Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

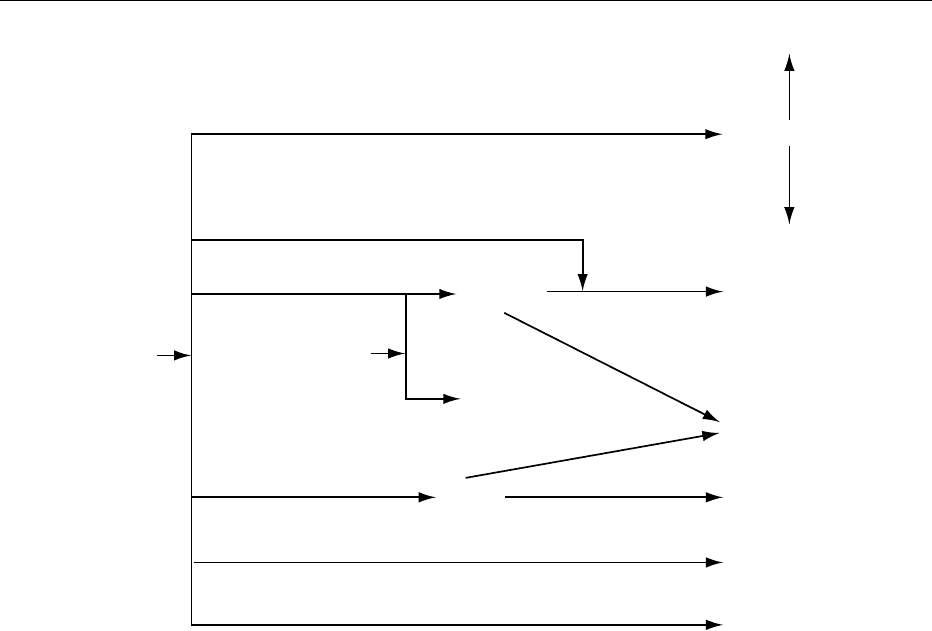
column using recycled hot water for removal of
higher alcohols (fusel oils), and a rectifying column
for final purification and concentration. For crude
industrial or fuel alcohol, only the boiling columns
and possibly a smaller side-draws purification
column are required. For anhydrous ethanol produc-
tion, additional azeotropic or extractive distillation
columns, a zeolite molecular sieve, or a pervaporation
membrane plant must be used.
Yeast Biomass
Single-cell Protein
0011 Yeast biomass has been produced commercially
by whey fermentation from the 1940s until the
present. Strains of K. marxianus var. lactis or var.
marxianus are those most commonly used, although
other strains may be produced successfully. In
some operations, ethanol is also obtained as a co-
product by lowering the aeration rate during the
fermentation.
0012 The requirements of a suitable strain for biomass
production are summarized as follows.
1.
0013High specific growth rate and biomass yield to
insure a high productivity
2.
0014The strain must not be affected by whey proteins if
these are present
3.
0015The strain must be suited to continuous culture
4.
0016The strain must be acid-resistant. (To control con-
tamination, it is necessary to operate the process at
low pH or to wash the yeast at frequent intervals
with acid to remove contaminants)
5.
0017Large cell size and uniform morphology to aid cell
separation and concentration
6.
0018Adequate protein content and acceptability in
feeding trials
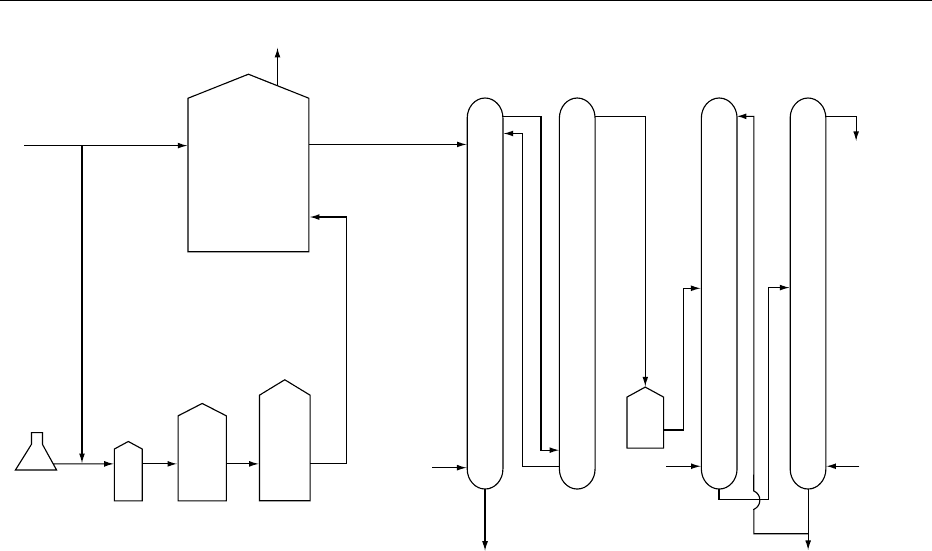
0019The Bel process (Figure 3), developed in France in
the late 1950s, is a frequently cited example of this
fermentation. Sweet cheese whey is first deproteinated
and diluted to a lactose concentration of 20–25 kg
m
3
. Whey is limiting in nitrogen sources for yeast
growth, so ammonium salts are added to maintain a
high nitrogen content and growth rate, and trace
metals (Fe, Cu, Mn, Zn) may also be added. The
culture has been shown to comprise three cooper-
ating yeast species. The continuous fermentation is
Yeast biomass
Yeast autolysate
(autolysis)
β-Galactosidase
Kluyveromyces or Candida spp.
(Hydrolysis)
Lactic acid
bacteria
NH
3
Ammonium lactate
Lactic acid
Bakers' yeast
Whey beverages
Acetic acid
Propionic acid
Methane
K. marxianus
Ethanol
Propionibacterium spp.
Mixed anaerobic bacteria
Whole or
deproteinated
whey
Saccharomyces
cerevisiae
Acetobacter spp.
fig0001 Figure 1 Major commercial products obtained by whey fermentation. Reproduced from Whey and Whey Powders: Fermentation of
Whey, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
WHEY AND WHEY POWDERS/Fermentation of Whey 6159
operated at a dilution rate of 0.33 h
1
and a tempera-
ture of 38
C. For a serum flow rate of 5–6m
3
h
1
, air
is added at about 1800 m
3
h
1
and provides both the
mixing and the aeration of the culture in an air-lift
fermenter. The residual sugar level is about 1 kg m
3
,
the biomass yield is 0.55–0.6 kg yeast (dry basis) per
kg lactose metabolized, and the biomass productivity
is approximately 4.5 kg m
3
h
1
. The yeast is separ-
ated and concentrated in a two-stage washing and
centrifugation process, plasmolyzed to render the
yeast protein more accessible, and dried.
0020 The composition and uses of biomass produced
from whey are similar to those of other food yeasts.
The crude protein content is about 50 wt% on a dry
basis and the only significant limitation is of the
sulfur-containing amino acids. Overall, the protein
value is similar to that of casein. Fermenting whole
whey and recovering a product containing both the
yeast biomass and the whey proteins may increase the
food value of the yeast.
Bakers’ Yeast
0021 Two processes have been developed for the produc-
tion of bakers’ yeast to overcome the limitation of
Saccharomyces cerevisiae not being able to utilize
lactose. In the first, the lactose is hydrolyzed using
b-galactosidase, and the glucose and galactose are
consumed simultaneously by the yeast in fed-batch
or continuous culture. The second process utilizes a
two-stage fermentation system. In the initial stage,
lactic acid bacteria convert lactose to lactate and
this is consumed in the subsequent stage by the
yeast. Although the bakers’ yeast so produced
appears comparable in quality to that from conven-
tional processes, only very limited quantities are
manufactured by these methods.
Products from Yeast Biomass
0022Yeast cells can be a useful source of several food
additives or processing aids. Following cell growth
and recovery, the biomass can be broken down mech-
anically or biologically to release cellular components
which may be purified or transformed to yield high-
value products. The major products that can be
derived from biomass are yeast autolysate, flavour
nucleotides, enzymes, and cell wall components such
as glucan and mannan.
Autolysate and Flavor Nucleotides
0023Autolysis is a process of self-digestion mediated by
degradative enzymes present within the cell. Elevated
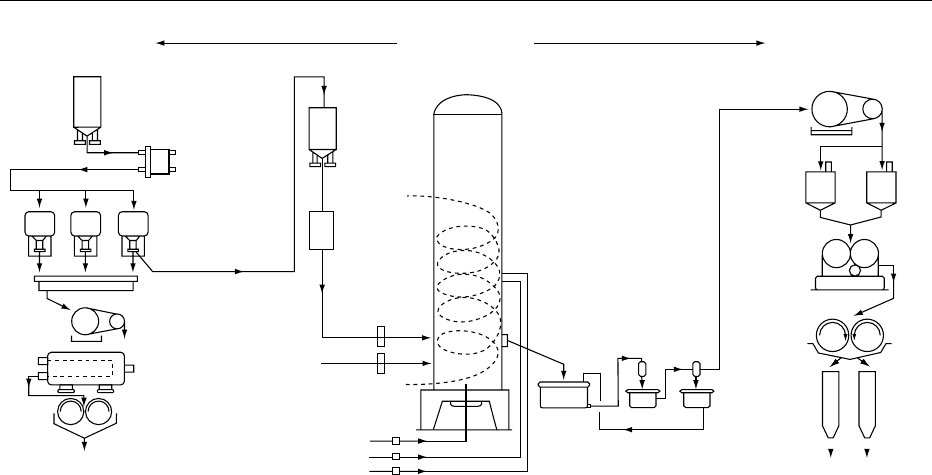
200 m
3
Vented
CO
2
Fermented beer
2.3% (v/v)
ethanol
Crude
ethanol
90% (v/v)
Final product
96.5% (v/v)
ethanol
SteamSteam
Steam
Hot water
to storage
Purified
ethanol
8% (v/v)
Still bottoms
to irrigation
Yeast propagation
25 l
0.5 m
3
10 m
3
Fermentation
Pasteurized whey
permeate
Boiling column 1
Boiling column 2
Extractive distillation column
Rectifying column
fig0002 Figure 2 Typical process for production of ethanol from natural-strength whey permeate. Reproduced from Whey and Whey
Powders: Fermentation of Whey, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
6160 WHEY AND WHEY POWDERS/Fermentation of Whey
temperatures and the presence of NaCl enhance the
rate of lysis during which protein is solubilized and
cell wall polymers are broken down. Optimal condi-
tions for autolysis of Klyveromyces strains are
50–55
C, pH 5.1–6.5, and 8–12 h; addition of salt
may not be necessary. Yeast nutrients are more readily
available in autolysates than in intact cells and the
autolysates find uses in foods, animal feeds, and
microbial cultures, e.g., for enhancing the growth of
dairy starter cultures.
0024 RNA can be isolated from lysed cells and used
to produce 5
0
-ribonucleotides which are important
flavor enhancers in food systems.
b-Galactosidase
0025 The enzyme b-galactosidase (EC 3.2.1.23), or lactase,
can be produced from selected strains of Kluyvero-
myces spp. following growth on diluted whey. The
enzyme is intracellular and is induced by lactose and
galactose. One study showed that expressed levels
may vary up to 60-fold between strains, but commer-
cial yield data are not available. The optimum condi-
tions for enzyme activity are pH 6–7and35–45
C,
and Mn
2þ
and Mg
2þ
are strong activators. b-
Galactosidase may be used to hydrolyze lactose to
overcome the problem of lactose intolerance and
to generate syrups for food use which are sweeter
than lactose and which do not crystallize as readily
(hence reducing ‘sandy’ defects). Oligosaccharides,
which have useful nutraceutical properties, are
alternative and interesting byproducts of the hydroly-
sis process.
Organic Acid Fermentations
Lactic Acid and Ammonium Lactate
0026Whey has been a traditional feedstock for lactic acid
production by fermentation, but the process struggles
to be economic compared to the utilization of corn
syrups. Lactic fermentations have typically been con-
ducted in batch mode using homofermentative strains
of Lactobacillus such as L. delbrueckii sbp. bulgari-
cus. Complex nutritional sources such as corn steep
liquor, malt sprouts, and malt or yeast extracts may
be supplemented. The pH is controlled in the range
5.5–6.5 by addition of Ca(OH)
2
or CaCO
3
and the
optimum temperature is about 43
C. The medium is
usually pasteurized, but contamination is not usually
a serious problem. For natural whey the fermentation
will be complete in less than 24 h with a yield of
90–95%. The productivity of the fermentation is
typically 1–3kgm
3
h
1
.
0027Recovery and purification of lactate represents a
major cost in the process and are complicated by the
highly corrosive nature of the product. This requires
all equipment to be 316 stainless steel or better and
removal of metal ions introduced by corrosion may
be required for some grades of product. In traditional
processes, the fermentation liquor is initially heated
BEL LACTIC YEAST
Filtration
Fermentation
tower
Cooler
Deproteinized
whey 70˚
Heat
exchanger
15˚C
Deproteination
Pasteurization
Whey from
cheese plant
WHEY PROTEINS
Decanting
Cloth
Filtration
Drying
Grinding
LACTALBUMIN
H
2
O
Air
Nitrogen salts
Nitrogen salts
Yeast
suspension
Water
Separation
and washing
Packaging
BEL LACTIC YEAST
Grinding
Drying
Plasmolysis
FLOW DIAGRAM
fig0003 Figure 3 Fromageries Bel process for yeast production from deproteinated whey. Reproduced from Whey and Whey Powders:
Fermentation of Whey, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993,
Academic Press.
WHEY AND WHEY POWDERS/Fermentation of Whey 6161

to 80–100
C and the pH is raised above 10 to solu-
bilize calcium lactate, kill all microorganisms, and
precipitate calcium phosphate and proteins. The
liquor is then decanted, filtered, and treated with
carbon to increase the purity. Following evaporation
under vacuum, a technical-grade lactic acid can be
recovered by acidification with sulfuric acid, filtra-
tion of the precipitated CaSO
4
, and further carbon
and purification treatments. To obtain higher-grade
products, calcium lactate crystals may be recovered
following cooling of the liquor; these are then
washed, redissolved, and carbon-treated in several
cycles. Several alternative recovery processes are
available, e.g., both solvent extraction and distilla-
tion of lactate esters are practiced commercially.
0028 Lactic acid has attracted much recent interest as a
base for the manufacture of biodegradable polylac-
tate plastics. A large-scale pilot plant was commis-
sioned in Wisconsin, US, but full-scale production did
not proceed.
0029 Ammonium lactate is produced by the addition of
liquid ammonia to the fermentation broth. After
completion of the fermentation, the broth is evapor-
ated, neutralized by addition of further ammonia,
and processed to a range of liquid or solid products.
These are used as animal feeds and are especially
suitable for ruminants. Ammonium lactate is superior
to urea and similar to soybean meal in its nutritive
value and digestibility.
Acetic Acid
0030 Following the ethanol fermentation of whey, the alco-
hol can be further metabolized to acetic acid by Acet-
obacter spp. This process has been commercialized,
most notably by Kraft, and the resulting whey vinegar
may be used in salad dressings and other foods. The
manufacturing process is assumed to be similar to
that for conventional vinegar production. The pro-
duction of acetic acid from whey as a base for synthe-
sis of other chemicals has also been considered.
Recent attention has focussed on production of
acetate deicers as a possible market.
Propionic Acid
0031 Propionic acid is added as a fungistatic agent to bread
and bakery products and can be produced by whey
fermentation using strains of Propionibacterium
freudenreichii ssp. shermanii. Whole whey is typically
used and the fermentation must proceed under
sterile conditions at around 30
C and pH 6.5–7.5.
Nutritional supplements, such as yeast extract,
considerably enhance propionate production and
a typical yield of about 40% of the lactose fer-
mented is achieved after 60–70 h. The culture fluid
is spray-dried to obtain a powder containing both the
acid and whey proteins.
Methane Fermentation
0032The microbial production of methane from whey
offers the major benefits of an efficient waste treat-
ment process coupled with the production of a z-
convenient energy source that can be utilized on-site.
The methane fermentation, or anaerobic digestion,
requires a limited input of energy and nutrients and
produces very little sludge for ultimate disposal
in comparison to conventional aerobic treatment
processes. A number of bioreactor (digester) configur-
ations are available for the treatment of high-strength
effluents such as whey. All of these rely on retaining
the microorganisms mediating the fermentation in the
digester, so that the hydraulic residence time of
the waste stream can be very much less than the
biological solids (or biomass) residence time. This
separation is necessary as the cells grow only slowly,
yet large volumes of waste must be processed daily.
0033Several large-scale whey or whey permeate
digesters are in operation and many laboratory or
pilot-scale trials have been reported for a variety of
retained-cell systems. Mesophilic operation at
30–37
C is favored. Overall COD (chemical oxygen
demand) reductions of about 90% are possible for
loading rates of up to 20 kg m
3
day
1
, although
loading rates in large-scale operations are typically
lower, at 1.5–10 kg m
3
day
1
. Gas production is
about 35 l
1
of whey processed and has a methane
content of 55–65%. These figures are consistent with
the theoretical yield of 350 litres CH
4
at standard
temperature and pressure per kg COD removed.
0034Success has also been achieved with treatment of
slops from whey distilleries by anaerobic digestion.
The reduced strength of the slops from ethanol
fermentation can promote more stable operation of
the digester, although the total methane yield is
less. However, regardless of the initial substrate,
some aerobic polishing of the effluent is required
before digester effluent can be discharged to natural
waterways.
Fermented Whey Beverages
0035Whole or deproteinated whey can be fermented to
produce a range of beverage products. The major
advantages offered by whey as a substrate for bever-
age production are that whey drinks have a greater
nutritive value and are more thirst-quenching than
most soft drinks, and are less acidic than fruit juices.
The marketing of these products generally empha-
sizes the health and nutritional benefits of the
6162 WHEY AND WHEY POWDERS/Fermentation of Whey

beverage, especially if they still contain the whey
proteins. A variety of whey drinks are available in
many countries, although they are most popular in
Europe.
0036 Lactic or alcoholic fermentations can provide de-
sirable properties in the beverage. Lactic fermenta-
tions use conventional starter organisms or probiotic
strains and Kluyveromyces yeast strains are com-
monly used for the alcoholic fermentation. A com-
bined lactic–ethanol fermentation may be conducted
with a kefir-type culture, but whey health drinks usu-
ally have a low (< 1%) or negligible alcohol content.
A typical recipe for preparation of a fermented-fruit
whey beverage is outlined in Table 1. Whey ‘wines’
can be manufactured by fermentation of deprotei-
nated wheys and can serve as a base for wine coolers.
Other Fermentation Products
0037 Many alternative fermentation processes have been
investigated for whey utilization. Possible products
include mixed solvents (acetone, butanol, and etha-
nol), yeast oils and specialized lipids, glycerol, extra-
cellular polysaccharides, other organic acids (citric,
acrylic), vitamins, poly(b-hydroxybutyrate), amino
acids, flavoring agents, pigments, and lantibiotics
such as nisin. Of these, polysaccharides (xanthan
gum) and flavoring agents (based on diacetyl) have
been commercially produced in limited quantities,
and the production of lantibiotics using lactic acid
bacteria also appears feasible.
See also: Casein and Caseinates: Methods of
Manufacture; Cheeses: Chemistry of Gel Formation;
Effluents from Food Processing: On-Site Processing of
Waste; Composition and Analysis; Enzymes: Uses in
Food Processing; Fermented Milks: Other Relevant
Products; Lactic Acid Bacteria; Lactose; Vinegar
Further Reading
Belem MAF and Lee BH (1998) Production of bioingredi-
ents from Kluyveromyces marxianus grown on whey: an
alternative. Critical Reviews in Food Science and
Nutrition 38: 565–598.
Driessen FM and van den Berg MG (1990) New develop-
ments in whey drinks. Bulletin of the International
Dairy Federation 250: 11–19.
Kilara A and Patel MT (1992) Whey and lactose fermenta-
tion. In: Zadow JG (ed.) Whey and Lactose Processing,
pp. 409–448. London: Elsevier Applied Science.
Litchfield JH (1996) Microbiological production of lactic
acid. Advances in Applied Microbiology 42: 45–95.
Mawson AJ (1994) Bioconversions for whey utilisation and
waste abatement. Bioresource Technology 47: 195–203.
Meyrath J and Bayer K (1979) Biomass from whey. In: Rose
AH (ed.) Microbial Biomass. Economic Microbiology,
vol. 4, pp. 207–269. London: Academic Press.
Sienkiewicz T and Riedel C-L (1990) Whey and Whey
Utilization, 2nd edn. Gelsenkirchen-Buer: Verlag Th.
Mann.
Yang ST and Silva EM (1995) Novel products and new
technologies for use of a familiar carbohydrate, milk
lactose. Journal of Dairy Science 78: 2541–2562.
Principles of Dialysis
J J Roberts, University of Pretoria, Pretoria, South
Africa
J F Mostert, ARC-Animal Nutrition and Animal
Products Institute, Irene, South Africa
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001Dialysis is the oldest, most natural, and flexible
technique for separating components from a solu-
tion. Electrodialysis (ED), however, has been known
and studied since the beginning of the twentieth
century, but it was not until around the end of the
1940s that stable membranes of low electrical resist-
ance were developed, allowing the commercial appli-
cation of ED using membrane stacks with several
membranes in series. The modern process of ED has
been made possible by the development of highly
selective membranes for anions and cations. Both
processes may be used in conjunction with other
tbl0001 Table 1 Production of a fermented-fruit whey drink from whey
Recipe
Stabilizer 030%
Fruit concentrate 2–7%
Sour whey 857%
Sugar 6–10%
Flavor and color As required, to 100%
Possible technical pH range 36–42
Method
1. Coarse filtration of sour whey
2. Disperse stabilizer in the sour
whey, avoiding lumps
3. Maintain gentle agitation for 30 min
to allow the stabilizer to swell
4. Heat to 85
C and cool to incubation
temperature (c.42
C)
5. Inoculate with Lactobacillus helveticus
6. Incubate to pH 40
7. Cool
8. Add fruit and sugar
9. Check pH and adjust, if necessary,
with 50% citric acid solution to
pH36–42
10. Thermal treatment
11. Place in chill
WHEY AND WHEY POWDERS/Principles of Dialysis 6163

membrane-separation processes (osmosis, reverse
osmosis, ultrafiltration, and microfiltration) in ex-
perimental and large-scale industrial applications. A
major disadvantage is that an equilibrium is quickly
reached. However, specific molecules may be com-
pletely separated, provided a high concentration gra-
dient is maintained, and the permeate is constantly
removed. (See Membrane Techniques: Principles of
Reverse Osmosis; Applications of Reverse Osmosis;
Principles of Ultrafiltration.)
Theory of Dialysis and Electrodialysis
Dialysis
0002 Dialysis is a separation process whereby smaller
molecules are segregated from larger molecules
(macromolecules) in a solution by virtue of their dif-
ferent rates of diffusion through a specific membrane
(or by nondiffusion, which is the criterion of the
colloidal state and forms the basis of separation of
colloidal materials from crystalloids). Alternatively,
dialysis may be regarded as a membrane transport
process in which solute molecules are exchanged be-
tween two liquids separated by a membrane. Hence,
the primary driving force is the difference in concen-
tration of the permeable species between the solution
in the dialysis membrane system and that on the
outside. The membrane might be natural, such as a
pig bladder, or artificial (i.e., consisting of materials
such as cellulose derivatives or collodion).
0003 To illustrate the principles of dialysis, a test solu-
tion can be placed in a sack or bag that may consist of
a membrane with, for example, a 24-nm pore diam-
eter. The bag is closed/knotted and soaked in distilled
water at a given temperature and for a given time; the
bags are usually agitated. The small molecules diffuse
out into the water, while the larger molecules remain
in the solution in the bag. This method is commonly
used to separate proteins from salt solutions. In add-
ition, it is an indispensable technique in the recovery
and purification of materials in food, chemical,
biological, and pharmaceutical preparations.
Electrodialysis
0004 ED is a unit operation that uses semipermeable mem-
branes for the separation or concentration of electric-
ally charged particles (ions) from nonionic particles
or species in a solution. The key to the process is the
use of ion-selective membranes. These membranes are
ion-exchange resins cast in sheet form, which allow
the passage of positively charged cations (e.g., sodium
or potassium) or anions (e.g., chloride or phosphate).
To achieve separation by means of ED, cation and
anion membranes are alternated with plastic spacers
in a stack configuration with the anode at the one end
and the cathode at the other. Spacers are usually made
of low-density polyethylene and arranged in the
membrane stack so that all the mineralized streams
are manifolded together, and all the concentrated
streams are manifolded together. The spaces between
the membranes represent the flow paths of the demin-
eralized and concentrate streams. Hence, a repeating
section, called a cell pair, consists of a cation transfer
membrane, demineralized water flow spacer, anion
transfer membrane, and concentrate water flow
spacer. A typical membrane stack may have from
300 to 500 cell pairs.
0005A direct electric current (d.c.) (or an electrostatic
potential or gradient) applied across the electrodes
creates a driving force. This induces anions to migrate
in the direction of the anode (þ) and cations to
move in the direction of the cathode (). The ion-
selective membranes form barriers to ions of opposite
charge. The net result is that the anions attempting to
migrate to the anode pass through the anion barrier
or membrane but are stopped by the cation mem-
brane. Likewise, cations trying to migrate to the cath-
ode pass through the cation membrane but are
stopped by the anion membrane. The overall effect
is that the barriers form alternate compartments of
ion-diluting cells and ion-concentrating cells. There-
fore, ED relies primarily on voltage or electromotive
force and the use of ion-selective membranes to effect
separation between charged particles. Hence, on cir-
culation of a specific liquid product or test solution
through the diluting cells and a brine solution through
the concentrating cells, free mineral ions leave the test
solution and collect in the brine stream. The level of
demineralization achieved depends upon the initial
ash content, current density, and the duration of
time the test solution is within the membrane cells.
0006Figure 1 shows a schematic diagram of a typical ED
process for the demineralization of whey in an ED
membrane stack. One stream enters the membrane
stack and flows in parallel only through the deminer-
alizing compartments, while a brine stream enters the
membrane stack and flows in parallel only through
the concentrating compartments. Note that the solu-
tions flow across, not through, the membranes. When
a d.c. voltage is applied across the electrodes, the
electrical potential gradient created causes anions to
move in the direction of the anode and cations
to move in the direction of the cathode. The ion-
selective membranes form barriers to ions of opposite
charge. The result is that anions attempting to
migrate to the anode pass through the anion mem-
brane but are stopped by the cation membrane;
cations trying to migrate to the cathode pass through
the cation membrane but are stopped by the
6164 WHEY AND WHEY POWDERS/Principles of Dialysis
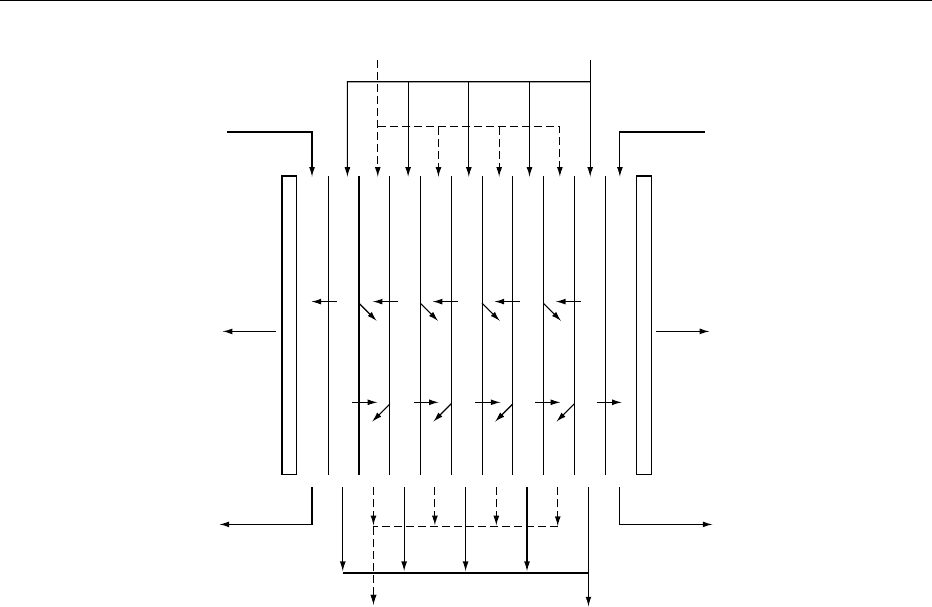
anion membrane. Hence, the membranes form
alternate compartments of ion-diluting cells and
ion-concentrating cells. By circulating whey through
the diluting cells and a brine solution through the
concentrating cells, free mineral ions leave the whey
and collect in the brine stream. The level of deminer-
alization achieved depends on the initial ash content,
current density, and duration of time the whey solu-
tion is within the membrane cells.
000 7 For a better comprehension of the process of ED
dialysis (especially the efficiency of the mass-transfer
reaction), the functioning of an electrolytic cell will be
discussed briefly. A pair of electrodes as well as an
electrolytic solution (i.e., a solution containing ions)
are placed in such a cell. When a voltage is applied to
the electrodes, a migration of cations and anions
takes place. According to Faraday’s law, the number
of mass equivalents transported by this mechanism
is directly proportional to the amount of electricity
which passes through the cell. In mathematical terms,
this can be expressed as follows:
Current time ¼ F E ðequivalent mass transported Þ,
ð1 Þ
where E ¼ number of moles valency, and Faraday’s
constant F ¼ 96490 A s per equivalent or 26.8 A h per
equivalent.
0008Faraday’s law states that if, for example, a current
of 26.8 A is applied in an electrolytic cell for 1h, it
will transport one equivalent of matter from the solu-
tion to the electrodes. Hence,
i ¼ FE ðJ
j
Z
j
Þð2Þ
where i ¼ current density (A m
2
), J
j
¼ molar flux of
ion j (mol s
1
m
2
), and Z
j
¼ valency of ion j.
0009Eqn (2), therefore, states that the total current in an
electrolytic cell is equivalent to the sum of the current
conveyed by each species in the cell.
0010Similarly, the amount of electricity necessary to
produce separation by means of electrodialysis can
be calculated by means of eqn (1). First, we must
consider the cell efficiency coefficient (N
c
), which
accounts for the nonideality of the cell stack. This is
due mainly to the presence of the selective mem-
branes, but also to the loss of current through pipes
and manifolds as well as the diffusion of electrolyte
through the membranes. Thus, for a stack with n cell
pairs:
Brine solution
CACACACACA
Whey feed
Electrode rinse
solution feed
Anode
(+)
To positive pole
of rectifier
Electrode rinse
effluent
Partially
demineralized
product
Concentrated
brine effluent
Electrode rinse
effluent
To negative pole
of rectifier
Cathode
(−)
Electrode rinse
solution feed
1234567891011
K
+
CI
−
CI
−
CI
−
CI
−
CI
−
CI
−
CI
−
CI
−
CI
−
K
+
K
+
K
+
K
+
K
+
K
+
K
+
K
+
fig0001 Figure 1 Schematic diagram of the electrodialysis process showing the demineralization of whey. From Batchelder BT (1987)
Electrodialysis applications in whey processing. In: Trends in Whey Processing, Bulletin of the International Dairy Federation No. 212,
pp. 84–90. Brussels: International Dairy Federation, with permission.
WHEY AND WHEY POWDERS/Principles of Dialysis 6165

n current time ¼ FE ðequivalents removed Þ=N
c
,
ð3Þ
N
c
can be expressed as the product of three efficien-
cies.
N
c
¼ N
m
N
l
N
w
ð4Þ
N
m
accounts for the imperfection of the permselective
membrane and is also called the Coulomb efficiency;
N
l
corresponds to the inefficiency due to current
leakage through pipes and manifolds; N
w
accounts
for water transport, which always follows the
migration of ions. The latter is also referred to as
electroosmosis. For commercial equipment N
c
is
about 50%, and for laboratory equipment 90%. By
manipulating eqn (3):
N
c
¼ F ðQ
in
C
in
Q
out
C
out
Þ=ðIn Þ:ð5Þ
where Q
in
¼ input flow (m
3
s
1
); Q
out
¼ output
flow (m
3
s
1
); C
in
¼ input concentration (equivalents
m
3
); C
out
¼ output concentration (equivalents m
3
).
0011 Owing to electroosmosis, Q
in
and Q
out
are gener-
ally different.
0012 The energy consumed by an electrodialysis system
may be computed from
E ¼ RI
2
t :ð6Þ
where E ¼ electric energy (W s ¼ J), R ¼ total resist-
ance of the system (ohm), I ¼ electric current (A), and
t ¼ time (s). From eqn (3), the number of equivalents
removed ¼ItN
c
n/F. Hence from eqn (6) the energy
per unit of equivalent removed is
e ¼ RIF=N
c
ðW s per equivalentÞ:
Types of Electrodialysis Membranes and
Fouling Problems
0013 The following types of membranes are used in ED:
.
0014 anion-permeable membranes (—NH
3
þ
) (A), which
contain fixed cationic groups and repel cations;
.
0015 cation-permeable membranes (C), which contain
fixed anionic groups (—SO
3
) and repel anions; and
.
0016 nonselective membranes (N), which are permeable
to both anions and cations.
Commercially available ED membranes are manufac-
tured as heterogeneous and homogeneous membranes.
The first type is made by mixing ion-exchange resins
with a solution of polymeric materials, which is used
as a thin coating material on a fine cloth or mesh as a
mechanical reinforcement. Anionic or cationic mem-
branes are obtained by using the corresponding ion-
exchange resins. In homogenous membranes, the
active cationic or anionic groups are induced or
created in the polymeric material. Nonselective mem-
branes are usually manufactured from cellulose. Pore
sizes of the membranes are in the range 1–2 nm.
0017The desired characteristics of a good-quality mem-
brane for electrodialysis include:
.
0018good electrical conductivity;
.
0019good mechanical strength;
.
0020high ionic permselectivity;
.
0021chemical stability;
.
0022resistance to fouling by organic molecules;
.
0023insolubility in aqueous solutions;
.
0024resistance to chemical change in pH from 1 to 10;
.
0025long life expectancy;
.
0026impermeability to water under pressure;
.
0027operation at temperatures in excess of 46
C.
0028A major problem with ED is that of membrane
polarization, also known as ‘limiting current density.’
Limiting current density is usually expressed as (CD/
N
d
)
lim
, where CD ¼current density (the amount of
current carried by a unit area of membrane surface),
and N
d
¼normality of the demineralized outlet
stream. This limit is a function of the fluid velocity
in the flow path, the stream temperature, and the
types of ions present. This condition may arise when
too large a driving potential is applied to a system.
The latter results in a rapid depletion of both anions
and cations from the region immediately in contact
with the membranes of the feed cell. Hence, ions are
removed from the boundary layers through the mem-
branes more rapidly than they can be replaced by
diffusion from the bulk feed fluid, so that nothing
remains to conduct the current. This polarization
potential is a combination of applied voltage, diffu-
sion characteristics of the ions, flow characteristics of
the feed, and the ionic concentration of the feed. Note
that polarization does not usually become significant
at both membranes at the same time. Other typical
problems with ED systems include current leakage,
back diffusion, and membrane poisoning and fouling.
Membrane poisoning is the result of ions passing into
the membrane, attaching irreversibly, thereby ob-
structing the passage of following ions. In contrast,
fouling is a surface phenomenon and is the result of
the deposition on the surface of a fouling layer that
forms a barrier to diffusion. These effects are largely
eliminated in an electrodialysis reversal system.
Electrodialysis Reversal
0029The electrodialysis reversal (EDR) system is the first
commercially available membrane process that is
symmetrically reversible. This means that the direc-
tion of ion movement through the membranes and the
6166 WHEY AND WHEY POWDERS/Principles of Dialysis

identity of the concentrating and demineralizing com-
partments are reversed at periodic intervals (e.g.,
every 20 min). To bring this about, the d.c. polarity
is reversed. The mechanism of EDR differs from that
of unidirectional ED in a very simple but profound
way, and the advantages of EDR system are many.
The process is more tolerant than other commercially
available membrane processes of a wide range of
troublesome organic, inorganic, colloidal, and bio-
logical contaminants. The single most important
advantage of EDR is that any scale or film build-up,
common in unidirectional processes such as electro-
dialysis and reverse osmosis, is limited. There is a
tendency for constituents that are capable of forming
slimes and precipitates to be deposited at the mem-
brane/solution interface, and over a period of time,
these deposits affect the membrane desalting/filtra-
tion performance. The self-cleaning characteristic of
EDR systems is analogous to that of reversing
heat exchangers used in the cryogenic industry for
removal of carbon dioxide and water scale from
low-temperature surfaces.
Transport Depletion
0030 The fouling of anion exchange membranes by nega-
tively charged organic collodial matter is a serious
problem in conventional ED systems. This problem
can be avoided by replacing anion membranes with
nonselective (neutral) membranes, with the order of
the membranes being –C–N–C–N–C–, so that con-
centration polarization is avoided.
Ion Substitution
0031 Ion substitution is another variant of ED in which one
species of ion is removed and substituted by another.
Instead of the simple, alternating –anionic–cationic–
anionic–(–A–C–A–)– membrane arrangement, the
sequence –A–C–C–A– solution to be demineralized
is fed into the C–C compartment.
Ion Replacement
0032 Another modification of classical electrodialysis is
ion replacement, where the membranes used are
anionic or cationic only. In this application, the
solutions are neither enriched nor depleted in
ions; only an exchange in specific types of ions is
accomplished.
Advantages of Electrodialysis
0033 The many advantages of electrodialysis, e.g., in pro-
tein separations, include fast and controlled removal
of salts, no product dilution, low membrane area
requirements, negligible product adsorption, easy
salt recovery in the same unit, and the use of lower
concentrations of salting-out agents than are required
by conventional direct addition processes.
See also: Membrane Techniques: Principles of Reverse
Osmosis; Applications of Reverse Osmosis; Principles of
Ultrafiltration
Further Reading
Batchelder BT (1987) Electrodialysis applications in whey
processing. In: Trends in Whey Processing, Bulletin of
the International Dairy Federation No. 212, pp. 84–90.
Brussels: International Dairy Federation.
Brocklebank MP (1987) Large scale separation and isol-
ation of proteins. In: King RD and Cheetham PSJ (eds)
Food Biotechnology – 1, pp. 139–192. New York:
Elsevier Applied Science.
Glover FA (1986) Modifications to the composition of
milk. In: Robinson RK (ed.) Modern Dairy Technology,
vol. 1, pp. 235–271. New York: Elsevier Applied
Science.
Lo
´
pez-Leiva MH (1988) The use of electrodialysis in food
processing. Part 1: Some theoretical concepts. Lebens-
mittel Wissenschaft und Technologie 21: 119–125.
Applications of Dialysis
J F Mostert and L Frylinck, ARC-Animal Nutrition and
Animal Products Institute, Irene, South Africa
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The concentration, removal, or separation of com-
ponents in aqueous mixtures by membrane tech-
niques, e.g., dialysis and electrodialysis, have many
applications in the processing of liquid foods and
areas closely related to food.
0002Dialysis is a technique employing the difference in
concentration as a driving force to separate large
particles from small ones in a solution, for example,
proteins from salts. The solution to be treated is
placed on one side of a membrane, and a solvent
(water) on the other side. The membrane has pores
of a diameter that allows the small salt molecules to
pass through, but is too small for the protein mol-
ecules to pass (Figure 1). A disadvantage of dialysis is
that the rate of diffusion varies with the difference in
concentration. Dialysis can, however, be speeded up
if the solvent on the other side of the membrane
is changed frequently.
WHEY AND WHEY POWDERS/Applications of Dialysis 6167
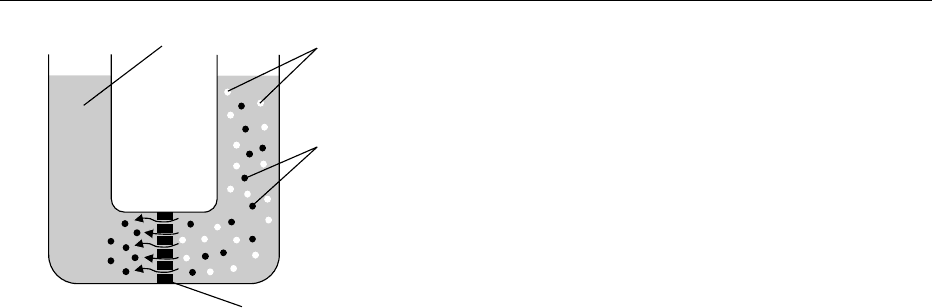
0003 Electrodialysis (ED), on the other hand, is defined
as the transport of ions through nonselective semi-
permeable membranes under the driving force of a
direct current and an applied potential. The mem-
branes used have both anion and cation exchange
functions, thus making the electrodialysis process
capable of reducing the mineral content of a process
liquid, e.g., whey or sea water. The most important
use of ED is in the desalination of brackish water for
the production of potable water as well as in the
production of table salt from sea water. ED is a classic
whey demineralization process with a well-proven
technology and with a multitude of systems operating
worldwide. Other important applications are found
in the dairy, wine, and beverage industries. The
integration of dialysis and ED in food-processing
systems, together with new advances in fermentation
systems, has a profound effect on new biotechnolo-
gical applications for the manufacture of specialized
and highly differentiated food products with en-
hanced functional properties.
Dialysis in the Dairy Industry
0004 In the dairy industry ED is primarily used for the
demineralization (desalting) of whey. Large volumes
of whey are produced as a byproduct from cheese-
making and, to a lesser extent, from casein manufac-
ture. For each tonne of cheese produced, about 8
tonnes of whey are left over, resulting in huge volumes.
In the past whey has been returned to farmers for
animal feeding, irrigation of lands and pastures, or
discharged directly into rivers, waterways, or sewage
systems. Considerable quantities of whey are still
being discharged into the environment worldwide.
0005 Whey is the liquid residue of cheese and casein
production and is, to a certain extent, one of the
biggest reservoirs of high-quality food protein not
fully utilized in human consumption levels. The com-
position of whey will vary substantially depending on
several factors, including the process involved in the
production of whey. On average, whey contains
about 65 g kg
1
of solids, comprising about 50 g lac-
tose, 6 g protein, 6 g ash, 2 g nonprotein nitrogen, and
0.5 g fat. The whey protein fraction of bovine milk
contains four main proteins: b-lactoglobulin (b-Lg,
50%), a-lactalbumin (a-La, 20%), blood serum albu-
min (10%), and immunoglobulins (lg, 10%; mainly
lgG
1
, with lesser amounts of lgG
2
, lgA, and IgM).
Wheys are broadly classified as ‘sweet’ wheys pro-
duced from rennet-coagulated cheeses and acid
wheys, which have a similar composition but with a
higher acidity and ash content.
0006The unique technological, nutritional, physio-
logical, and even nutraceutical properties of whey
proteins, together with the high disposal cost of
dairy effluent, stimulated research in whey utilization
within the food industry. Industrial whey processing
began with fluid whey being concentrated and dried.
New membrane techniques and processes revolution-
ized whey processing by providing breakthrough
methods for the separation of dissolved or suspended
solids, for the removal of water and, especially, for
the combination of these. Nowadays demineralized
whey powders, whey protein concentrates, whey pro-
tein isolates, individual proteins and derivates, as well
as permeate products/lactose derivates are manufac-
tured and are available on the market.
0007It is not easy, from an economic point of view, to
process whey into products of higher commercial
value. As whey has a fairly high salt content – about
8–12% calculated on dry weight – its usefulness as
an ingredient in human foods is limited. By having
the whey demineralized, various fields of application
can, however, be found for whey, which is partially
(25–30%) or highly (90–95%) demineralized. Par-
tially demineralized whey concentrate can, for
example, be used in the manufacture of icecream
and bakery products or even in quarg, whereas highly
demineralized whey concentrate or powder can be
utilized in formulas for infants and also in a wide
range of other products.
0008The two main methods used commercially for
high-degree demineralization of whey, namely ion
exchange and ED, result in products of somewhat
different composition. The ion exchange process is
relatively nonselective and removes both monovalent
and polyvalent ions, whereas ED is more dependent
on ionic mobility and tends preferentially to remove
monovalent ions. Economically, only about 90% de-
mineralization is possible with ED; 50–75% is
regarded as being more viable from a practical and
Permeable membrane
Water
Salt
Protein
fig0001 Figure 1 The separation of protein molecules from a salt solu-
tion, using a dialysis membrane. Reproduced from Bylund G
(1995) The chemistry of milk. In: Dairy Processing Handbook,
pp. 17. Lund, Sweden; Tetra Pak (Processing System Division),
with permission.
6168 WHEY AND WHEY POWDERS/Applications of Dialysis
