Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

Wine Making
0118 This is very rudimentary. The grape harvest takes
place in the second fortnight of October. About
90% of the production is mechanically harvested.
Then the grapes are pressed in horizontal presses.
The continuous presses (called the Archimedes
screw) are prohibited because they may induce herb-
aceous tastes. Sulfur dioxide is not used because it
binds ethanal which is then released during distilla-
tion and tastes pungent. Nor is there any racking of
the lees before fermentation (de
´
bourbage), and
addition of sugar to the must to increase the potential
alcoholic strength (chaptalization) is strictly
prohibited.
0119 Sometimes, alcoholic fermentation starts badly in
cold and rainy years, but generally it is very fast (8
days for the first vat), then 3–5 days, when the wine
material has been used and allows seeding of musts.
0120 The wines are kept on the lees, and malolactic
fermentation is conducted in the month following
alcoholic fermentation, even if the pH is low, because
absolutely no sulfur dioxide is used.
0121 Distillation can immediately take place after the
end of the fermentations, and the best brandies are
those which are distilled first. Ethyl butyrate levels
increase with time of conservation and especially
when the temperature increases. This is explained by
the total absence of disinfectants to protect the wine
against oxidation and bacterial accidents. As long as
the wine ferments, the medium is reduced (low oxi-
doreduction potential) and there is no risk. However,
at the end of fermentation, the wine must be stored in
full, hermetically closed vats. The most frequent acci-
dent is the browning of wine (casse brune) caused by
an enzymatic oxidation of the phenols in quinones in
years of rot. It seems that this has no effect on the
quality of the spirits since the polyphenol does not
distil.
0122The bacterial risks are the bacterial degradation of
tartaric acid call tourne (which is far from frequent),
and the problem of bitterness due to acrolein which
arises from the decomposition of glycerol in prope-
nal. This is not very frequent but is very harmful
for the brandies, which then become undrinkable.
Several cases occurred in the past when the tanks
were situated under the ground; the wine cooled too
slowly and lactic bacteria degraded the glycerol and
butanediol. Since the use of disinfectants is pro-
hibited, it is obviously necessary to work with per-
fectly clean wine-making equipment because of
possible contamination. The screw of the press must
be lubricated with paraffin oil because mineral oil
could give a tainted taste to the Cognac.
Wines
0123The alcoholic strength is generally relatively low
(8–10% vol). The wines are too acid for direct con-
sumption at pH 3 or even less, and total acidity ranges
CHALAIS
MONTLIEU
BEAUVOIR
BARBEZIEUX
BLANZAC
ANGOULEME
LA ROCHEFOUCAUD
JARNAC
JONZAC
MARENNES
GEMOZAC
ST PORCHAIRE
ROCHEFORT
TONNAY CHARENTE
PONS
ROYAN
OCEAN ATLANTIQUE
GIRONDE
SEUDRE
CHARENTE
COGNAC
LA ROCHELLE
SAINTES
AULNAY
ST JEAN D ’ANGELY
MACQUEVILLE
MATHA
RUFFEC
AIGRE
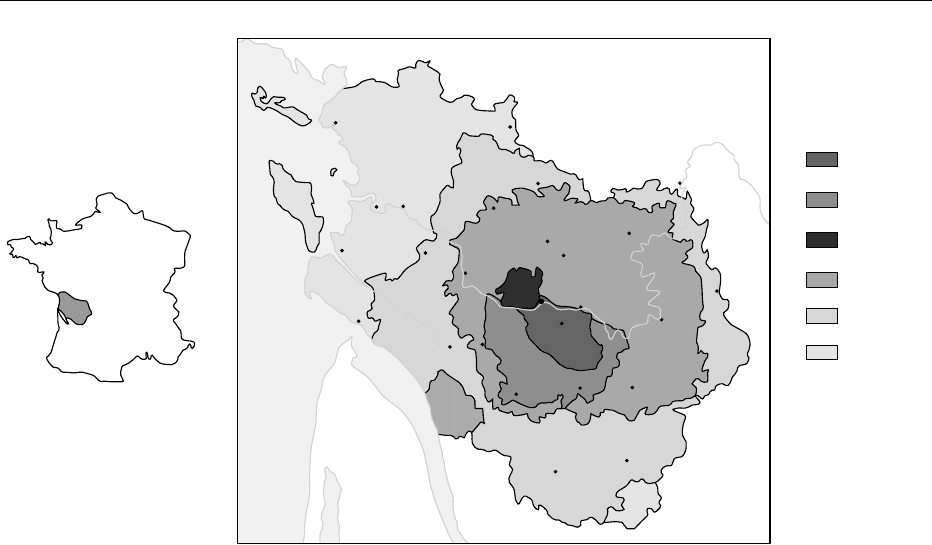
GRANDE
CHAMPAGNE
PETITE
CHAMPAGNE
FINS BOIS
BONS BOIS
BOIS
CORDINAIRES
BORDERIES
SEGONZAC
ILE DE RE
ILE D ’OLERON
fig0009 Figure 9 (see color plate 11) Cognac in France and the different appellation areas. See text for further details.
BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture 595
from 7 to 10 g l
1
(expressed as tartaric acid), even if
malolactic fermentation has taken place. The acidity
of the must reaches 15–20 g l
1
. This acidity makes it
possible to some extent to compensate for the absence
of sulfur dioxide. However, according to regulations,
the wines must be distilled before the end of March
because after that time heat would lead to inevitable
deterioration caused by the growth of lactic bacteria.
These wines contain only very little ethanal since
sulfur dioxide is not used.
0124 The flavor must be very neutral. When the specific
fruitiness of the type of grape is too marked, it is not
possible to obtain fine brandies. For example, musca-
delle or sauvignon cultivars would not be suitable to
obtain good Cognac. In contrast, with the very neu-
tral Ugni blanc, the flavor comes from fermentation
byproducts. This flavor is probably mainly due to
higher alcohols which are present in high quantities,
since fermentation occurs in the presence of the less of
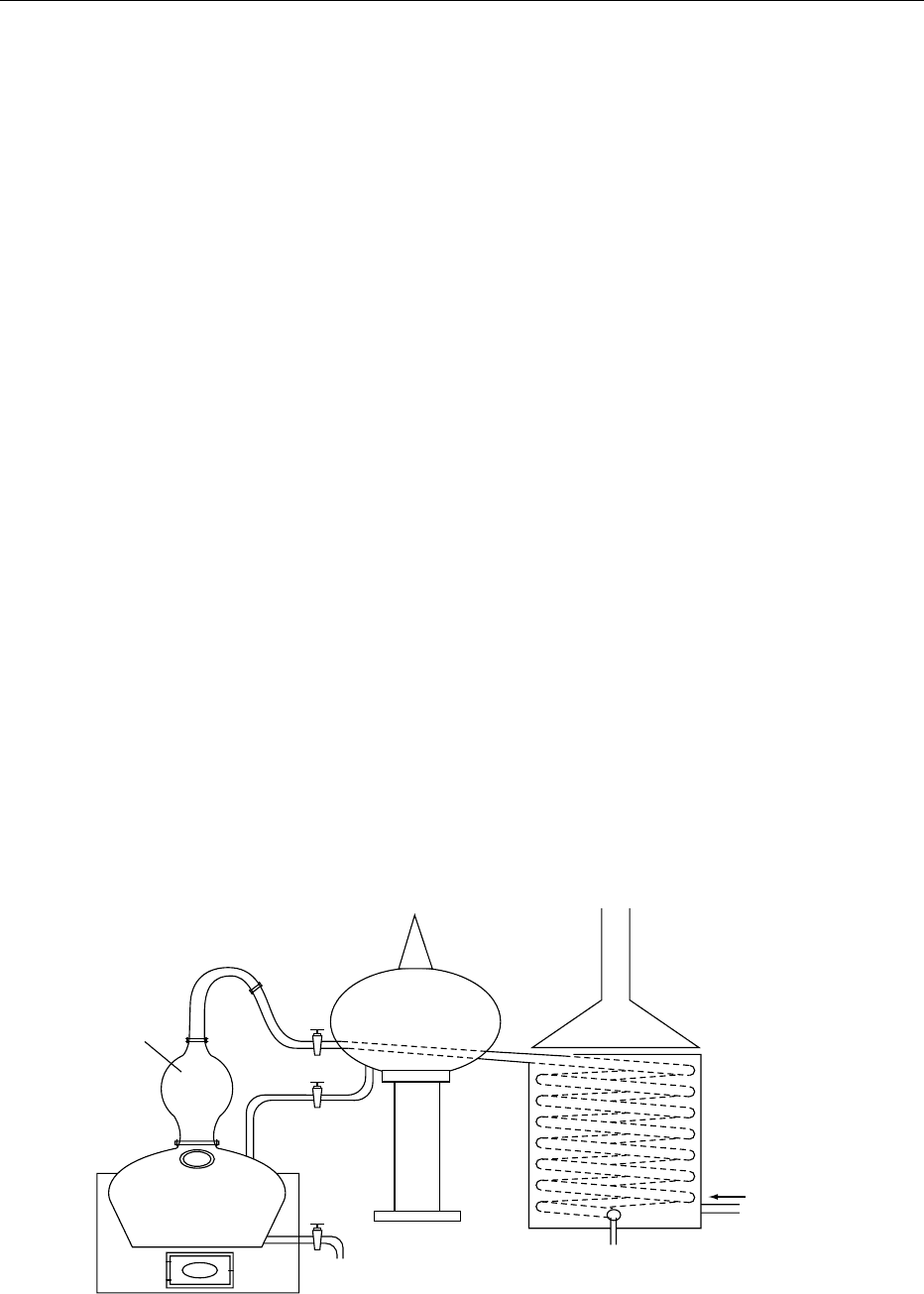
the must (see Table 1). The esters of fatty acids, ethyl
caproate, caprylate, caprate, and laurate are also
important because of a relatively low fermentation
temperature (late grape harvest, low sugar content).
0125 The herbaceous tastes in Cognac are due to car-
bonyl compounds with six carbons. They may occur
when the harvested grapes contain too many leaves or
when they are crushed too hard by the machines. Oily
tastes are due to 1,1,6-trimethyl-1,2-dihydronaphta-
lene (TDN) for the same reasons. This chemical sub-
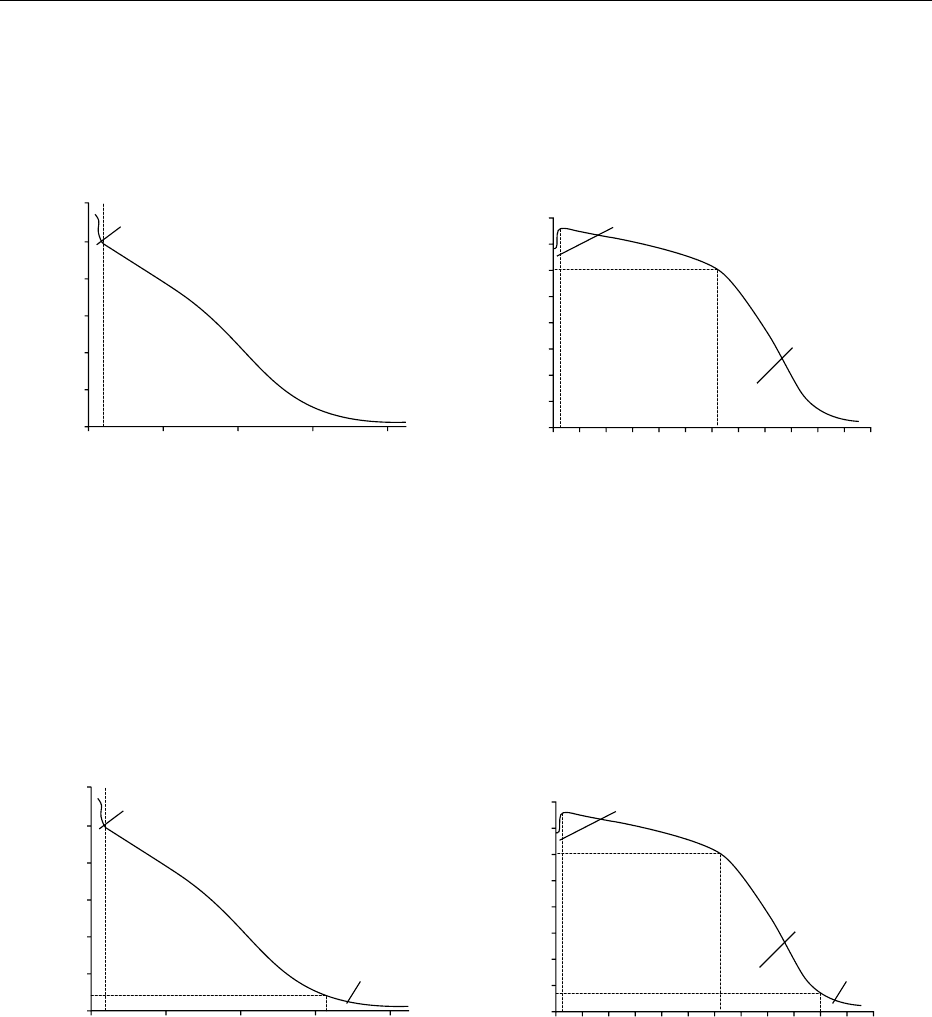
stance seems to be more or less specific to Ugni blanc,
especially when it is not ripe enough.
0126 Volatile acidity is generally very low (0.2–0.4 g l
1
expressed in acetic acid). Ethyl acetate levels are also
generally low (30–60 mg l
1
). The dry extract varies
between 15 and 24 g l
1
. The quantity of unfermented
sugar is very low, and often nil (less than 50 mg l
1
).
Distillation
0127The charentais still (Figure 10) The charentais still
is made of copper of electrolytic quality (no hydrogen
bubbles in metal). Copper is essential since it is a good
conductor, is not attacked by wine acids, and fixes the
traces of H
2
S arising from the late treatment of the
vine with sulfur to combat oidium infection. Copper
combines a part of the caprylic, caproic, and lauric
fatty acids, whose odor resembles that of cheese, and
long-chain fatty acids which could give insoluble
soaps (eliminated by filtration). Distillation in a stain-
less-steel boiler gives poor-quality brandies. The pro-
cess can be improved by adding turnings of copper or
copper sulfate. The copper is rolled and hammered to
increase its hardness and to make its surface
smoother.
0128There are two shapes of stills according to the
shape of the boiler, which is either straight or onion-
shaped. The volume of the boiler is 30 hl maximum
for the second distillation called the bonne chauffe;
exceptionally, the volume of the boiler may be
150 hl but only for the distillation of the wine (first
distillation).
0129The upper part is called the chapiteau (olive or
onion form). Its volume represents 10% of the
volume of the boiler and it is topped by the swan
neck. A wine heater generally makes it possible to
recover the calories provided by the alcoholic vapors.
The temperature is about 50
C before the boiler is
filled. The serpentine, which is also made of copper,
is about 30 m long. It is placed in the cooler, called
Swan neck
Wine heater
Cooler
Cold water
Alcohol meter holder
Washy wine
Furnace
Boiler
Chapiteau
fig0010 Figure 10 Charente still for the production of Cognac spirit.
596 BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture

la pipe, which is filled with water; its capacity is
approximately twice that of the boiler.
0130 The boiler is assembled on a solid mass of masonry.
The boiler is heated by direct flame (no steam or
electric heating). At the lower part is the furnace.
On the sides of the boiler are what is known as the
‘turns with fire’ in which the gases from the hearth are
burned before being evacuated towards the chimney.
From 600
C in the hearth, the evacuated gases drop
to no more than 250
C.
0131 The liquid in the boiler is at boiling point (between
90 and 102
C) and the temperature in the chapiteau
is lower than 5–8
C. Part of the vapor (3–5%) flows
back, which makes it possible to obtain a slight recti-
fication of the distillate. At 1 mm above the bottom of
the boiler, there occurs an overheating of about 5
C
before boiling begins. Excessive overheating induces a
certain bitterness of the spirit.
0132 Formerly wood was used as fuel, then coal, and
nowadays butane and generally propane. It is obvi-
ously necessary to use burners which work efficiently
without overheating. The adjustments are similar to
those of a central heating system.
0133 Distillation method Traditionally the wines are dis-
tilled with their lees. However, in recent years only
the finest lees (yeast) have been used, so it is possible
to speak of wines with fine lees or even wines free of
lees. This has repercussions on the ester content
(Table 4).
0134First distillation or distillation of the wine, known as
chauffe du brouillis The boiler is filled to 95% of its
volume. The wine boils after 1.5 h of heating, and the
distillate which runs out first contains 55–60% vol of
alcohol. Distillation takes place until all alcohol is
distilled and is stopped when the distillate contains
2% vol. The liquid thus collected is called the brouil-
lis, its alcoholic strength ranges from 26 to 31% vol.
It is obtained in approximately 12 h. The temperature
of flowing distillate must be about 15
C.
0135Second distillation, called the bonne chauffe This is
carried out under the same conditions. After 1.5 h of
heating, the distillate starts to run out. It contains
75–80% vol of alcohol: 1% of total volume collected
constitutes the heads which are separated and mixed
with wine or brouillis. The distillate is collected up to
60% vol of alcohol; this is the heart, whose average
alcoholic strength is nearly 70% vol. The distillate
which then runs out is called the seconds (which taste
of fat); these seconds are mixed with wine or brouillis
to be redistilled.
0136The temperature of the distillate which runs out of
the cooler must be about 18
C to obtain a good
brandy. The rate of flow is 1 l min
1
.
0137The seconds can either be mixed with the wine and
in this case the alcoholic strength is not of major
importance (first principle, Figure 11), or they may
be added to the brouillis, in which case the strength is
28% vol. Therefore, it may be necessary to reduce the
distillation of the brouillis according to the alcoholic
strength of the initial wine (second principle, Figure
12). In this case the seconds are redistilled only once,
which increases the strength of the brouillis.
0138The second distillation is performed at a lower
temperature, thus obtaining better rectification of
the spirit. Here, the tails of the brouillis need to be
cut.
0139There are also variants of these two methods. Vary-
ing the intensity of heating is important according to
the strength of brouillis required. Slow distillation
gives good rectification. An odorous fine brandy is
obtained but with dryness that may be detected on
tasting due to the lack of certain products of tail
distillation (e.g., ethyl lactate, diethyl succinate). In
contrast, fast heating involves the formation of a
marrowy brandy with little bouquet. Excessive
heating results in a heavy taste.
Aging
0140The cognac ages in barrels of between 200 and 600 l.
The barrels must be in oak of special quality. Trad-
itionally these oaks came from the forests of Tronc¸ais,
Allier, Limousin, and the Vosges in France. The stave
woods, i.e., pieces of wood used to make the barrels,
tbl0004 Table 4 Effect of the lees of the wine on the composition of the
distillate
Fewleesin thewine Withleesin the wine
Ethyl caproate 6.76 8.3
Ethyl caprylate 8.95 23.6
Ethyl caprate 13.8 63
Ethyl laurate 12.45 36.2
Ethyl myristate 5.4 9.8
Ethyl palmitate 9.77 13.2
Ethyl palmitoleate 1.44 1.8
Ethyl stearate 0.59 0.61
Ethyl oleate 1.19 1.22
Ethyl linoleate 7.69 9.2
Ethyl linolenate 1.86 2.58
Isoamyl caprylate 0.42 2.48
Isoamyl caprate 1.67 5.76
Isoamyl laurate 0.78 1.83
2-phenylethyl caprylate Traces 1.2
2-phenylethyl caprate 0.25 1.55
Summ of aromatic esters 73.02 186.65
(þ150%)
Results as mg l
1
of the distillate at 70% vol.
Reproduced from Cantagrel R, Lurton L, Vidal JP and Galy B (1992) La
distillation caharentaise pour L’obtention des eaux-de-vie de Cognac. In:
Bertrand A (ed.) Les Eaux-de-vie Traditionnelles d’Origine Viticole, pp. 60–69.
Paris: TEC & DOC, with permission.
BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture 597
must be stored for 3 years outside so that the bitter
substances that they contain are transformed by en-
zymatic reaction. The meta-digallic acid is hydro-
lyzed into the less aggressive gallic acid. The same
occurs with aesculine and scopoline, glucosides that
are transformed into aesculetine and scopoletine,
which are less bitter. The transformations can take
place only with time; fast drying obtained by stoving
(heating) is not sufficient and the brandies placed in
such barrels are bitter.
0141During aging the brandy is oxidized slowly. Its
acidity grows by oxidation of alcohol into volatile
0
0
(a) (b)
10
20
30
40
50
60
2
% vol.
% vol.
46
hh
Brouillis
920 l 30% vol.
Wine: 2227 l at 9% vol.
+ heads 19 l at 66.5% vol. (10 l 57% vol. + 25 l 78% vol. 3 4/11)
+ seconds 254 l at 30% vol. (700 l 3 4/11)
Heads, 10 l 57% vol.
Mixture: 2500 l at 11.6% vol. Brouillis 2500 l at 30% vol.
80
0
10
20
30
40
Heart
(Cognac)
730 l 70% vol.
50
60
70
80
Heads 25 l 78% vol.
Seconds
700 l 30% vol.
123456789101112
fig0011 Figure 11 Distillation of the cognac, first principle. (a) First distillation of the wine, to obtain the broiullis; (b) second distillation,
bonne chauffe. Eleven brouillis must be distilled to give four bonnes chauffes.
0
0
(a) (b)
10
20
30
40
50
60
2
% vol.
% vol.
46
Tails
120 l 3% vol.
hh
Brouillis
800 l 27% vol.
Wine: 2324 l at 9% vol.
+ heads: 21 l at 66% vol. (10 l 55% vol. + 24 l 76% vol. 3 4/9)
+ tails: 155 l at 3% vol. (120 l + 78 l 3 4/9)
Heads, 10 l 55% vol.
Mixture: 2500 l at 9.1% vol. Mixture: 2432 l at 27.8% vol.
Brouillis: 1800 l at 27% vol.
+ seconds 632 l at 30% vol.
80
0
10
20
30
40
Heart
(Cognac)
662 l 70% vol.
50
60
70
80
Heads 24 l 76% vol.
Seconds
632 l 30% vol.
123456789101112
Tails
78 l 3% vol.
fig0012 Figure 12 Distillation of the cognac, second principle. (a) First distillation of the wine to obtain the brouillis; (b) second distillation,
bonne chauffe. Nine brouillis must be distilled to give four bonnes chauffes, and all the tails are mixed with the wine.
598 BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture
acids and by dissolution of the acid substances in the
wood. Moreover, acetals are formed, and their odors
are softer than those of aldehydes. The odor charac-
teristic of young brandy becomes blurred, and even-
tually disappears, to be replaced by a vanilla odor
induced by vanillic aldehyde, together with other
phenolic aldehydes and acids arising from the alco-
holysis of lignin in oak wood. The color becomes
brown by dissolution of tannin. Moreover, the taste
softens with the appearance of sugars arising from the
hydrolysis of wood hemicelluloses.
0142 There is also the appearance of a rancid taste by
oxidation of fatty acids. Several stages in aging may
be distinguished:
.
0143 From 1.5 to 5 years the main process is dissolution
of substances in the wood.
.
0144 By 5–10 years astringency decreases and the
brandy becomes rounder.
.
0145 From 10 to 35 years a rancio taste appears.
.
0146 After 40 years one should no longer keep brandies
out of the barrel.
A barrel can yield substances to the cognac for about
40 years.
0147 During aging, there is a loss of volume known as
‘the angels’ share.’ This represents 3% on average per
year, with a 1% vol reduction in the alcoholic
strength.
0148During conservation there is little evolution of the
volatile substances. The alcohols concentrate, the
esters are slightly hydrolyzed, and the unsaturated
fatty acids oxidize, which gives the rancio taste. The
Cognac ages by slow oxidation in barrels. In bottles
there is no further evolution.
Analysis
0149Traditional analyses Their purpose is to determine
real and raw volume percentages of alcohol (titra-
tion), dry extract, total acidity, and the ratio of non-
alcoholic elements, i.e., volatile acidity, aldehydes,
esters, furfural, and higher alcohols; methanol values
are determined separately.
0150Table 1 shows values for Cognac compared to
those of brandy and Armagnac. The sum of volatile
substances of Cognac is very high because the distil-
lation process does not eliminate higher alcohols;
however some tail products such as volatile acidity
are noticeably lower than in Armagnac.
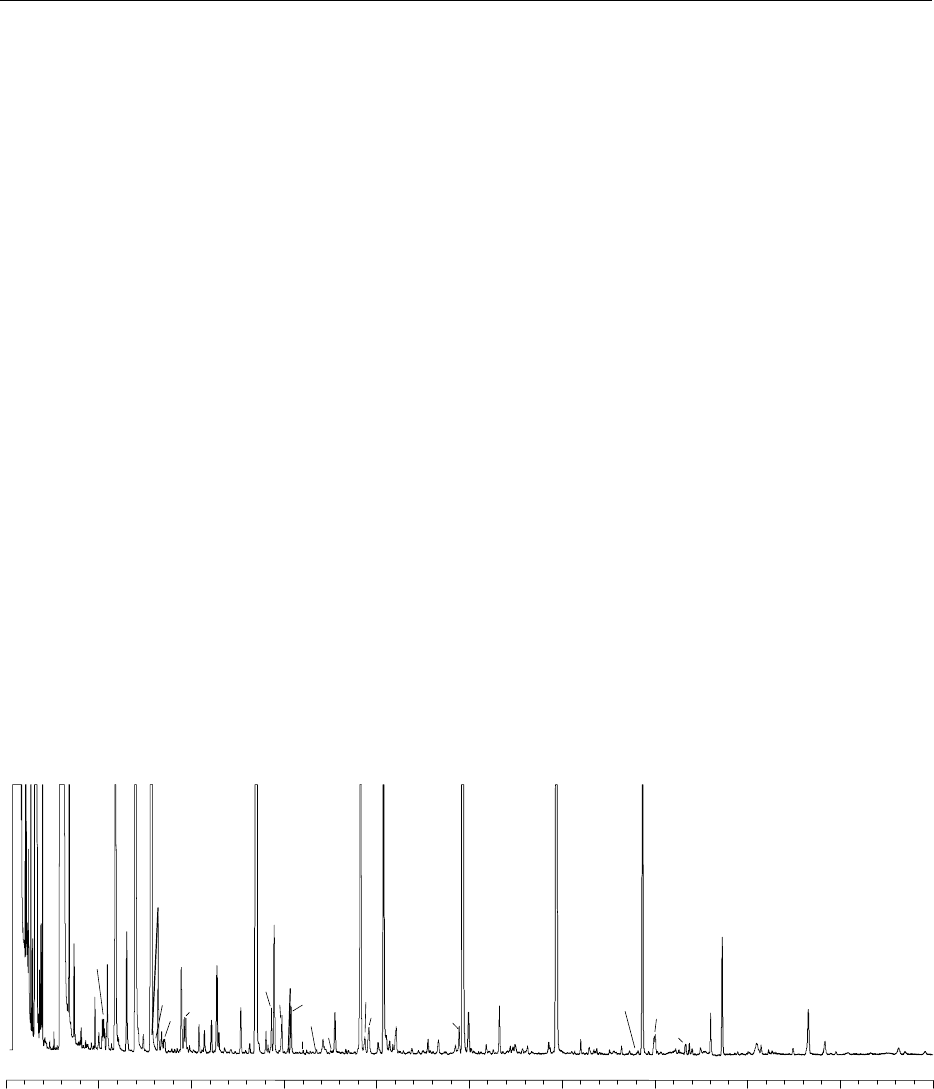
0151Gas chromatography Gas chromatography is used
to analyze volatile compounds. As an example, the
chromatogram in Figure 13 shows some of the vola-
tile alcohols, esters, acids, and various aromatic com-
ponents contained in an ether-hexane extract of
VSOP cognac. Other analyses can be carried out by
injecting the spirits directly into the chromatograph.
min
0 10 20 30 40 50 60 70 80 90
1
2
3
4
5
6
7
8
9
10
11
13
14
15
16
19
20
17
18
21
22
23
24
25
26
27
28
29
30
31
32
33
34
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
35
fig0013 Figure 13 Chromatogram of the extract of a Cognac by ether hexane (concentration about three times); column FFAP
50 m 0.22 mm; injection according to splitless mode; temperature programming from 40 to 200
C. Identification of the peaks: 1,
Ethyl butyrate; 2, 2-methylpropan-1-ol; 3, isoamyl acetate; 4, isoamyl alcohols; 5, ethyl hexanoate; 6, hexyl acetate; 7, styrene; 8,
acetoin; 9, ethyl heptanoate; 10, ethyl lactate; 11, hexan-1-ol; 12, trans-hex-3-en-1-ol; 13, cis-hex-3-en-1-ol; 14, octan-3-ol (internal
standard 1); 15, trans-hex-2-en-1-ol; 16, ethyl octanoate; 17, trans-linalol oxide (furane); 18, furfural, 19, acetic acid; 20, benzaldehyde;
21, vitispirane; 22, linalol; 23, ethyl decanoate; 24, butyric acid; 25, 3-methylbutyric acid; 26, diethyl succinate; 27, a-terpineol; 28, 1,1,6-
trimethyl-1,2-dihydronaphthalene (TDN); 29, methionol; 30, unknown; 31, unknown; 32, damascenone; 33, phenylethyl acetate; 34, ethyl
dodecanoate þhexanoic acid; 35, benzyl alcohol 36, trans-whisky lactone; 37, 2-phenylethanol; 38; cis-whisky lactone; 39, 4-ethylgaia-
col; 40, nerolidol þethyl myristate; 41, octanoic acid; 42, 4-allylgaiacol (eugenol); 43, 4-ethylphenol; 44, ethyl palmitate; 45, decanoic
acid; 46, ethyl stearate; 47, ethyl oleate; 48, dodecanoic acid; 49, ethyl linoleate; 50, ethyl linolenate; 51, unknown; 52, myristic acid; 53,
palmitic acid; 54, palmitoleic; 55, linoleic acid.
BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture 599

The high levels of esters and fatty acids indicate that
wine is distilled with lees (yeast).
Disturbances and Deposits
0152 These can be:
.
0153 of organic nature: insolubilization of certain sub-
stances when the temperature decreases; to avoid
this accident the brandies are filtered cold after
cooling at 5
C.
.
0154 of mineral origin due to calcium arising from the
barrels (1–1.5 mg in 15 years’ storage) or from
the filter plates, thus requiring the use of plates
without CaCO
3
. Beyond 3 mg l
1
the brandy de-
posits at cold temperature. Certain treatments by
ion exchange resins could be used to eliminate the
excess of calcium and copper.
Preparation for Commercialization
0155 During aging, once a year, the cognac is racked and
all the barrels of the same production are mixed
together. The alcoholic strength is gradually dimin-
ished by adding demineralized water to finally obtain
an alcoholic strength of 40.0% vol in commercialized
bottles (never less). During aging it is common to mix
spirits of different origins, quality and age in order
always to obtain the same odor and taste for a given
brand name. For instance, some Cognac firms use up
to 60 different brandies to prepare their blend but the
brand name (i.e., ***, VSOP) must consider only the
youngest spirit of the blend.
0156 The color can be adjusted with the addition of
caramel. The taste can be adjusted with the addition
of woody water extract obtained from small pieces of
oak wood to give more body to the spirit, more
astringency, and a little bitterness. On the other
hand, excessive hardness can be diminished by the
addition of sucrose syrup, generally less than 8 g l
1
.
Generally, all these procedures should be carried out
at least 2 or 3 months before bottling.
Tasting
0157 Just as for wines, the length of a brandy can be
measured by taking it into the mouth, spitting it out,
and counting the number of seconds during which an
intense aromatic persistence may be perceived, just
before the appearance of a certain dryness. The word
‘caudalies’ is used to indicate the number of seconds
elapsed. For a Grande Champagne Cognac the sensa-
tion lasts 7–12 s; for Bois ordinaire Cognac it is
only 2 s.
0158 The Grande Champagne In addition to the length
of aromatic persistence, this brandy is characterized
by a bouquet of great smoothness and distinction,
odors of vine flower, dried lime, flowers and dry
vine shoots.
0159The Petite Champagne The same characters are
found but are less accentuated.
0160Borderies The persistence is the same as in Petite
Champagne. These brandies have a developed odor
which is more suave than that of the champagnes. At
the time of the folle blanche before 1880, brandies
had an odor of violet. Unlike in Armagnac, this is not
considered a quality in Cognac spirits. They are less
light than the Champagnes.
0161Fins bois The fins Bois brandies are rounder than
the Champagnes. They are more marrowy but have
a heavier odor more reminiscent of the fruit than
the flower. From the point of view of character, the
length decreases. They age faster, so together with
the bons bois producting *** and VSOP Cognac,
they constitute the most readily available of the
Cognacs.
Acknowledgments
0162The author is grateful to the Bureau Interprofession-
nel de l’Armagnac for its collaboration and financial
support in the form of research contracts, and to his
students at the Institute of Oenology: P. Wildbolz,
Ph. Jadeau, Miss M.C. Segur, Miss S. Biau, M.K.
Bertsch, Miss R. Vanderlinde, and E. Herve.
See also: Alcohol: Properties and Determination;
Metabolism, Beneficial Effects, and Toxicology; Alcohol
Consumption; Brandy and Cognac: Chemical
Composition and Analysis of Cognac; Grapes; Sensory
Evaluation: Aroma; Taste
Further Reading
Bertrand A (1988) Role of the continuous distillation pro-
cess on the quality of Armagnac. In: Piggott JR and
Paterson A (eds) Distilled Beverage Flavour. Recent
Developments, pp. 97–115. Edinburgh: Ellis Horwood.
Bertrand A (1989) L’analyse chimique et sensorielle d’eaux-
de-vie d’Armagnac. Conse
´
quence de la technologie
sur la qualite
´
. In: La Re
`
glementation Communautaire
des Eaux-de-vie et des Liqueurs, vol. 9, pp. 101–111.
Verona: Bollettino del Cideao OIV (ed.).
Bertrand A and Segur M-C (1991) L’alambic armagnacais.
In: Bertrand A (ed.) Les Eaux-de-vie Traditionnelles
d’Origine Viticole, pp. 70–74. Paris: Lavoisier-TEC &
DOC.
Bertsch K (1992) Les Eaux-de-vie d’Armagnac, Crite
`
res
Analytiques de Qualite
´
,e
´
tude sur le Carbamate
d’E
`
thyle. Thesis: l’ Universite
´
de Bordeaux II.
600 BRANDY AND COGNAC/Armagnac, Brandy, and Cognac and their Manufacture

Cantagrel R, Lurton L, Vidal JP and Galy B (1992) La
distillation caharentaise pour l’obtention des eaux-
de-vie de Cognac. In: Bertrand A (ed.) Les Eaux-de-vie
Traditionnelles d’Origine Viticole, pp. 60–69. Paris:
TEC & DOC.
Casamayor P and Cousteaux F (1985) Le Guide de l’Ama-
teur d’Armagnac. Toulouse: Laffont/Briand.
Caumeil M (1983) Le Cognac. Pour la Science December:
48–57.
Dufor H (1982) Armagnac – eaux-de-vie et terroir. Tou-
louse: Privat.
Guigon D and Cogat P (1991) Elimination des de
´
fauts dans
les eaux-de-vie de vin traditionnelles. In: Bertrand A
(ed.) Les Eaux-de-vie Traditionnelles d’Origine Viticole
pp. 110–113. Paris: Lavoisier-TEC & DOC.
Jadeau P (1987) Incidence du De
´
bourbage des Mou
ˆ
ts et de
la Fermentation Malolactique des Vins sur la Compos-
ition des Eaux-de-vie d’Armagnac. Thesis: Universite
´
de
Bordeaux II.
Lafon J, Couillad P and Gay-Bellyle F (1973) Le Cognac, sa
Distillation. Paris: JB Bailliere.
Marche M and Joseph E (1975) Etude the
´
orique sur le
cognac. Revue Francaise d’Oenologie 57: 1–22.
Mariller C (1925) Distillation et Rectification des Liquides
Industriels. Paris: Dunod.
Puech J-L (1986) Le vieillissement de eaux-de-vie. In: Les
Aro
ˆ
mes des Vins. Montpellier: Journe
´
e de Rencontres
Œnologiques.
Roget J and Garreau C (1991) Recherches sur l’origine de la
distillation et la pre
´
paration de l’alcool. In: Bertrand A
(ed.) Les Eaux-de-vie Traditionnelles d’Origine Viticole,
pp. 54–59. Paris: Lavoisier-TEC & DOC.
Segur M-C (1988) Approche de la De
´
gustation Analytique
des Eaux-de-vie d’Armagnac. Interpre
´
tation Statistique.
Thesis: Universite
´
de Bordeaux II.
Sempe A (1988) La Grande Messe de l’Armagnac. Paris:
Laffont.
Chemical Composition and
Analysis of Cognac
R Cantagrel, Bureau National Interprofessionnel du
Cognac (BNIC), Cognac, France
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Cognac is one of the most prestigious products in
the world. It has received an ‘Appellation d’Origine
Contro
ˆ
le
´
e’ or registered designation of origin, and
the term ‘Cognac’ must only be used for spirits from
Cognac or the Charente region. It is subject to strict
production regulations to guarantee origin and
quality.
0002Cognac is characterized by a rich aroma and flavor,
which distinguishes it from other spirits distilled from
wine, the production of which is not governed by
such strict regulations.
Chemical Composition and Sources of
Variations
0003The aroma of Cognac is the result of the characteristic
aromas of many of its constituents, enhanced or
modified by synergistic or masking effects. More
than 500 substances have been detected in spirits;
they belong to a large number of chemical classes:
alcohols (ethanol, methanol, higher alcohols, etc.),
aldehydes, esters, volatile acids (acetic acid, fatty
acids, etc.), ketones, acetals, nitrogen-, oxygen- and
sulfur-containing heterocyclic compounds, phenolic
acids, and aldehydes. These different aromatic con-
stituents are contributed by:
.
0004the grape (primary aromas);
.
0005wine-making (fermentation aromas);
.
0006distillation (specific aromas produced by the
heating process);
.
0007aging (aromas imparted by the oak wood).
The main aim in the production of a spirit is to form
pleasant, distinctive flavors and fragrances to be
conveyed by the alcohol. (See Wines: Production of
Table Wines.)
0008The quality of a given Cognac depends on a
number of factors:
1.
0009In the production process, variations can be due
to:
.
0010the weather conditions, which may vary from
year to year, causing differences in the chemical
composition of the resulting wines and spirits;
.
0011the diversity of grape producers;
.
0012the different ‘crus’ or growth regions within the
area of designated origin;
.
0013the several hundered distillers;
.
0014the several types of stills used with different
distillation methods.
2.
0015In the aging process, variations can be due to:
.
0016the origin of the oak wood used for the barrels or
casks;
.
0017cask-making techniques;
.
0018the layout and geographical location of the cellar
(chai) where the spirits are aged;
.
0019the length of the aging period.
Variability of the Raw Material
0020The wines used in producing Cognac spirits are made
essentially from the Ugni-blanc grape variety, which
BRANDY AND COGNAC/Chemical Composition and Analysis of Cognac 601
produces wine with a relatively low alcohol content
(generally 8–10% v/v) and a fairly high acidity (6–
12 g of sulfuric acid per liter). This high degree of
acidity ensures good conservation before distillation.
Most of the constituents that make up a Cognac are
present in the wine and lees used in the distillation.
0021 Weather conditions are never the same from one
year to the next. They determine the size of the grape
harvest and the different qualities of wine produced
by the vineyard. For example, the average alcoholic
strengths (v/v) of wines over the last 12 years in
Cognac were:
.
0022 1987, 7.5%;
.
0023 1988, 9.6%;
.
0024 1989, 10.9%;
.
0025 1990, 9.8%;
.
0026 1991, 9.6%;
.
0027 1992, 8.0%;
.
0028 1993, 7.8%;
.
0029 1994, 8.6%;
.
0030 1995, 9.7%;
.
0031 1996, 9.8%;
.
0032 1997, 10.3%;
.
0033 1998, 9.2%.
As a result, the chemical composition of the resulting
spirits varies from year to year.
0034 The figures below illustrate the diversity of the raw
material obtained:
.
0035 the total area of the registered designation region is
80 000 ha, comprising six growth zones (‘crus’)
within the designation region;
.
0036 the number of grape growers producing the white
wine sold for Cognac is 8000;
.
0037 the number of producers with vineyards larger than
3 ha is 5000;
.
0038 the number of industrial-scale distillers is 150;
.
0039 the number of growers distilling on their own
premises is 2500.
Cognac is an internationally recognized product,
highly specific, and yet obtained from a number of
different sources.
0040 During the distillation period (about 5 months), the
composition of the wines varies (see Table 1). The
higher alcohol and polyol content does not change,
but there is a significant decrease in the content of
esters with aromatic properties of interest (isoamyl,
hexyl and phenylethyl acetates, ethyl caproate,
caprylate, caprate and laurate) and an increase in
ethyl acetate, ethyl lactate, diethyl succinate, acetal-
dehyde (ethanal) and acetic acid. For this reason, the
distillation is not performed in the same way in
November, when the fermentation is completed, as
in March.
Charente Distillation Method
0041The distillation period in Charente begins as soon as
the alcohol fermentation ends (generally the begin-
ning of November) and continues until March 31.
0042The Charente method of distillation is performed
in two stages. From the first distillation stage, the
brouillis (27–30% (v/v) alcohol) is obtained; the
second distillation stage yields the bonne chauffe
(70% (v/v) alcohol). The purpose of this is threefold:
1.
0043To extract the volatile compounds contained in
the wines used. Besides water and alcohol (etha-
nol), other compounds contribute more through
their intense characteristics rather than their
quantities; they represent 0.3–1% (v/v) of the
alcohol.
2.
0044To select (rectification) from among the volatile
substances present with the alcohol. Certain
highly volatile products are undesirable at high
doses (ethanal, ethyl acetate, acetal, etc.).
3.
0045To perform certain chemical transformations
favorable to the quality of the spirit. The heating
time has a decisive influence on the combination
and decomposition of compounds.
This reactive function of the Charente pot still is the
source of chemical reactions such as:
0046Esterification/hydrolysis:
Acid þ Alcohol Ð Ester þ Water:
0047Acetal formation:
Aldehyde þ Alcohol Ð Acetal þ Water:
0048Maillard reaction:
Sugar þ Amino acid ! Pyrazine; Furans ðcocoa
flavorsÞ:
tbl0001Table 1 Variations in the concentration of wine constituents
during storage for 5 months
Constituents Variation (%)
Ethyl acetate þ24.1
Isoamyl acetate 1
a
55.7
Hexyl acetate 2
a
60.7
Phenylethyl acetate 3
a
62.1
Ethyl caproate 4
a
22.0
Ethyl caprylate 5
a
14.2
Ethyl caprate 6
a
18.9
Ethyl laurate 7
a
52.0
Total esters 1–7
a
25.8
Ethyl lactate þ49.1
Diethyl succinate þ226
Acetaldehyde (ethanal) þ117
Acetic acid þ18.8
a
Organoleptically aromatic esters present in wine in low quantities. Ethyl
acetate and ethyl lactate concentrations are much higher.
602 BRANDY AND COGNAC/Chemical Composition and Analysis of Cognac

0049 Strecker degradation:
a-Amino acid ! Aldehydes ! Acetals:
0050 In order to prevent a coarse character appearing in
the spirits, the wines are generally racked in order to
leave only part of the lees. In keeping with tradition
and local custom, the natural wine is distilled on fine
lees. But of course, each firm gives its distillers spe-
cific instructions in order to obtain products in keep-
ing with the distinctive types they each market. These
instructions concern in particular the proportion of
fine lees that must be kept in the wines used. Obvi-
ously, as a result, there are considerable analytical
differences among the various finished products.
The presence of lees results in a greater quantity of
aromatic esters, which have a high sensory impact,
and produces spirits higher in floral aromas (Table 2).
Care must be taken that the lees are of good quality; if
not, they are a major source of defects.
0051 Given the very high number of chemical classes
present in wines, all wine aromas that are volatile or
carried over by water–alcohol vapors do not pass
over with the same speed during distillation.
0052 Obtaining a good-quality spirit necessarily implies
a harmonious balance of all the constituents: the
distiller’s entire art is required to achieve this balance.
Aging Cognac
The Contribution to Product Diversity
0053 Aging is a basic factor in the quality of Cognacs; it is
also the most costly. Cognac can be kept for many
years in oak barrels; the longer the aging, the more
the intrinsic quality is enhanced. In order to maintain
this quality, the producer is very careful in producing
and aging the product.
0054Aging is an active process, and there are several
parameters that must be controlled:
.
0055the cellar or chai;
.
0056the humidity;
.
0057the temperature;
.
0058the type of container used for aging.
0059The cellar may have an earthen floor, which pro-
vides good humidity, or it may be made of cement, in
which case, the humidity is lower. The degree of
humidity influences the evaporation rate. In a dry
cellar, evaporation will consist mainly of water loss.
In a very humid cellar, evaporation will affect mainly
the alcoholic strength as alcohol is lost; Cognacs will
then be smoother and mellower, and have a greater
‘rancio’ taste.
0060Difference in Cognac temperature from 7
Con
average in winter to 22
C in summer are reasonable.
This is one of the factors in the ability of a cellar to age
spirits. Chemical reactions occurring during aging
depend on the temperature (esterification, for
example).
0061There are several types of containers. There are
1000- and 50 000-l barrels (for delivery of Cognac
and blends) and casks of 100, 270, 350, 400, and
550 l (aging). Currently, most casks are of the 350-l
type manufactured by coopers (cask makers). The
cask performs several functions during aging. (See
Barrels: Wines, Spirits, and Other Beverages.)
Quality Factors
0062The oak imparts aromatic elements to the Cognac in
greater or lesser amounts according to:
.
0063the choice of oak – coarse grain oak imparts
tannins more easily than fine grain;
.
0064the amount of time the staves are allowed to dry
and their thickness;
.
0065the method of heating when bending the staves to
make the casks;
.
0066the alcoholic strength of the spirits during aging;
.
0067the age of the cask or barrel;
.
0068the grape production zone (‘crus’) and the distilla-
tion method.
Oak wood consists of cellulose (wood fiber), hemi-
celluloses, lignin, and tannins. Tannins are highly
soluble in spirits.
0069The first notable modification during aging is the
change in color. This phenomenon is directly linked to
the extraction and oxidation of tannins. As a direct
result, a young Cognac in a new cask will color
tbl0002 Table 2 Effect of lees on ester contents (mg l
1
spirit at 70%
vol.)
Distillation
Constituents With fewlees Withlees
Ethyl caproate 6.76 8.3
Ethyl caprylate 8.95 23.6
Ethyl caprate 13.8 63.0
Ethyl laurate 12.45 36.2
Ethyl myristate 5.4 9.8
Ethyl palmitate 9.77 13.2
Ethyl palmitoleate 1.44 1.8
Ethyl stearate 0.59 0.61
Ethyl oleate 1.19 1.22
Ethyl linoleate 7.69 9.52
Ethyl linolenate 1.86 2.58
Isoamyl caprylate 0.42 2.48
Isoamyl caprate 1.67 5.76
Isoamyl laurate 0.78 1.83
2-Phenylethyl caprylate Trace 1.20
2-Phenylethyl caprate 0.25 1.55
Total aromatic esters 73.02 182.65
(¼þ150%)
BRANDY AND COGNAC/Chemical Composition and Analysis of Cognac 603
quickly, even excessively, if kept there for a long time,
whereas a young Cognac in an old cask will change
color more slowly, remaining clear even after several
years. Excess tannin (bitter taste) is often associated
with a very high degree of extracted colour. (See
Tannins and Polyphenols.)
0070 The aromatic aldehydes, mainly vanillin, appear
when the lignin breaks down. They are produced by
aging and are very important for the bouquet of
Cognac. Different types of casks result in substantial
variations to their contents of these substances. These
aldehydes, predominantly vanillin, contribute a
vanilla note, which is not too heavy and is greatly
appreciated.
0071 The stave acts as a selective membrane enabling
exchanges to take place between the Cognac within
the cask and the air in the cellar by way of the evap-
oration of the most volatile and smallest molecules, a
phenomenon that contributes to oxidation.
0072 The quality of a Cognac’s aging is measured by its
‘rancio taste’ (hydrolyses of fatty acid esters together
with oxidation and transformation into ketones). The
formation of the rancio taste during aging is accom-
panied by the continual extraction of tannins, slow
oxidation, and various chemical reactions. (See Fatty
Acids: Properties.)
0073 The conditions necessary to make a good cask
container are:
.
0074 the use of good-quality wood;
.
0075 the use of wood dried in the open air, exposed to
inclement weather for at least 3 years;
.
0076 the careful preparation of a new cask and the judi-
cious racking into older casks after proper storage
of the recently distilled Cognac in new casks.
0077 Important research work on the aging of Cognac
spirits has been a major contribution in identifying
the molecules extracted from the oak by the spirits
and in understanding the reaction mechanisms under-
lying the aging process.
0078 The development of gas–liquid chromatography
(GLC) and high-performance liquid chromatog-
raphy (HPLC) techniques has enabled the analytical
changes noted during aging to be quantified with
greater detail. Several aspects of variations observed
in the aging of Cognacs are shown in Tables 3 and 4
by way of example. The extraction of gallic acid is
much greater in a cask made of coarse grain oak,
35.7 mg l
1
compared with 14.5 mg l
1
in a cask
made of fine grain wood. In an old cask, the extration
of phenolic compounds is less (Table 3), but the
oxidation and exchange with the atmosphere con-
tinue to occur. (See Pesticides and Herbicides: Types,
Uses, and Determination of Herbicides; Phenolic
Compounds.)
0079Cognac is sold throughout the world mainly under
commercial designations; among those currently in
use are III, VSOP, Napoleon, and X.O. These
commercial qualities correspond with products of
different ages. The price increases with age. These
commercial products are obtained through blends
(also called ‘coupes’) of different cognacs. Each dealer
has their own blending technique and style. The blend
of different Cognacs is above all a subjective choice. It
enables a representative product of constant and re-
producible quality to be obtained. Blending is there-
fore an important step in the production of Cognac,
since it is the key link between production and
marketing.
Sensory Analysis
0080Cognac spirits are obtained from different ‘crus’ or
growth zones within the area of designated origin:
Grande Champagne, Petite Champagne, Borderies,
Fins Bois, Bons Bois, and Bois Ordinaires. They
show major sensory differences (see Table 5). The
blending of several crus influences the quality of the
product. (See Sensory Evaluation: Sensory Character-
istics of Human Foods.)
tbl0003Table 3 Effect of cask on a spirit aged 13 years
Concentrations (mg l
1
)
Constituent New cask Old cask
Gallic acid 15.3 5.4
Vanillic acid 2.8 1.0
Syringic acid 7.0 2.2
5-Hydroxymethylfurfural 6.3 1.8
Furfural 21.3 10.8
5-Methylfurfural 1.6 0.2
Vanillin 8.8 2.9
Syringaldehyde 17.6 4.6
Coniferaldehyde 6.7 0.6
Sinapaldehyde 17.0 1.7
tbl0004Table 4 Effect of length of aging period
Concentrations (mg l
1
)
Constituent 0.7 years 5 years 13 years
Gallic acid 4.6 9.0 15.3
Vanillic acid 0.3 1.4 2.8
Syringic acid 0.6 2.6 7.0
5-Hydroxymethylfurfural 4.2 4.2 6.3
Furfural 26.8 24.7 21.3
5-Methylfurfural 1.5 1.4 1.6
Vanillin 0.9 4.4 8.8
Syringaldehyde 2.25 8.9 17.6
Coniferaldehyde 3.65 5.9 6.7
Sinapaldehyde 9.45 17.8 17.0
604 BRANDY AND COGNAC/Chemical Composition and Analysis of Cognac
