Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


For the selection of the operational conditions, it is recognized that
hydrophobic interactions can be weakened by several factors, such as a
temperature decrease, pH change, presence of organic solvents, non-ionic
detergents, and polyols such as PEG.
Usually, desorption is performed by means of a decreasing salt gradient,
so that the solutes are eluted according to the increasing order of their
surface hydrophobicity. The remaining proteins can also be eluted by the
application of buffers with increasing concentrations of, for example,
PEG. The operational conditions during the process are mild, since the
salts used in HIC exert a stabilizing effect on proteins (Ladisch, 2001).
HIC has a high adsorption capacity and provides high recoveries,
making it a popular technique for large-scale applications (Kumpalume
and Ghose, 2003). A limitation, however, is the high cost of using large
amounts of salt. Its selectivity is not high, being lower than that of affinity
chromatography. HIC is suitable when combined with ion exchange and
molecular exclusion chromatography steps (Maugeri Filho and Mendieta-
Taboada, 2005).
Reverse phase chromatography (RPC)
Currently, the most widely used adsorbents in RPC are silica resins,
containing a hydrophobic phase, usually octyl (C8), octyldecyl (C18),
methyl (C1), or phenyl groups. Additionally, new adsorbents based on
organic materials such as methacrylate, polystyrene, and copolymers of
styrene and divinylbenzene have been developed (Hearn, 1998).
Due to its high hydrophobicity, the adsorbent interacts strongly with
proteins, requiring low salt concentration during the adsorption, and
increasing gradients of organic solvents, such as methanol, isopropanol,
and acetonitrile, during elution (Harrison et al., 2003).
Despite being widely used in protein analysis, peptide mapping, and for
purification of low molar mass molecules, RPC is not often used for
protein purification on a large scale (Ladisch, 2001).
12.4.5 Separation processes based on specificity of ligands
Some proteins function through specific non-covalent binding to other
molecules, termed ligands. Ligands can be small molecules, such as
enzyme substrates, or larger molecules, such as hormones. Protein inter-
action with the ligand is determined by the size and shape of the ligand, as
well as by the number and distribution of complementary regions. These
regions combine charged and hydrophobic portions, presenting other
short range interactions, such as hydrogen bonds.
Protein–ligand interaction, which is stereo-specific and consequently
presents high affinity, can be used for the isolation of a given protein from
a complex mixture. This provides a high degree of purification. The most
widespread technique is affinity chromatography. Affinity ligands have
also been used to increase the resolution and the selectivity of other
techniques such as precipitation (Lali et al., 1998), liquid–liquid extraction
(Johansson, 1998), and filtration assisted by macroligands (Romero and
Zydney, 2002).
314 Animal Cell Technology
Affinity chromatography
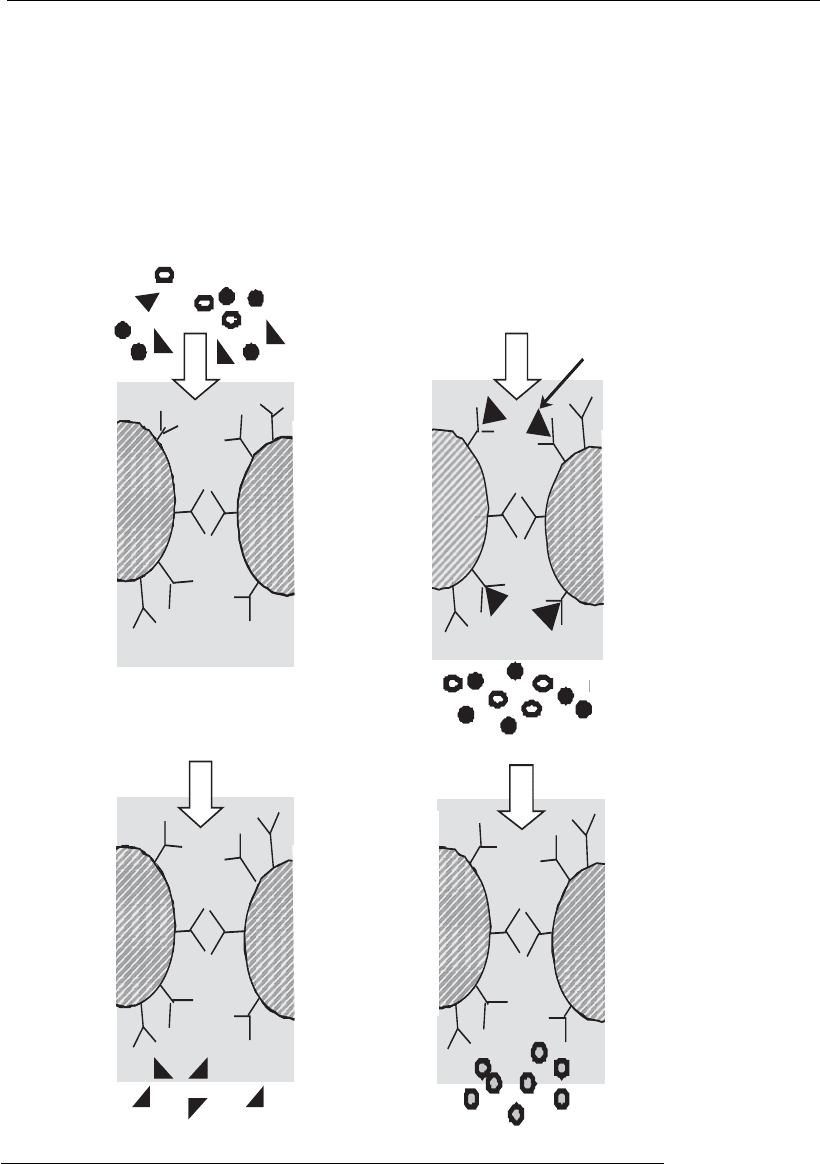
Affinity chromatography is an adsorption method based on the recogni-
tion between a ligand immobilized on a solid matrix and a biomolecule to
be separated. The main difference between this method and other chroma-
tographic techniques is the high interaction specificity between molecules
and stationary phase.
As shown in Figure 12.5, during the adsorption step interactions occur
between the target biomolecule and the ligand located in the adsorbent
-
Elution
buffer
3. Elution
Sample
loading
1. Adsorption
Adsorption
buffer
Regeneration
buffer
4. Regeneration
Target
protein
2. Washing
Figure 12.5
Principles underlying affinity chromatography.
Produc t purification processes 315

pores, whereas the remaining molecules flow through the adsorbent with-
out interacting. Next, in the washing step, a buffer (usually the same used
in the equilibration or adsorption steps) is pumped through the column,
for the removal of molecules retained in the interstitial space or weakly
adsorbed by non-specific interactions. During the elution step, desorption
of the target biomolecule is promoted by changes in the medium (pH,
ionic strength, etc.) intended to weaken the interactions between the ligand
and the target biomolecule, or by the addition of counter-ligands with a
strong affinity to the ligand or to the adsorbate. Finally, regeneration is
carried out by washing the column with a specific solution, to recover the
adsorbent for its reuse in a new cycle.
Vijayalakshmi (1989, 2002) classifies the ligands used in affinity chroma-
tography in two groups: biospecific and pseudo-biospecific. Biospecific
ligands interact with the target biomolecule through functional affinity,
exactly as happens naturally, for example in antigen–antibody, glycopro-
tein–lectin, and hormone–receptor interactions. Biospecific ligands pro-
vide high specificity and high adsorption capacities, with possibilities of
achieving purification factors up to 1000-fold (Roper and Lightfoot, 1995).
A common example of the application of biospecific ligands is the
purification of monoclonal antibodies (mAbs) from cell culture super-
natants using protein A and G as the ligands (Castilho et al., 2002a;
Rasmussen et al., 2005; Hahn et al., 2006). However, biospecific ligands
are usually high cost molecules with a fragile three-dimensional structure.
The interaction can sometimes be so strong that adsorbate desorption is
only possible under drastic pH or ionic strength conditions.
Pseudo-biospecific ligands, such as metal chelates, amino acids, and dyes
are simpler and less expensive molecules with a structural affinity to
biomolecules (group specificity). Since they have simpler structures, they
can be immobilized by stable, well defined chemical reactions to the
chromatographic matrix. Their disadvantages are their lower specificity, as
compared with biospecific ligands. However, there are many examples of
molecules obtained from animal cell cultures that have been successfully
purified using pseudo-biospecific ligands (El-Kak and Vijayalakshmi,
1991; Atkins et al., 2005; Serpa et al., 2005; Kumar et al., 2006).
Due to the central role of the ligand in affinity chromatography, some
factors should be considered when selecting a ligand for the purification of
a protein:
(i) specificity: the ligand should be able to selectively recognize the target
protein;
(ii) reversibility: the ligand should form a reversible complex with the
protein to be purified, and the complex should be resistant to the
composition of the feeding stream or washing buffers, but easily
dissociated during elution without denaturation;
(iii) stability: the ligand should be stable under the conditions employed
during immobilization and chromatography;
(iv) immobilization feasibility: the ligand should contain a functional
group suitable for covalent binding to the support, without affecting
interaction properties with the protein.
316 Animal Cell Technology
The interaction between the ligand (L) and the protein (P) to form the
reversible complex (PL) can be described by:
P þ L > PL
where the dissociation constant, K
D
, is given by Equation (4):
K
D
¼
P
½
L
½
PL
½
(4)
In general, in affinity techniques K
D
values are in the range of 10
–8
to
10
–4
M, but the K
D
determined for free ligands can differ from those
determined for immobilized ligands. Many authors report their data in
terms of the association constant (K
A
), which is equal to K
D
–1
.
After selecting the ligand, a suitable matrix should be chosen for its
immobilization. The effectiveness of an immobilized ligand can be depen-
dent on the matrix structure. Different criteria are used to select the solid
matrix. The matrix should have high porosity and a suitable pore size, so
that the target protein can access the ligands immobilized in its interior. It
should be chemically stable during activation, ligand coupling, adsorption,
elution, and regeneration. The matrix should also be mechanically resistant
and rigid to allow suitable mobile phase flow, and should tolerate wide
pH and temperature ranges. The matrix should have a uniform structure,
possess high concentration of functional groups for ligand coupling, and
should not promote non-specific adsorption.
For a particular application, the relevance of these criteria may change.
Widely used matrices are agarose and synthetic polymers also used in ion
exchange and molecular exclusion chromatography. The ligand can be
coupled to the solid matrix through spacer arms, especially when the
ligand is small and stereo hindrance can affect its interaction with the
target protein. The length of the spacer molecule is important and, also,
it should have reactive ends enabling its coupling to the matrix and to
the ligand. The spacer molecule should not interact with proteins and
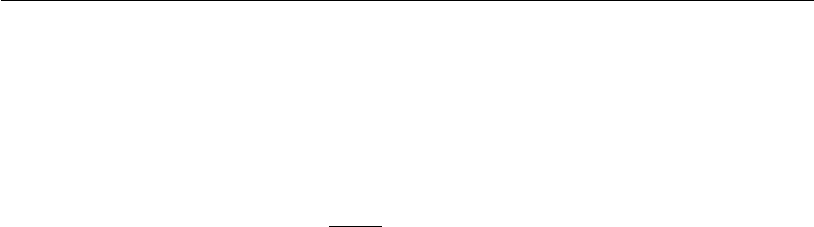
should preferably be hydrophilic. The coupling of a ligand to a matrix
by means of a spacer arm is based on two general procedures, described
in Figure 12.6.
Once a suitable matrix is obtained, with the ligand coupled to it, and
properly characterized with regard to ligand concentration, the effective-
ness of the protein–ligand interaction can be evaluated. A sample contain-
ing the target protein is applied to the column, which is washed and
evaluated for retained protein.
In affinity chromatography, there are several elution methods. One
involves the elution by affinity based on the addition of high concentra-
tions of a free ligand in the buffer. The eluted complex can later be
dissociated by changing the buffer salt concentration or by the addition of
a surfactant that decreases hydrophobic and van der Waals interactions,
such as a non-ionic detergent. Another possibility is elution based on high
salt concentration. In this case, non-hydrophobic interactions between the
protein and the immobilized ligand are broken, including biospecific ionic
forces and other polar forces. Other elution methods are based on pH and
Produc t purific ation processes 317
temperature changes (lower temperatures weaken hydrophobic inter-
actions) or on the addition of chaotropic agents.
A major concern in affinity chromatography is the release of the ligand
from the matrix and its consequent loss in the column outlet stream. This
can happen due to instability of the ligand–matrix binding or to dissolu-
tion of the matrix. Released ligands can contaminate the product and
decrease the adsorbent performance. This reduces the possibilities for
adsorbent reutilization, which has an impact on process costs.
For washing and storing, the majority of adsorbents used in affinity
chromatography can be regenerated with solutions of high salt concentra-
tion (2 M KCl) and stored in the presence of antimicrobial agents.
Affinity chromatography offers several advantages over other types of
chromatography because of its remarkable levels of purification and high
yields, which can come close to 100%, at least at laboratory scale (Walsh
and Headon, 1994). Because of its high selectivity, it allows the isolation
of a molecule present in low concentration in a complex mixture, enabling
the processing of large solution volumes and desorption with reduced
*
*
(A)
⫹ Activating
agent
⫹Spacer arm ⫹ Ligand
Ligand
spacer arm
⫹
⫹Activating
agent
*
*
(B)
Figure 12.6
Alternative routes for the coupling of ligands to a matrix. (A) The spacer arm is coupled to the matrix
and then the ligand is covalently attached to the support; (B) the spacer arm is first attached to the
ligand, and then it is attached to the matrix.
318 Animal Cell Technology

eluent volumes. This provides high purification and concentration factors
in a single step, in short times, and with high recoveries of biologically
active product.
12.4.6 Other developments
Expanded bed adsorption
Expanded bed adsorption (EBA) was derived from studies in the 1970s,
aimed at carrying out chromatography techniques in fluidized beds
(Kilikian and Santos, 2005). However, unlike fluidized beds, in an EBA
column the bed expansion is stable and predictable. This occurs because
the adsorbent usually consists of hybrid particles of high density such as
quartz, covered with a polymeric material such as cross-linked agarose to
which the immobilized ligands are attached (Figure 12.7; see color section).
By manipulating the proportion of these two materials, an apparent
density distribution in the range of 1.15–1.20 g cm
–3
is obtained. The
particles have a size distribution, usually in the range of 100–300 m.
When subjected to an ascending stream of the mobile phase, the particles
segregate within the column at different equilibrium positions, according
to their sizes and apparent densities.
In EBA, it is common to characterize the column and adsorbent through
the experimental determination of expansion curves, which show bed
expansion as a function of the superficial velocity of the ascending mobile
phase. The degree of expansion is defined as the ratio between the height
(H) of the expanded bed at a given superficial velocity, and the height of
the packed bed (H
0
), before pumping the mobile phase. The expansion
curve of the adsorbent Streamline-rPrA in a Streamline Direct 24 column
is shown in Figure 12.8A. For the characterization of the adsorption
performance of a column, breakthrough curves can be determined for
different superficial velocities (GE Healthcare/Amersham Biosciences,
2002), and for different size columns, if scale-up is intended.
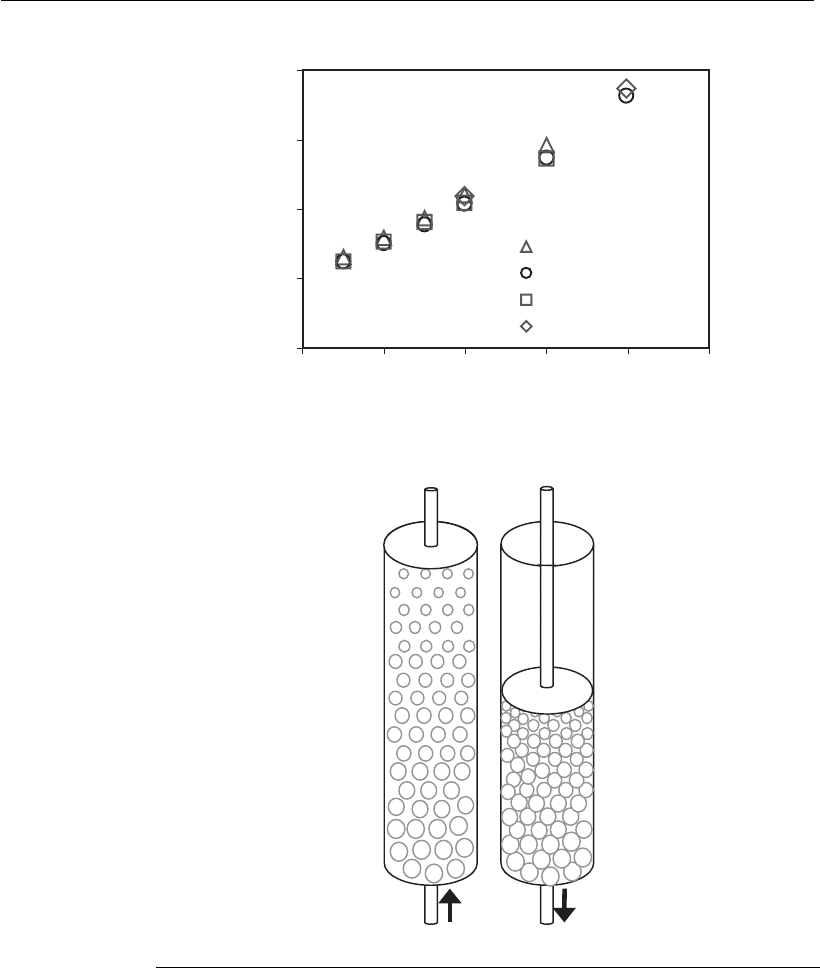
After adsorption, the column is washed with the bed still in the
expanded form, whereas elution is usually performed with the bed in the
packed form (Figure 12.8B), to minimize the elution volume, and thus
achieving a high concentration factor for the target protein.
EBA is basically an operation mode that is suitable for different types
of chromatography, such as ion exchange, affinity, and hydrophobic
interaction. Its main advantage is that, due to bed expansion, it allows the
application of cell suspensions, without loss in chromatography perform-
ance (Anspach et al., 1999). Therefore, EBA allows the elimination of the
solid–liquid separation steps that usually precede a chromatographic
process, reducing the number of downstream steps, with a decrease in
processing time and an increase in overall yield. Such advantages, com-
bined with purification and concentration factors that are adequate for
primary purification steps, explain why this technique has been used
increasingly in recent years on laboratory as well as on an industrial
scale.
Interesting results obtained on a pilot plant scale, for the direct purifica-
tion of a mAb from 100 L of a hybridoma-containing suspension, were
Produc t purification processes 319
reported by Ameskamp et al. (1999). Examples of EBA applications in the
industrial processing of vaccines and recombinant proteins from CHO
cells have also been reported in the literature (GE Healthcare/Amersham
Biosciences, 2000). For the direct application of cell suspensions, it is
(B)
(A)
0
1
2
3
4
Superficial velocity (cm/h)
H/Ho
7 rpm, without cells
14 rpm, without cells
17 rpm, without cells
14 rpm, with cells
0 100 200 300 400 500
Figure 12.8
(A) Expansion curve of the adsorbent Streamline-rPrA
1
, in a Streamline Direct 24
1
column, for different stirring velocities (located on the bottom of the column), in
the presence or absence of cells in the feed. H: height of the expanded bed;
H
o
: height of the packed bed. (B) Illustration of the adsorption step (left) in
expandedbedmode,andelution(right)inpackedbedmode.
320 Animal Cell Technology
recommended to use a specific column equipped with a stirrer positioned
at the bottom, which helps to prevent aggregation of adsorbent particles
and cells.
Membrane adsorbers
Membrane adsorbers derive from the technological developments in the
membrane separation field and in column chromatography of proteins.
They combine the high selectivity of chromatographic separations and the
high productivity usually obtained in membrane separation processes.
It differs from conventional chromatographic adsorbents in that the
support for ligand immobilization is composed of microporous mem-
branes, usually polymeric, with a nominal pore size generally in the range
of 0.4–3 m. Depending on the nature of the immobilized ligand, the
membrane is characterized as an ion exchange, affinity, or hydrophobic
interaction adsorber (Tho
¨
mmes and Kula, 1995; Charcosset, 1999; Haupt
and Bueno, 2000; Klein, 2000; Bueno and Miranda, 2005). Like chromato-
graphy with resin matrices, the selectivity of separation is determined by
the ligand–adsorbate pair and depends on pH, ionic strength, and tem-
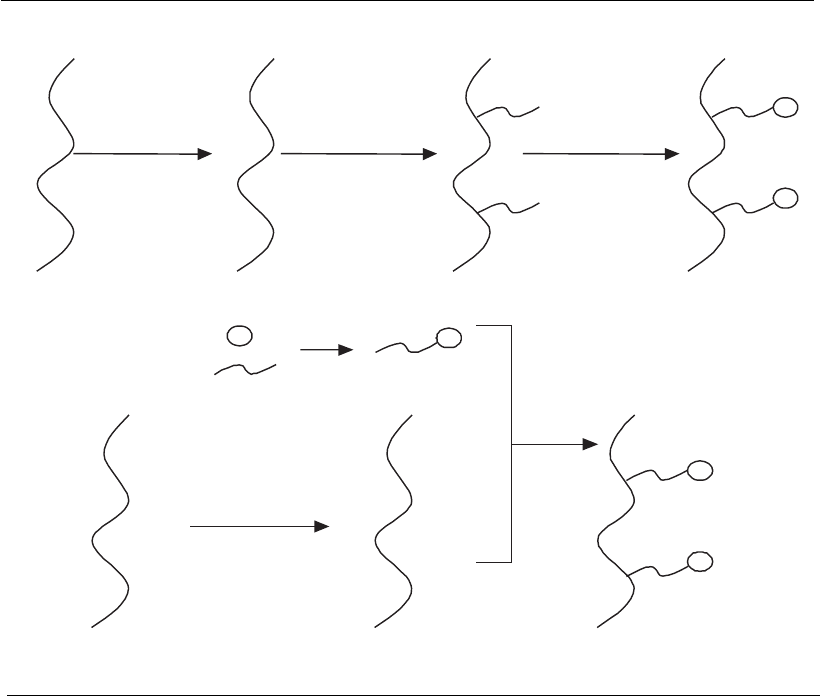
perature. Adsorption isotherms in Figure 12.9 show the differences pre-
sented by a poly(sulfone) membrane containing the synthetic peptide
TG19318 as the ligand, concerning the affinity for immunoglobulins (Ig)
from different classes, in the presence of different buffers.
0
5
10
15
20
25
c* (mg/mL)
q* (mg/mL)
Human IgM, 50 mM Bis-Tris, pH 7
Rat IgG, 50 mM Bis-Tris, pH 7
Human IgG, 50 mM Bis-Tris, pH 7
Human IgG, 50 mM phosphate, pH 7.4
Human IgG, 100 mM phosphate, pH 7.4
0.0
0.5 1.0 1.5 2.0
Figure 12.9
Adsorption isotherms of human IgM, human IgG, and rat IgG using the membrane
Ultrabind-TG19318, in different buffers (Castilho, 2001). q*, equilibrium
concentration of Ig adsorbed onto the membrane; c*, equilibrium concentration
of Ig in the liquid phase.
Produc t purification processes 321
One of the major limitations in chromatographic processes carried out
in packed columns with porous resins is the slow solute diffusion within
the particles. The limiting step is the diffusion of the solute towards the
immobilized ligands located within the pores, which implies the use of
relatively low flow rates (Brandt et al., 1988).
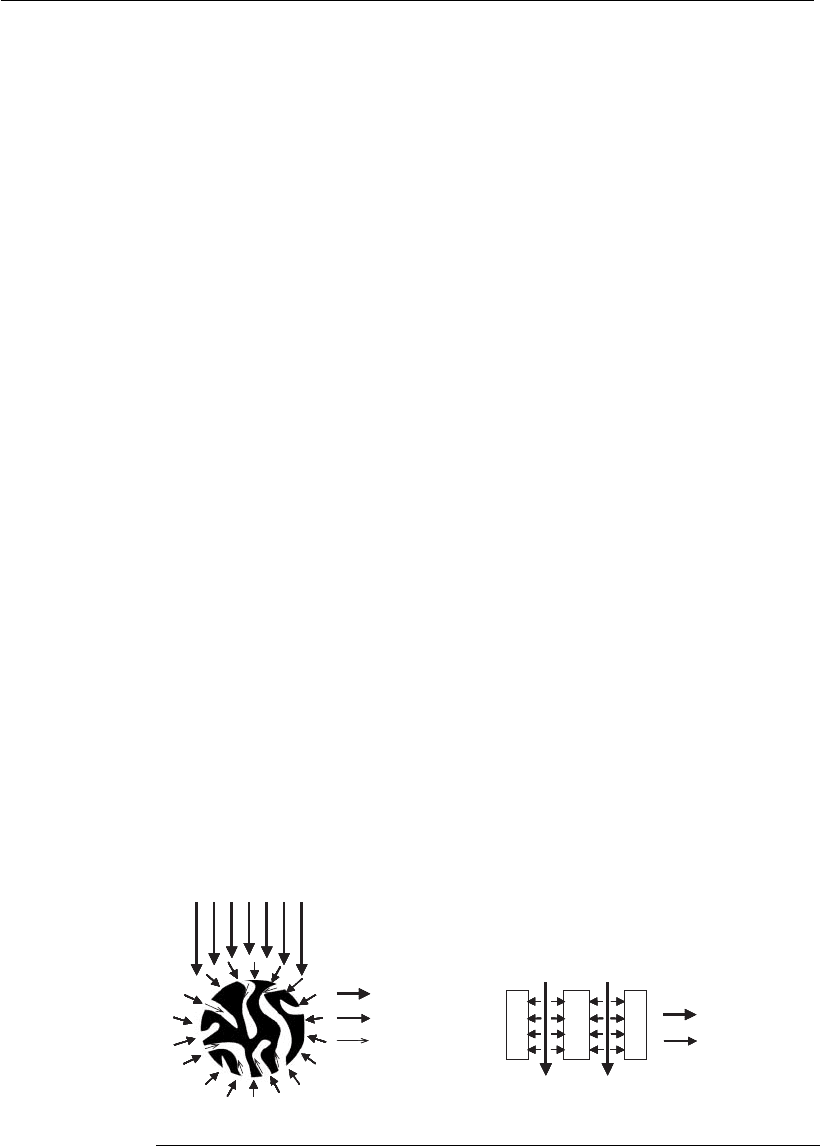
The use of microporous membranes as a chromatography matrix avoids
intraparticle diffusional limitations, since their pores, around two orders
of magnitude larger than those of conventional resins, are accessed mainly
by convection (Figure 12.10). This enables the operation at relatively high
flow rates, with relatively low pressure drops. Additionally, membrane
adsorbers present better mechanical resistance than gels, with no deforma-
tion and bed compaction problems. Also, the systems are usually modular
and easy to scale up (Klein, 2000; Bueno and Miranda, 2005).
One of the major advantages of membrane chromatography, similarly
to EBA, is the possibility for direct processing cell suspensions, enabling
an integration of the clarification and primary purification steps. However,
this is only possible by the use of membrane modules with suitable
hydrodynamics, capable of effectively avoiding membrane fouling (Bel-
ford, 1988; Castilho and Anspach, 2003).
Examples of integrated processes using affinity membranes are avail-
able in the literature. Vogel et al. (2002) purified recombinant human
tissue plasminogen activator (rhtPA) directly from a CHO cell suspen-
sion, using L-lysine affinity membranes, achieving a yield of 86% and a
removal of 95% of contaminant proteins. Castilho et al. (2002b) pro-
posed the use of protein A affinity membranes for an integrated perfu-
sion process with simultaneous primary product purification. CHO cells
producing an anti-HIV mAb were cultivated in a perfusion bioreactor
coupled to a rotating disk filter containing affinity membranes, which
promoted not only separation and recycling of the cells to the bioreactor,
but also product adsorption. Through periodic elution and regeneration
cycles, the authors obtained, in only one step, a product with high purity
and 14-fold more concentrated than in the bioreactor (Castilho et al.,
2002b).
(A) (B)
Convection
Film diffusion
Pore diffusion
Convection
Film diffusion
Chromatography in
porous particles
Chromatography in
membrane adsorbers
Figure 12.10
Comparison of solute transport to the adsorption sites: (A) in conventional porous
resins and (B) in membrane adsorber s. Adapted from Ghosh (2002).
322 Animal Cell Technology
12.5 Conclusions
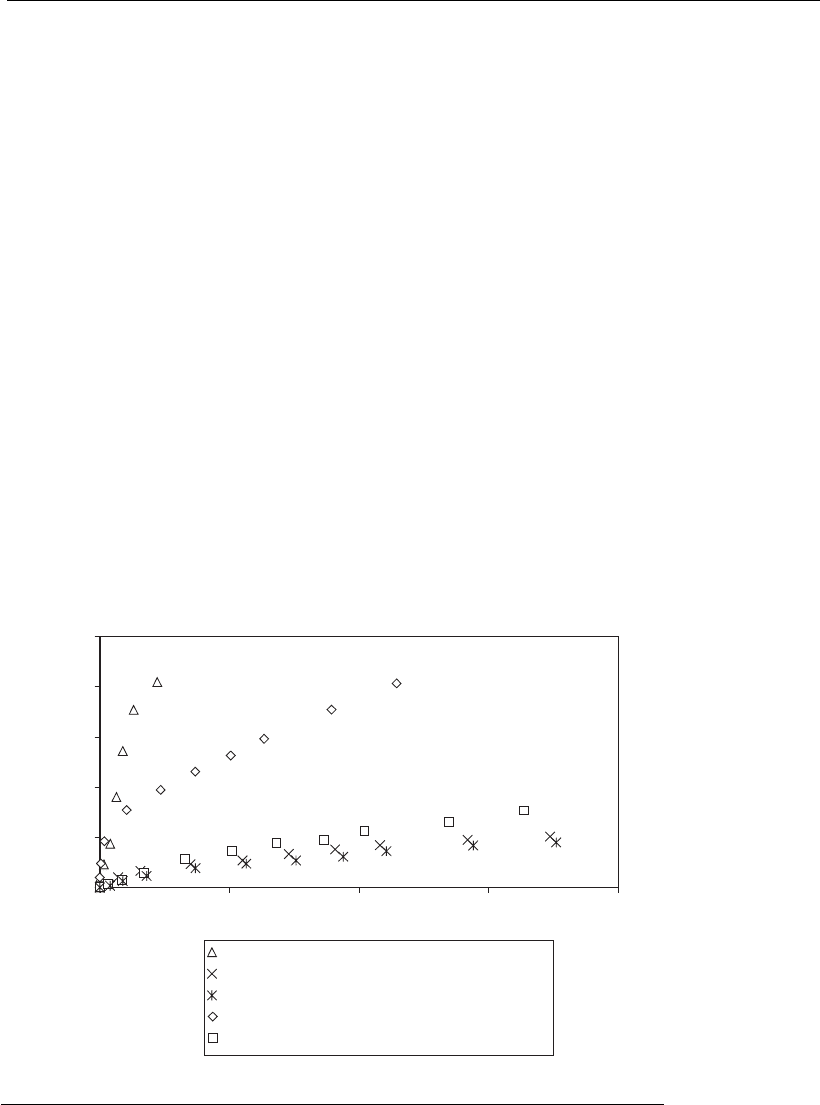
Figure 12.11 shows the methods most commonly used as consecutive steps
in the downstream processing of bioproducts. Precipitation and ion
exchange chromatography are commonly employed as the first step,
whereas, for such processes composed of five steps, molecular exclusion
chromatography is used predominantly as the last step. As shown in
Figure 12.12, affinity chromatography is the technique with the highest
purification factor, followed by hydrophobic interaction chromatography.
Despite being frequently used, precipitation has low selectivity and
provides a low purification factor.
Downstream processing has an important impact on the final product
cost, in same cases reaching 90% of the total production costs
(Cunha et al., 2003). There are many reasons for this, and one of them is
the high number of steps usually needed to achieve the required purity.
Due to an inherent product loss in every step, the global yield of the target
protein is usually low. The implementation of redundant steps for facilitat-
ing process validation is an additional reason for the high costs of down-
stream processing.
Therefore, in spite of the large availability of methods for the recovery
and purification of proteins, there is still a need for studies in this area,
aiming to improve the existing techniques and to develop novel methods
to satisfy new demands. The optimization of the recovery and purification
process for a given bioproduct may involve conflicting objectives
and, therefore, a careful balance should be made of the advantages and
123
4
5
10
20
30
40
50
60
Precipitation Ion exchange
Affinity
0
Frequency (%)
Molecular exclusion
Purification step
Figure 12.11
Frequency of use of different purification methods in processes composed o f five
purification steps. Estimates are based on the analysis of 100 articles in
international scientific journals published from January 1992 to January 1994
(adapted from Freitag and Horva
´
th, 1996).
Produc t purification processes 323
