Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


12
Pr oduct purification
pr ocesses
A
ˆ
ngela Maria Moraes, Leda dos Reis Castilho,
and Sonia Maria Alves Bueno
12.1 Introduction
The development of methods and techniques for the separation and
purification of biological macromolecules such as proteins has been a
prerequisite for advances in bioscience and biotechnology during the last
five decades.
The recovery and purification of a bioproduct is carried out to isolate it
from its production system (cell culture, plant or animal tissues), and to
obtain the required purity and formulation. Before establishing a strategy
for recovery and purification, it is essential to collect all available informa-
tion related to the protein and to the medium where it is found. Usually,
not only the theoretical information, but also preliminary experiments are
needed. It is also noted that the feasibility of a process on a laboratory
scale does not guarantee its feasibility on an industrial scale.
With the use of high performance materials and automated instruments,
protein separation is becoming a more controllable process. However,
some problems persist even with the use of sophisticated instruments.
Many difficulties are still found in determining the optimal extraction and
purification conditions, as well as in selecting suitable methods for detect-
ing the protein and quantifying its biological activity.
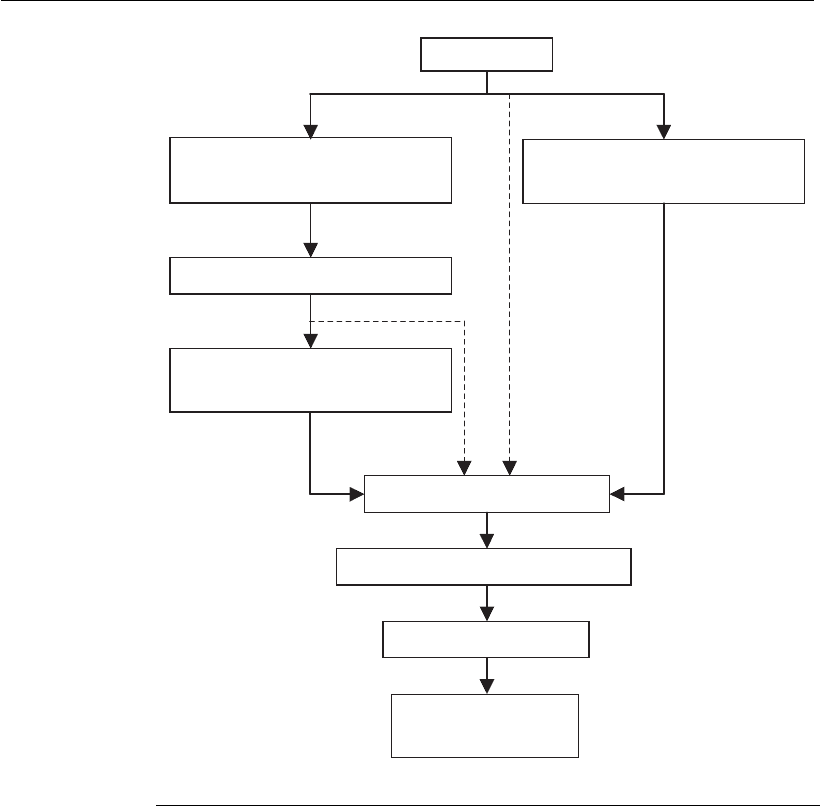
A general scheme of the recovery and purification process for a
bioproduct is shown in Figure 12.1. Despite the lack of a universal
strategy, some general directions (listed below) should be followed when
establishing the purification protocol:
(i) choose separation methods based on different physical principles;
(ii) choose methods based on physical properties related to the greatest
differences between products and impurities;
(iii) first remove contaminants that are most dissimilar to the product
and/or those that are most abundant;
(iv) assign the most demanding process to the end of the sequence.
12.2 Basic considerations
Before developing an isolation and purification process for a protein, some
considerations should be made regarding the final application of the
product, the most suitable source material and the available information
about the product, including existing data on manipulation and quantifica-
tion (Harris and Angal, 1989).
From these preliminary data, the objectives and performance criteria of
the purification sequence can be defined and, thus, the fundamental basis
for designing the most suitable strategy is established.
12.2.1 Final application of product
Usually protein purification represents the route for obtaining the product
in a pure form, suitable for different applications such as: activity and
structural studies, studies of the structure–function relationship of the
protein, and its clinical or industrial applications. The purpose of the
application determines the required purity of the protein, the acceptable
Solid–liquid separation
(cell extraction)
Cell disruption
Solid–liquid separation
(removal of debris)
Solid–liquid separation
(cell removal)
Bioreactor
Primary isolation
Intermediate purification
Final purification
Formulation
and packing
Intracellular
products
Extracellular
products
Figure 12.1
General scheme of a bioproduct recovery and purification process. The dotted
arrows indicate integrated processes (see Section 12.4.6).
296 Animal Cell Technology

loss of activity, and also, the acceptable time and cost of the purification
process.
Therefore, for activity-related studies, relatively small amounts of pro-
tein are needed and high purity is certainly not crucial, provided that the
activity-interfering species are removed. Cost in this case has relatively
low relevance, but speed is important to minimize activity loss.
Structural studies, on the other hand, require large amounts of highly
purified protein. Cost and time are of secondary importance, except in
studies regarding structure–function relationship, for which maintenance
of biological activity is important and thus, the purification speed is
important.
Purification cost and time are most relevant for industrial scale produc-
tion. The required amounts and purity levels are determined based on the
final application of the protein. For example, for therapeutic applications
high purity is crucial, but the required amounts are relatively small.
The amount of purified protein obtained depends not only on the
amount of the raw material used, but also on the yield of the process. At
every stage of the purification process, some product loss occurs. There-
fore, to maximize the yield, a minimum number of stages should be used.
However, decreasing the number of stages can affect the final purity of the
protein. Some purification methods give a higher yield than others and
thus, a systematic selection should be made that is aimed at maximizing
the yield at each stage, but also insuring the required final purity is
obtained.
The purity of the target protein can be expressed in terms of the
percentage of total protein, although other types of contaminants can also
be important. For enzymatic studies, 80–90% of purity is usually suitable,
not taking into account the activity-interfering contaminants, which
should be removed. For structural studies, the required purity should be
equal to or higher than 95%. For therapeutic applications, any type of
contaminants should be removed and purity higher than 99.9% is often
required.
Additional purification steps lead not only to additional yield losses, but
also to an increase in processing time and cost. For research purposes, the
process scales are usually small and the cost of an additional step may not
be important. On the other hand, at an industrial scale, the introduction of
an additional step may make a purification process uneconomic. Removal
of residual contaminants is, in most of the cases, significantly more
difficult than the earlier purification steps and involves several additional
steps, reducing the final yield and increasing the processing cost and time.
In most cases, except for structural studies such as sequencing, the
purified protein should be as native and as active as possible. Attempts to
minimize denaturation and proteolysis should be made, avoiding typically
harsh conditions.
12.2.2 Selection of the protein source
As discussed in the initial chapters of this book, genetically modified
animal cells are commonly used to obtain proteins for therapeutic,
prophylactic, or diagnostic applications. The problem is that obtaining
Produc t purification processes 297

bioproducts from animal cell cultures is limited by the low protein
concentration observed in these systems, making the purification process
difficult and expensive. On the other hand, animal cells can secrete
recombinant proteins into the extracellular medium, allowing easier and
cheaper purification, as compared with heterologous proteins found as
insoluble intracellular aggregates in bacteria.
To facilitate the purification, the target biomolecule can be modified by,
for instance, coupling to it a peptide segment that can be recognized and
captured by an affinity ligand attached to a chromatographic matrix. After
purification, the inserted fragment can be removed by chemical or enzy-
matic cleavage (Scheich et al., 2003).
The culture medium containing the bioproduct should be processed as
soon as possible, to avoid the possibility of degradation, for example, by
oxidation or the action of proteases. When immediate processing of
extracellular products is not possible, it is recommended to remove the
cells and freeze the material, provided the bioproduct is stable to freezing.
12.2.3 Protein properties and manipulation
Knowledge of the physical and chemical properties as well as the cellular
location of the target protein helps in designing the separation process.
Whether the protein is intra- or extracellular, soluble or insoluble in the
cytoplasm, bound to the cell membrane or located within the organelles,
will affect the selection of the separation method and the type of buffering
system. Proteins bound to the cell membrane require detergents or organic
solvents for their solubilization.
Apart from the general problems of solution handling, protein solutions
should be handled to prevent denaturation, aggregation, and degradation.
Adequate cleaning and sanitization of all equipment is essential in the
manipulation of such biomolecules.
12.3 Cell disruption
Bioproducts are usually secreted from animal cells in culture, and can be
purified after cell removal by solid–liquid separation techniques (see
Chapter 11). However, the product can sometimes be found within the
cell and this requires its extraction from the cellular mass, which contains
numerous molecular species that can have high viscosity and proteolytic
activity, which increases the difficulty of sample handling.
The extraction of an intracellular protein usually involves a compromise
between recovery and purity. Optimization of the extraction conditions
should maximize the release of the target protein, while minimizing the
contaminants, which may be difficult to remove. For this, it is important
to determine the conditions under which denaturation or degradation
occurs.
There are several available methods for disrupting cells or tissues. The
operational conditions can be optimized through the systematic variation
of parameters such as medium composition, time, temperature, stirring
rate, and size and shape of the blades. Selection of a suitable procedure
298 Animal Cell Technology

demands preliminary tests in which aliquots of the sample are taken at
different time intervals and analyzed for product concentration and
activity.
The recovery of intracellular proteins involves distinct cell disruption
procedures, depending on the cell characteristics. For the processing of
animal cells, which do not have a cellular wall, mild and moderate
techniques are commonly used. Mild techniques include cell lysis by
enzymatic digestion, chemical solubilization or autolysis and the use of
manual homogenizers and grinders, whereas the moderate techniques
involve blade homogenizers and abrasive grinding.
When the protein is present within a cellular organelle, these methods
can still be suitable. However, they may be preceded by isolation of the
organelles. Sometimes, the protein has low solubility in the extraction
medium, and produces a particulate system requiring specific techniques.
These proteins can be extracted through thermal, chemical, or enzymatic
treatments, and in some cases detergents are needed for solubilization. In
any case, it is essential that a suitable solvent is selected for protein
extraction.
In all purification steps it is recommended that the pH of the protein
solution is controlled, to avoid denaturation or deactivation of the target
biomolecule. It is recommended that an operational pH condition in which
the target protein presents maximum stability is determined, although this
value may be different from the optimal for protein extraction.
Several factors affect the selection of the buffer solution, such as: the
optimum pH; the buffer anionic or cationic species (which can interfere in
the subsequent purification steps); the pH variation with ionic strength or
temperature; the buffer reactivity with the proteins in solution; the bio-
logical activity (e.g. phosphates can inhibit or activate a protein in bio-
logical reactions); the interaction of the buffer with other components; the
buffer permeation in biological membranes; the toxicity; the light absorp-
tion at 280 nm; the cost (especially if used in large-scale processes); and the
protein solubility.
In many cases the target protein is bound to membranes or particles or
is aggregated as a consequence of its hydrophobic characteristics. In these
cases, detergents and chaotropic agents can be used to weaken these
interactions during cell disruption and extraction steps. The detergent
performance is highly dependent on pH and temperature.
Intracellular proteins usually expose thiol groups, which can oxidize
during the purification process. Such groups may be protected by reducing
agents such as 1,4-dithiothreitol (DTT), 1,4-dithioerythritol (DTE), and
mercaptoethanol. Usually, reagent concentrations of 10–25 nM are suffi-
cient to protect the thiol groups without reducing the internal disulfide
bonds.
The presence of metal ions may be harmful to a biologically active
protein owing to two main factors; the increase in oxidation of the thiol
groups by molecular oxygen, and the complexation with specific groups.
The chelating agent most commonly used to complex these metal ions is
EDTA (ethylenediaminetetracetic acid) at a concentration of 10–25 nM.
However, in some cases, application of metal ions such as calcium and
magnesium may be needed to stabilize certain proteins.
Produc t purification processes 299

The presence of proteases is a threat for protein stability. A simple
strategy for protection against proteolytic degradation is based on the fast
handling of the sample and use of low temperatures. One additional
precaution is the addition of protease inhibitors, especially during disrup-
tion and extraction steps. In some cases, there is a need for a combination
of inhibitors. Sometimes, pH adjustment is effective in inactivating pro-
teases without affecting the stability of the protein product.
Despite being more critical for intracellular products, protease action
may also occur in extracellular media. For example, in the expression of a
baculovirus vector, which is a lytic system, proteases are often expressed
and secreted during recombinant protein production, and the problem
may assume a critical level. A similar problem occurs in serum-free culture
systems. The absence of proteins such as albumin and macroglobulin
reduces the protection against proteolysis. In these cases, addition of an
antiproteolytic agent to the culture medium is recommended.
In order to avoid microbial growth in the protein solution during the
purification process, buffer solutions should be routinely sterilized by
filtration. Buffers such as phosphate, acetate, and carbonate at neutral pH
are highly susceptible to microbial growth. Buffers with pH values lower
than 3 or higher than 9 usually prevent bacterial growth, but can allow
fungal growth. To circumvent such problems, it is recommended to
proceed with the purification steps as fast as possible, always using fresh
and filtered buffers, storing solutions at 48C and, if possible, adding an
antimicrobial agent to the buffer solutions.
12.4 Protein purification methods
After the extraction of the target biomolecule, purification is the next step.
It is possible to select the entire sequence of purification steps for isolating
a particular protein based on its properties, and on the fact that every
protein presents a unique combination of characteristics. A strategy should
be established to minimize the number of stages, leading to the maximum
protein yield, minimum cost, and the required purity for the product’s
final application.
Table 12.1 shows the most commonly used purification techniques and
related protein properties to provide separation. Every technique should
be evaluated with regard to different parameters such as:
(i) the capacity, i.e. the sample amount that can be handled;
(ii) the resolution, i.e. how efficiently proteins are separated from each
other;
(iii) the yield;
(iv) the cost.
A balance should be made between process parameters and performance
expectation of each purification stage. In the early stages, capacity and cost
are important parameters, whereas in the final stages, high resolution is
more relevant.
The sequence of stages in a purification process usually starts with low
resolution steps such as precipitation and liquid–liquid extraction, which
300 Animal Cell Technology

are low cost and allow the processing of large amounts of material and
high total protein concentrations. In the intermediate steps, a combination
of chromatographic techniques is usually employed, followed by a final
purification step focusing on the removal of product-related impurities
(e.g. aggregates or isoforms) and/or those existing at very low concentra-
tions. In this final stage, affinity chromatography and molecular exclusion
are very usual processes.
The currently most relevant separation and purification techniques for
processing biomolecules from animal cell cultures are discussed in the
sections that follow. They are classified according to the protein character-
istic on which the separation is based, such as solubility, molar mass,
electrical charge, adsorption properties, and biological affinity for ligands.
12.4.1 Separation processes based on solubility
Protein precipitation
Precipitation is used as a separation step during the early stages of a
purification process, usually followed by chromatographic separations,
and also as a concentration method prior to an analysis or to a subsequent
purification step.
The solubility of a protein in an aqueous solution relies on the distribu-
tion of the hydrophilic and hydrophobic groups on its surface. Protein
precipitates are formed by the aggregation of protein molecules caused by
changes in pH, ionic strength, or solvent dielectric properties, as well as by
the addition of a miscible organic solvent, other inert solvents, and
Table 12.1 Characteristics of typical purification methods: properties on which separation is
based and their performance parameters
Principle of
separation
Technique Capacity Yield Resolution Cost
Solubility Liquid–liquid extraction High High Low Low
Fractional precipitation High High Low Low
Size Microfiltration, ultrafiltration, dialysis High Medium Low Low
Molecular exclusion chromatography Medium-low High Medium-low Medium
Electrical SDS-PAGE Very low High High Medium
charge Electrofocusing Very low High High Medium
Ion exchange chromatography Medium Medium Medium Medium
Specific
interaction
with ligands
Affinity chromatography Medium-low Low Very high High
Surface Reverse phase chromatography Medium Medium High High
hydro-
phobicity
Hydrophobic interaction
chromatography
Medium Medium Medium Medium
Adapted from Wheelwright, 1991.
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Produc t purification processes 301

polymers. Precipitation is based on the fact that proteins are electrolytes
of high molar mass. The method is suitable for fractionation of protein
mixtures, since each protein is composed of different amino acids that
define its behavior. The precipitates can be recovered by filtration or
centrifugation, and the remaining traces of both the precipitating agent
and the redissolved precipitate can be removed by dialysis, diafiltration, or
desalinization in a molecular exclusion column. Detailed reviews on the
topic are given by Scopes (1994) and Kumar et al. (2003).
The most widely used precipitation agents for protein purification are
ammonium sulfate and polyethylene glycol (PEG), which are discussed
below.
Salting-in and salting-out
Neutral salts have a pronounced effect on the solubility of proteins,
especially if they are globular. At low concentration, salts increase the
solubility of many proteins in a phenomenon known as salting-in. This
solubilization is a function of the solvent ionic strength, which depends on
the concentration and on the electrical charge of the cations and anions
that constitute the salt. These effects are caused by changes in the ioniza-
tion of dissociating groups of the protein.
However, as the ionic strength is gradually increased, protein solubility
begins to decrease. At high ionic strengths, the protein may be precipitated
from the solution. This effect is known as salting-out. This phenomenon
occurs when a salt is added to a system and the ions are solvated by water
molecules displaced from the protein. Therefore, at high salt concentration
the hydrophobic protein groups are exposed, enabling the formation of
hydrophobic interactions between the protein molecules, thus causing
aggregation. Larger proteins or those with more available hydrophobic
regions will aggregate faster and this can be the basis of fractionation. The
effectiveness of the salt is largely dependent on the anion, multivalent
anions being the most effective. The order of selectivity may be described
by the Hofmeister series (Ersson et al., 1998):
Anions: PO
4
3–
. SO
4
2–
. CH
3
COO
–
. Cl
–
. Br
–
. NO
3
–
. ClO
3
–
. I
–
. SCN
–
Cations: NH
4
þ
. K
þ
. Na
þ
. C(NH
2
)
3
þ
Ammonium sulfate is frequently used for protein precipitation, due to
its high solubility in water, which allows the solvent to reach very high
ionic strengths. Usually, protein precipitates produced through salting-out
do not denature and the protein activity is easily recovered. Addition of
neutral salts can also protect proteins against proteolysis and microbial
contamination.
Precipitation with polymers
Precipitation of proteins by the addition of anionic or cationic polymers
occurs through the neutralization of charges and formation of high molar
mass aggregates. Polymers commonly used for this purpose are carb-
oxymethylcellulose and chitosan. Neutral polymers, such as PEG and
methylcellulose can also be employed. In such cases, the mechanism is
302 Animal Cell Technology

similar to precipitation with organic solvents, that is, by change in the
dielectric constant of the medium, although at lower concentrations
(around 20%). Higher concentrations result in viscous solutions, making
the recovery of the precipitate difficult. Polymers used in protein precipi-
tation are expected to:
(i) present reversible solubility response to medium changes in pH and
temperature;
(ii) form compact precipitates;
(iii) have low cost; and
(iv) be inert and nontoxic.
The polymer must have a high molar mass. PEG at a range of 6–20 kDa
is commonly used (Ersson et al., 1998). Precipitation with PEG was used,
for instance, by Deml et al. (1999) as one of the purification steps for the
isolation of hepatitis B virus surface antigen (HBsAg) from the insect cell
culture supernatant of genetically modified DS-2 (Drosophila melanoga-
ster Schneider-2) cells.
Liquid–liquid extraction
Extraction is based on the transfer of a solute from one phase to another,
according to its partition properties between two immiscible phases. In
biotechnological processes, the most widely used extraction is liquid–
liquid involving the partitioning of biomolecules, organelles or cells be-
tween the phases (Harrison et al., 2003).
In a bioprocess, two types of liquid–liquid systems are used. When
molecules are stable in organic solvents, as occurs with low molar mass
molecules (such as antibiotics), the extracting liquid can be an organic
solvent immiscible with the aqueous phase originally containing the target
biomolecule. However, in the case of proteins with a tendency to denature
or degrade in the presence of organic solvents, aqueous two-phase systems
are used (Franco et al., 2005). Such systems are usually formed by a
polymer phase and a salt solution, or by two polymers, both soluble in
water but immiscible with each other. The most commonly used systems
are formed by PEG in one phase and a salt or other polymer (such as
dextran) in the other. Under ideal conditions, the protein of interest is
recovered in the upper phase (usually PEG) and the contaminants and
particles (cells, fragments of cells, and organelles) in the lower phase
(Ersson et al., 1998).
The partitioning coefficient K (equation 1) describes the distribution of
a solute, in equilibrium, between two liquid phases. This coefficient is a
function of several factors, such as the molecular size, pH, temperature,
concentration, and type of components in both phases. In Equation (1), y
and x are the solute concentrations in the extract and in the original
solution, respectively.
K ¼
y
x
(1)
Ideally, K should be maximized with a minimum volume of the extract-
ing phase. Partition coefficients close to 1 implicate in large solvent
Product purification processes 303
