an amino acid alteration affecting the observed peptide
profile (Figure 13.1; see color section).
13.4.6 Electrophoresis
Electrophoresis methods are among the most common and potent used in
the evaluation of protein purity and homogeneity. They are valuable
indicators of protein stability because they detect small molecular or
chemical changes in the product caused by denaturation, aggregation,
oxidation, deamidation, etc. One of the advantages is that they require
only microgram amounts of a sample.
Quality control of biotechnological produc ts 337

The two most widely used types of electrophoresis tests are: (i) sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE); and (ii)
isoelectric focusing (IEF).
SDS-PAGE
This test separates proteins according to their molecular weight (Laemmli,
1970). Firstly, the sample is denatured in a detergent, which breaks the
non-covalent intra- and inter-molecular links of the proteins, and then it is
separated electrophoretically by a polyacrylamide gel. The electrophoretic
separation must be performed under reduced and non-reduced conditions
to determine the existence of impurities with the same molecular weight.
The method under non-reduced conditions is normally used to estimate
the aggregation and/or oligomerization of the protein but only aggregates
or oligomers that are stable in the presence of SDS will be detected and
under the conditions used for the sample preparation and electrophoresis.
In the method validation, low concentrations of the product are used for
the detection of the target protein, while high concentrations are recom-
mended for the detection of impurities.
The sample detection after electrophoresis can be quantified by a
densitometric analysis of Coomassie brilliant blue stained spots. A silver
stain is available to provide higher sensitivities when detection of samples
in the nanogram range is required.
SDS-PAGE, together with the Coomassie brilliant blue coloration, is
used to quantitatively determine the sample purity with respect to dimers,
larger covalent aggregates, and polypeptide fragments. The SDS-PAGE
protein separation can be combined with the Western blot analysis, which
is used to determine the presence of an electrophoretic band (Figure 13.2;
see color section).
To calculate the percentage purity from a dilution series, the lane in
which the protein of interest is no longer visible is determined (Lm).
Similarly, for each band of impurity, the lane in which it is no longer
visible is determined (Li).
I(%) ¼
Pm
Pi
3 100
where I (%) is the estimated percentage of impurity, Pm the minor protein
mass applied which can be visualized and which corresponds to lane Lm-1
and Pi is the protein mass applied in lane Li-1. The sample’s percentage
purity is calculated by adding the impurity percentages corresponding to
the different impurity bands and deducting this result from 100. In order
to apply this principle it is assumed that there are no impurity bands at the
same position as the target protein.
The SDS-PAGE protein separation can be combined with Western blot
analysis, which is used to detect the band of the target protein.
After electrophoresis, proteins are transferred onto a nitrocellulose or
PVDF (polyvinylidene fluoride) membrane, which is immersed in a
solution of a selected antibody capable of combining with the target
protein. Then the reaction of antigen-specific antibody is detected through
a second antibody which is tagged enzymatically. Special attention must
338 Animal Cell Technology

be paid to impurities of the immunogen used in production of the anti-
body (Amadeo et al., 2004; Oggero et al., 2006).
Isoelectric focusing (IEF)
This separates proteins according to their charge in an electric field
(Svensson, 1961). The protein charges result from its amino acid composi-
tion and the presence of charged molecules from post-transductional
modification (e.g. sialic acid molecules in complex glycan structures
derived by glycosylation). However, there is a specific pH (the isoelectric
point, pI) for each protein, in which the opposite charges on each protein
molecule balance, providing a net charge of zero.
The IEF separation is performed with native proteins in polyacrylamide
with a large pore size or with agarose gel with ampholytes (amphoteric
ions of low molecular weight), which establish a pH gradient due to their
migration inside the polymer matrix when an electric field is applied. In
the presence of an electric field, sample proteins with a positive charge
migrate to the cathode and proteins with a negative charge migrate to the
anode. The migration ends when each protein reaches the pH value
corresponding to its specific pI value. Here, its net charge is zero. As the
protein’s migration depends on its amino acid composition, the altered
forms of proteins or non-target proteins will migrate to different points.
The IEF gels can be colored for the detection of bands with Coomassie
brilliant blue or silver stain. This technique can also be combined with an
immunological method through electro transference of the bands to a
nitrocellulose or PVDF membrane, after electrophoresis, and its later
reaction with a tagged antibody, either in an enzymatic or radioactive
form.
IEF is used as a purity test and for protein characterization, comparing
it with the relative position of an authentic band of the target protein. It
can also be used as a method to evaluate the stability of a biological
product. Small changes such as the deamination of an amino acid will
cause a change in the pI and a modification of the band pattern.
The results of the IEF gel can occasionally be difficult to interpret and
its applicability should be considered for each protein (Figure 13.3; see
color section).
High performance capillary electrophoresis (HPCE)
HPCE offers the advantage of high resolution protein analysis (Dolnik,
2006). Through this method it is possible to analyze the content of
isoforms recovered after the recombinant protein purification. Each peak
corresponds to a single isoform and the determination of the relative area
under each peak enables the determination of the percentage content of
each isoform. In some cases this can be used an as an assessment of a
protein’s quality, when the relationship between biological activity and
structure is known (Etcheverrigaray et al., 2005) (Figure 13.4; see color
section).
Quality control of biotechnological products 339

13.4.7 Carbohydrate determination
Glycosylation is a possible post-translational modification, characteristic of
recombinant proteins expressed from eukaryotic cell lines (see Chapter 6).
Glycosylation can influence pharmacokinetics and protein function, so
changes in the glycosylation profile can have a significant impact on the
pharmacodynamic characteristics and consequently therapeutic efficacy
(Sinclair and Elliot, 2004; Marini et al., 2005).
The glycosylation profile is affected by the cell culture conditions.
Ideally, a product should be characterized at least once to identify the
potential glycosylation sites as well as the specific carbohydrate at each
site.
The quantification of specific sugars and total carbohydrates should be
performed in each lot. In some cases, IEF and capillary electrophoresis can
be alternative methods for determining the percentage of each isoform in a
product sample lot. It is often important to determine the isoform profile
for each lot prior to release. It would be ideal, but not always possible, to
relate the isoform profile to the specific activity of the product.
Two main approaches can be taken to determine the sugar covalently
linked to the glycoprotein. Microheterogeneity of glycans is a common
phenomenon of glycoproteins giving rise to variability in glycoforms
between molecules. Therefore the information on carbohydrate content
represents either the average composition or representative structures. The
first approach is to determine the composition of glycoprotein-linked
sugars. The second approach is to release and separate the structures of
individual oligosaccharides covalently bound to glycoprotein. This ap-
proach serves to obtain an oligosaccharide map similar to the peptide map
for a protein (Chaplin and Kennedy, 1994).
More information about this subject can be obtained in Chapter 6
(Post-translational modification of recombinant proteins).
13.4.8 Potential impurities and contaminants of biotechnological
products
Table 13.1 describes the most frequent types of impurities and contami-
nants, indicating the most suitable detection methods as a guide.
The residual host cell DNA may represent a different threat as an
impurity for each product, since it depends on the host organism and the
purification process. The control of residual DNA is a measure of efficacy
and consistency in the purification process, as well as the product quality.
The levels of DNA should be quantified using methods of an adequate
sensitivity. Among the techniques used, the following can be mentioned.
(i) Slot blot hybridization, using specific probes tagged with radioactive
phosphorus (
32
P). This is the most sensitive and routinely performed
measurement (Pepin et al., 1990).
(ii) Biosensors technology. This methodology is more appropriate for the
determination of total DNA/nucleic acid impurities than specifically
for host cell DNA.
(iii) Polymerase chain reaction technology (PCR). Adequate primers
should be used.
340 Animal Cell Technology
13.5 Bioassays
Methods to determine the potential biological activity of products ob-
tained through recombinant DNA techniques are of fundamental impor-
tance. Despite the existence of numerous physicochemical techniques to
characterize the protein product structure and the presence of contami-
nants, they provide little, if any, information about its biological potency.
A bioassay is defined as a functional test, and no physicochemical test can
measure the function. However, for some peptide hormones, which are
less complex in structure than most cytokines, well defined physicochem-
ical tests may be used to estimate biological activity; for instance, the
capillary electrophoresis analysis of a protein’s isoform content if the
specific activity of each one is known.
A bioassay is an analytical procedure that uses a responsive biological
system (biological function) to measure the biological potency of a
product (Mire-Sluis et al., 1996). The most appropriate method to deter-
mine it is by comparing the biological activity of a sample with a well-
characterized reference standard.
The biological activity measured is often expressed in international
units, but should be recorded in relation to the product mass. This means
that the biological effect is measured as activity per unit of mass and
Table 13.1 Impurities and contaminants in processes to obtain biotechnolo-
gical products
Types Detection method
Impurities
Endotoxins Bacterial endotoxins test, pyrogen test
Host cell proteins SDS-PAGE, immune assays
Other protein impurities SDS-PAGE, HPLC, immune assays
DNA DNA hybridization, ultraviolet spectrophotometry,
PCR
Mutant proteins Peptide mapping, HPLC, IEF, mass
spectrophotometry, amino acids sequencing
Formyl methionine Peptide mapping, HPLC, mass spectrophotometry
Proteolytic cleavage IEF, SDS-PAGE, HPLC, amino acids sequencing
Protein aggregates SDS-PAGE, HPSEC
Deamination IEF, HPLC, mass spectrophotometry, amino acids
sequencing
Monoclonal antibodies SDS-PAGE, immune assays
Amino acids substitution Amino acids sequencing and analysis, peptide
mapping, mass spectrophotometry
Contaminants
Microbial contaminants
(bacteria, yeasts, fungi)
Hygienic control, sterility test, DNA
Mycoplasma PCR, DNA
Virus (exogenous and
endogenous)
CPE, Had (only exogenous virus), reverse transcriptase
activity, PMA
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; HPLC, high perform-
ance liquid chromatography; PCR, polymerase chain reaction; IEF, isoelectric focusing; HPSEC,
high performance size-exclusion chromatography; DNA, DNA-binding fluorochrome; CPE,
cytopathic effect; Had, hemadsorption; PMA, production of murine antibodies.
Quality control of biotechnological products 341

should be consistent within clearly specified limits. The activity expressed
per unit of mass is called specific activity and it constitutes a parameter of
identity and/or purity.
However, the bioactivity measured in an in vitro assay may not be
considered as a direct biological activity indicator in the human. Thus it
has been shown that a biological assay for a cytokine does not necessarily
reflect the clinical efficacy (Thorpe et al., 1997).
13.5.1 Bioassay types
The first bioassay type evaluated the response of cytokines directly in
animals. However there are many disadvantages to animal tests including
inter-animal variability, their expense, demand for intensive work, and
ethical concerns when many animals may have to be sacrificed to provide
statistically valid data. Significant variability can result from the sex, age,
species, and health of the animals used.
Cell culture technology can be a viable alternative to animal testing in
many cases, with the possibility of increasing significantly the number of
replicate samples and thus expanding the bioassay utility. Primary cell
cultures have the advantage that they maintain most of the characteristics
of the animal tissue from which the cells are derived. These would be ideal
for bioassays except that they cannot be kept in culture indefinitely. They
can be difficult and sometimes impossible to grow reproducibly, and are
subject to the inherent variability of the animal they come from.
Consequently, most bioassays for cytokines are performed using estab-
lished cell lines that can be grown indefinitely. There are many advantages
associated with the use of such lines. They are available from frozen cell
banks and do not require direct isolation from primary animal tissue. They
are generally clonal and so their growth is relatively consistent, allowing
relatively consistent results over time. As indicated earlier there is an
inherent variability associated with the use of living systems, whether
entire organisms, ex vivo tissue explants, or isolated cells. However, the
use of continuous cell lines would tend to minimize these problems. The
cells may lose cell function and suffer genetic changes over extended
culture passage. However, this can be controlled by maintaining a large
enough cell bank at low passage number for most experts and so any such
disadvantage is more than compensated for by their advantages.
Despite the diverse existing varieties of bioassays for cytokines, all are
based on the protein’s capacity to induce a measurable activity in cells and
tissues. The cell responds in various ways including: enhanced growth,
growth inhibition, expression of cellular markers, cytotoxicity, or antiviral
activity (Wadhwa et al., 1995).
The selection of the type of assay depends on the nature of the bio-
logical material available for assay. The bioassays can demonstrate the
consistency of the product’s biological activity by replicate analysis as well
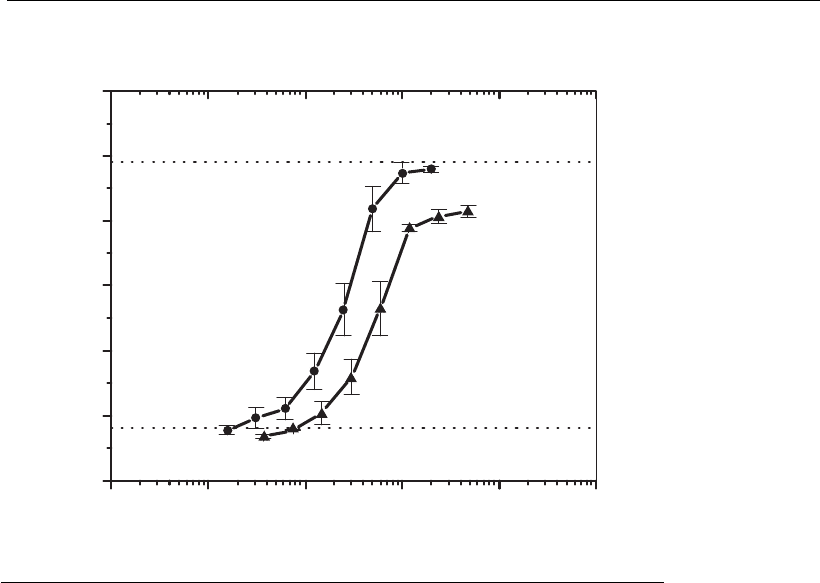
as define the potency of each lot. Figure 13.5 shows the analysis of
biological activity of a sample of recombinant human interferon-1a
(rhIFN1a).
342 Animal Cell Technology
13.5.2 In vitro bioassays
The major advantage of a bioassay is the capacity to detect only functional
molecules. However, despite this, there are also important disadvantages.
These include the low specificity, which may be attributed to the nature of
the molecules themselves, including their pleiotropic actions, their diver-
sity of target cells, and their ability to induce different effects on different
cell types. In addition, the same function may be performed by two
different cytokines, probably through the same cellular receptor. This is a
disadvantage when dealing with samples that may contain multiple cyto-
kines, such as clinical samples (Cannon et al., 1993; Whiteside, 1994).
However, the inclusion of specific neutralizing antibodies for target
proteins may permit the determination of the activity of a single protein
within a mixture.
Contamination (including mycoplasma) and differences in serum lots
for cultures can affect bioassay performance. Consequently, it is important
to maintain cell lines adequately and use consistent sources of material to
ensure reproducibility of test results. Bioassay data generally follow a
sigmoidal curve, which can complicate the interpretation of results.
1E 9⫺ 1E 8⫺ 1E 7⫺ 1E 6⫺ 1E 5⫺ 1E 4⫺
Dilutions of culture supernatant (dil )
⫺1
rhIFN 1a (UI/ml)β
0,01 0,1 1 10 100 1000
Absorbance ( 540 nm)λ ⫽
0,0
0,5
1,0
1,5
2,0
2,5
3,0
Figure 13.5
Determination of the in vitro biological activity of rhIFN1a (recombinant human
interferon-1a). Standard (d), production sample of rhIFN1a (m). The curves
corresponding to the standard and the sample were processed in triplicate and the
results are shown as the average valu e SD. The dotted horizontal lines indicate
the average values of the test’s negative and positive controls processed in
quadruplicate.
Quality control of biotechnological products 343
