Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Dual-Energy X-Ray Absorptiometry
0049 DEXA is a recent addition to the body composition
analysis field. It was originally developed to measure
regional and total-body bone mineral content, but it
is also capable of measuring fat mass. It is more
sensitive than dual-photon absorptiometry, which it
has now replaced, and exposes the subject to substan-
tially lower radiation doses than total-body calcium
measured by neutron activation analysis.
0050 The DEXA technique exposes the subject to low
energy irradiation at two different energies. As there
is differential absorption by tissues of different
densities (bone, lean and fat tissues), values for bone
mineral in the hip, spine, whole body, or specialized
regions, as well as values for fat or soft tissues, can be
derived.
0051 It is a sensitive technique for determining bone
mineral content and densities, and has become the
gold standard for clinical and research work in osteo-
porosis.
0052 Because the fat-free nonbone component of the
limbs effectively measures limb skeletal muscle
mass, of which the proportion to whole-body skeletal
muscle is fairly constant, DEXA can be used to meas-
ure skeletal muscle mass.
Other Techniques
0053 Other techniques for measuring body composition
include CT scanning and MRI. These techniques
have been used for accurate measurement of various
body compartments. These compartments include
visceral adipose tissue mass, increased amounts of
which are related to risk for diabetes and vascular
disease, and skeletal muscle volume. Whilst their clin-
ical and research use is limited by expense, availabil-
ity, and, in the case of CT scanning, patient exposure
to ionizing radiation, much helpful information has
been obtained from the research groups who have
utilized such technology.
Error of the Methods
0054 Each of the methods for measuring body composition
has intrinsic and biological errors. In general, these
are very comparable to the errors of biochemical
methods commonly performed in hospital labora-
tories (Table 3).
Effect of Disease Processes on Human
Body Composition
Obesity
0055 The fat compartment – expressed as mass or percent-
age fat – has importance as an expression of adiposity.
The association between increased adiposity and
morbidity is well documented. The incidence of
diabetes, hypertension, ischemic heart disease, and
gallbladder disease is increased at higher levels of
adiposity.
0056Fat distribution is being increasingly recognized as
a clinically relevant risk factor. Abdominal obesity,
expressed as the abdominal circumference-to-gluteal-
circumference ratio, has been shown, independent of
other factors, to represent an increased risk for mor-
bidity and mortality. First described in the 1950s, this
association has been verified and strengthened by
intense research interest in the last decade. More
recently, the abdominal circumference alone has also
been shown to predict morbidity and mortality. The
correlation is not just an epidemiological one: many
metabolic abnormalities, such as insulin resistance,
are associated with this condition.
0057With increasing adiposity, it is usual to find an
increase in lean mass. This occurs because of the
increased skeletal muscle mass required to carry the
increased fat mass.
0058In obese people who have undergone repeated
near-starvation dieting, there may be marked wasting
of skeletal muscle, even in the presence of adiposity.
Similarly, muscle wasting, or even marasmus, can
occur in the obese person who has concomitant severe
illness. For example, a chronic alcoholic with a poor
quality of food intake and liver impairment may be
obese (excess fat stores) and also have skeletal muscle
wasting.
Undernutrition
0059In global terms, undernutrition remains one of the
greatest determinants of health status. The most
common expressions of undernutrition are commonly
known as kwashiorkor and marasmus. Intermediate
forms are commonly seen. In marasmus, there is a
wasting of total-body protein without expansion of
the TBW compartment. In kwashiorkor, there is an
expansion of the extracellular component of TBW,
giving rise to peripheral edema and ascites. Exactly
why undernutrition results in these two different
forms remains unclear.
0060Both forms are associated with a reduction in the
capacity of the cell-mediated immune system of
the body, giving rise to an increased risk of infec-
tion. Where food intake is reduced, it is likely that
other nutrient deficiencies, such as iron, folate, or
vitamin A deficiency, will be present and may mask
the extent of the underlying body composition
changes.
0061In developing countries, these forms of undernutri-
tion usually arise from the combination of inadequate
food resources and chronic infection. In malnutrition
BODY COMPOSITION 555

caused by inadequate food intake, many studies
have shown an increase in the ratio of extracellular
fluid volume to TBW. Electrolyte abnormalities are
common. Reduced levels of sodium, potassium, and
magnesium may be found. Protein stores, as meas-
ured by IVNAA and expressed as nitrogen index, are
reduced.
0062 In developed countries, these conditions are fre-
quently seen in conjunction with malignancy, psychi-
atric conditions, organ failure, and conditions in
which self-feeding is difficult, e.g., stroke or arth-
ritis. (See Malnutrition: Malnutrition in Developed
Countries.)
0063 The wasting seen in many malignancies, even when
nutritional intake and absorption is apparently ad-
equate, may be caused by cytokines such as cachectin
and tumor necrosis factor.
0064 Visceral mass may be better preserved in cancer
patients, suggesting that loss of muscle accounts for
the major proportion of weight loss. This may be
the result of a difference in metabolic rate between
malignancy and anorexia; the rate is often increased
in patients with malignancy, but reduced in anorexia
nervosa. (See Anorexia Nervosa.)
0065 In anorexia nervosa, many of the body compos-
ition changes seen are similar to those found in
starved subjects. Total-body nitrogen, total-body
potassium, and blood volume are all reduced. Unlike
primary starvation, extracellular water may be re-
duced by relatively greater amounts than the reduc-
tion in TBW owing to induced vomiting or purging.
Interestingly, many patients with anorexia nervosa
maintain normal levels of hemoglobin and serum
albumin and rarely exhibit vitamin deficiencies or
develop edema.
Conclusion
0066 An understanding of body composition and its meas-
urement provides the clinician with further scientific
data on which to base a nutritional assessment.
Advances in this area are now available to provide
accurate measurements which previously could only
be estimated. Some of these techniques are easily
applicable to office general practice or field studies,
whilst others require more sophisticated equipment
which is only available in specialized centres. Body
composition changes in health and disease help to
explain the pathogenesis of illnesses which involve
alteration in food intake or absorption and
metabolism.
See also: Anorexia Nervosa; Malnutrition: Malnutrition
in Developed Countries; Osteoporosis
Further Reading
Alleyne GEO (1975) Mineral metabolism in protein-calorie
malnutrition. In: Olsen RE (ed.) Protein-Calorie Mal-
nutrition, pp. 201–208. New York: Academic Press.
Brozek J, Grande F, Anderson IT and Keys A (1963) Densi-
tometric analysis of body composition: revision of some
quantitative assumptions. Annals of the New York
Academy of Sciences 110: 113–140.
Durnin JVGA and Womersley J (1974) Body fat assessed
from body density and its estimation from skin fold
thickness: measurements on 481 men and women aged
from 16 to 72 years. British Journal of Nutrition 32:
77–97.
Forbes GB (1987) Human Body Composition, pp. 2–171.
New York: Springer-Verlag.
Forbes GB (1990) Body composition. In: Brown M (ed.)
Present Knowledge in Nutrition, 6th edn. Washington,
DC: International Life Sciences Institute.
Forbes GB, Kriepe RE, Lipinski BA and Hodgman CH
(1984) Body composition changes during recovery
from anorexia nervosa: comparison of two dietary
regimes. American Journal of Clinical Nutrition 40:
1137–1145.
Fowler PA, Fuller MF, Glasby CA et al. (1991) Total and
subcutaneous adipose tissue in women: the measurement
of distribution and accurate prediction of quality by
using magnetic resonance imaging. American Journal
of Clinical Nutrition 54: 18–25.
Hastings AB and Eichelberger L (1937) The exchange of
salt and water between muscle and blood. Journal of
Biological Chemistry 117: 73–93.
International Commission on Radiological Protec-
tion(1975) Report of the Task Group on Reference
Man, no. 23. Oxford: Pergamon Press.
Jelliffe EPP and Jelliffe DB (1969) The arm circumference as
a public health index of protein-calorie malnutrition
of early childhood. Journal of Tropical Paediatrics 15:
179–192.
Kvist H, Choudury B, Grangard U, Tyler U and Sjostrom L
(1988) Total and visceral adipose tissue volumes derived
from measurements with computed tomography in adult
men and women: predictive equations. American Jour-
nal of Clinical Nutrition 48: 1351–1361.
Lohman TG (1981) Skin-folds and body density and their
relation to body fatness: a review. Human Review 53:
181–225.
Lukaski HC (1987) Methods for the assessment of human
body assess human body composition. Journal of
Applied Physiology 60: 1327–1332.
Lukaski HL, Johnson PE, Bolonchuk WW et al. (1985)
Assessment of fat-free mass using bioelectrical imped-
ance measurements of the human body. American Jour-
nal of Clinical Nutrition 41(4): 810–817.
Mazess RB, Collick B, Trempe J, Barden HS and Hanson JA
(1989) Performance evaluation of a dual-energy x-ray
bone densitometer. Calcified Tissue International 44:
228–232.
Mazess RB, Barden HS, Bisek JP and Hanson J (1990) Dual
energy X-ray absorptiometry for total body and regional
556 BODY COMPOSITION

bone mineral and soft tissue composition. American
Journal of Clinical Nutrition 51: 1106–1112.
Mazess RB, Hanson JA, Trempe J and Bonnick SL (1991)
Ultrasound Measurement of the Os Calcis. Proceedings
of the 8th International Workshop on Bone Densitome-
try, 28 April, Bad Reichenhall, FRG.
Ross R, Goodpaster B, Kelley D and Fernando B (2000)
Magnetic resonance imaging in human body compos-
ition research: from quantitative to qualitative tissue
measurement. Annals of the New York Academy of
Science 904: 12–17.
Stroud DB, Borovnicar DJ, Lambert JR et al. (1990) Clin-
ical studies of total body nitrogen in an Australian hos-
pital. In: Yasurnura S (ed) In vivo Body Composition
Studies, Basic Life Sciences, vol. 55, pp. 177–182.New
York: Plenum Press.
Vague J (1956) The degree of masculine differentiation of
obesities: a fact for determining predisposition to
diabetes, atherosclerosis, gout, uric calculus disease.
American Journal of Clinical Nutrition 4: 20–34.
Wang ZM, Pierson RN and Heymsfield SB (1992) The five
level model: a new approach to body composition
research. American Journal of Clinical Nutrition 56:
19–28.
Widdowson EM and Dickerson JWT (1964) Chemical
composition of the body. In: Comari CL and Bronner F
(eds) Mineral Metabolism, vol. 2, part A, pp. 2–
247.London: Academic Press.
BONE
K D Cashman, University College, Cork, Ireland
F Ginty, MRC Human Nutrition Research, Cambridge,
UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Bone is a specialized connective tissue that makes
up, together with cartilage, the skeletal system. The
skeleton serves three important functions: (1) mech-
anical; support and site of muscle attachment for
locomotion; (2) protective; for vital organs and
bone marrow; and (3) metabolic, as a reserve for
ions, especially calcium and phosphate; for the
maintenance of serum homeostasis, which is critical
to life.
0002 In bone, as in all connective tissues, the fundamen-
tal constituents are the cells and the extracellular
matrix (the osteoid). Osteoid is particularly abundant
in bone and is composed of collagen fibers and non-
collagenous proteins. Bone is distinguished from
other forms of connective tissue by the fact that it
becomes extremely hard, owing to the deposition
within a relatively soft organic matrix of a complex
mineral substance, largely composed of calcium,
phosphate, and carbonate. Bone is built up during
youth to a peak reached probably in the 20s. It then
diminishes again, first slowly, then more rapidly,
especially in women after the menopause, and in
both sexes in the elderly. The regulation of this skel-
etal growth and development is complex and involves
genetic, mechanical, hormonal, and nutritional influ-
ences. At a cellular level, bone metabolism or remod-
eling is coordinated by a multiplicity of interacting
local hormones, cytokines, and growth factors. Such
bone-associated factors may play a role in the patho-
physiology of bone disorders.
Anatomical Features of Bone
0003Anatomically, two types of bone can be distinguished
in the skeleton: flat bones (i.e., the skull bones,
scapula, mandible, and ilium) and long bones (tibia,
femur, humerus, etc.). External examination of a long
bone (Figure 1) shows the two wider extremities (the
epiphyses), a more or less cylindrical tube in the
middle (the diaphysis or midshaft), and a develop-
ment zone between them (the metaphysis). In a grow-
ing bone, the epiphysis and the metaphysis, which
originate from two independent ossification centers,
are separated by a layer of cartilage, the epiphyseal
cartilage or growth plate, which has an important
role in the longitudinal growth of bones. The external
part of bone is formed by a thick and dense layer of
calcified tissue, the cortex or compact bone, which in
the diaphysis encloses the medullary cavity where the
hematopoietic bone marrow is housed. Cortical bone
makes up about 80% of the total skeleton. Toward
the metaphysis and the epiphysis, the cortex becomes
progressively thinner, and the inner space is filled
with a network of thin, calcified trabeculae: this is
cancellous bone, also named spongy or trabecular
bone. The spaces enclosed by these thin trabeculae
also are filled with hematopoietic bone marrow and
are in continuity with the medullary cavity of the
diaphysis. Cancellous bone is also found in the verte-
brae and the majority of flat bones. It makes up about
20% of the total skeleton. The relative proportions of
cortical and cancellous bone differ at different sites in
BONE 557
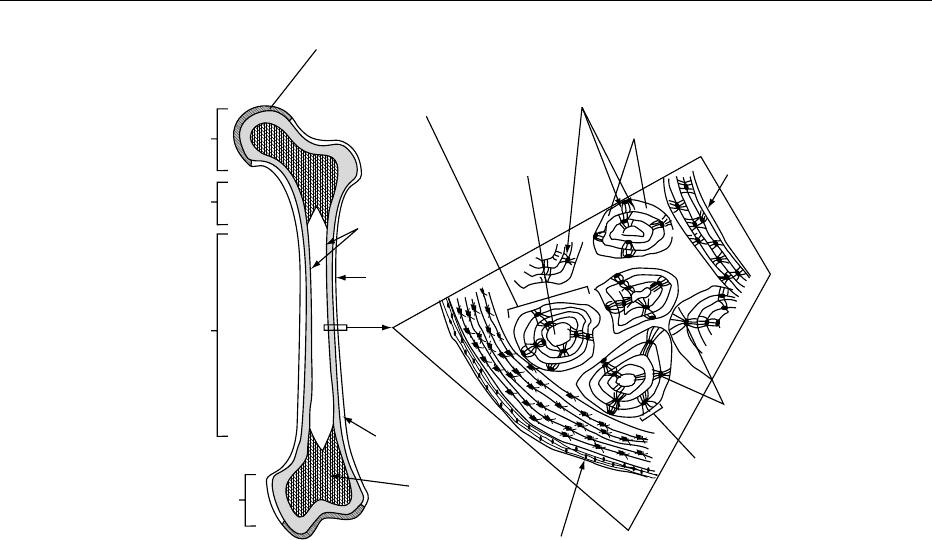
the skeleton (e.g., cancellous bone comprises > 66%
of the total bone in the lumbar spine, but only 25% of
the total bone in the neck of the femur). The bone
surfaces at the epiphyses that take part in the joint are
covered with a layer of articular cartilage that does
not calcify.
0004 There are two bone surfaces at which the bone is in
contact with the soft tissues (Figure 1): an external
surface (the periosteal surface) and an internal surface
(the endosteal surface). The surfaces are lined with
osteogenic cells organized in layers, the periosteum
and the endosteum. Cortical and trabecular bone are
constituted of the same cells and the same matrix
elements, but there are structural and functional dif-
ferences. The primary structural difference is quanti-
tative: 80–90% of the volume of compact bone is
calcified, whereas only 15–25% of the trabecular
bone is calcified (the remainder is occupied by bone
marrow, blood vessels, and connective tissue). The
result is that 70–85% of the interface with soft tissues
is at the endosteal bone surface, which leads to the
functional difference: the cortical bone fulfills mainly
a mechanical and protective function, while the trab-
ecular bone has a metabolic function. Therefore, at a
macroscopic level, bone can be regarded as an outer
cortical sheath and an inner three-dimensional
trabecular network, which together allow optimal
mechanical and biochemical function with minimal
weight.
Bone Matrix and Mineral
0005Chemically, bone is made up of one-third protein
matrix and two-thirds mineral. Bone matrix is
formed by type I collagen fibers (which make up
90% of the total protein of bone) and numerous
noncollagenous proteins (which make up the
remaining 10% of total protein of bone; Table 1).
Type I collagen is a highly cross-linked fibrillar
protein which, through its tridimensional structure
similar to rope, gives bone its tensile strength. The
functions of the noncollagenous proteins are diverse
and some are still poorly understood (Table 1). The
urinary excretion and the plasma or serum con-
centrations of some of these components of the
matrix are used chemically to assess bone metabolism
(Table 1). Spindle- or plate-shaped crystals of
hydroxyapatite Ca
10
(PO
4
)
6
(OH)
2
are found on and
within the collagen fibers. They tend to be oriented in
the same direction as the collagen fibers.
0006The preferential orientation of the collagen fibers
alternates in adult bone from layer to layer, giving to
this bone a typical lamellar structure. The lamellae
can be parallel to each other if deposited along a flat
surface (trabecular bone and periosteum), or concen-
tric if deposited on a surface surrounding a channel
centered on a blood vessel. In cortical bone, the tissue
is mainly organized as Haversian systems which rep-
resent its basic structural building blocks (Figure 1).
Epiphyseal cartilage
Haversian system
Canaliculi
Lamellae
Haversian canal
Endosteum
Osteoblast
Lacuna
Periosteum
Cancellous bone
Compact bone
Periosteum
Endosteum
Epiphysis
Epiphysis
Metaphysis
Diaphysis
fig0001 Figure 1 Schematic view of a longitudinal section through a long bone (insert; transverse section through the diaphysis).
558 BONE

Each Haversian system is made up of a central canal,
the Haversian canal, which contains an artery, a vein,
a lymphatic vessel, a nerve, and some bone cells. This
is surrounded by successive rings of lamellae. Lying
between the lamellae are a number of small cavities,
the lacunae, which contain bone cells, or osteocytes.
Fine protoplasmic processes from the osteocytes
branch profusely throughout the matrix in very
small canal-like structures, the canaliculi (Figure 1).
These serve to interconnect the lacunae. Lamellar
bone is the form present in adult cortical and cancel-
lous bone. However, when bone is being formed very
rapidly (during embryonic development and fracture
healing), there is no preferential organization of the
collagen fibers. They are not so tightly packed and
found in somewhat randomly oriented bundles. This
type of bone is called woven bone. This immature
bone is usually replaced by lamellar bone, so that it
is practically absent from the adult skeleton, except
for some that may persist near tendon insertions and
ligament attachments.
Bone Cells
0007Bone is a dynamic connective tissue, comprising an
exquisite assembly of functionally distinct cell popu-
lations that are required to support its structural,
biochemical, and mechanical integrity and its central
role in mineral homeostasis. Bone tissue (both cor-
tical and cancellous bone) is constantly turned over
by two cell-mediated processes, bone modeling and
bone remodeling. In the former, new bone is formed
at a location different from the one where it was
destroyed (resorbed). This allows bone to elongate
and widen, altering the shape of the skeleton. This
change takes place principally in the child and ado-
lescent, allowing the development of a normal archi-
tecture during growth. It is also the process by which
vertebrae increase in size during life. During adult-
hood, bone remodeling is the primary process of bone
turnover, however, modeling does continue to take
place at certain skeletal sites such as the periosteum.
The physiological repair of fractured bone is also
tbl0001 Table 1 Principal noncollagenous proteins in the bone matrix
Protein type/name Potential function(s)
Serum proteins
Albumin Transports proteins; inhibits hydroxyapatite crystal growth
a-2HS glycoprotein Promotes endocytosis; chemoattractant for monocytic cells; mineralization inhibitor
Glycoproteins
Alkaline phosphatase
a
A phosphotransferase; potential Ca
2þ
carrier; hydrolyzes inhibitors of mineral deposition
Osteonectin May mediate deposition of hydroxyapatite; binds to growth factors; may influence cell-cycle
antiadhesive protein
Tetranectin Binds to plasminogen; may regulate matrix mineralization
RGD-containing glycoproteins
Thrombospondins (I,II,III,IV) Cell attachment
Fibronectin Binds to cells
Vitronectin Cell-attachment protein
Osteopontin Binds to cells; inhibits mineralization; may regulate proliferation; inhibits nitric oxide
synthase; may regulate tissue repair
Bone sialoprotein
a
Binds to cells; binds Ca
2þ
with high affinity; may initiate mineralization
BAG-75 Binds to Ca
2þ
; may act as a cell-attachment protein; may regulate bone resorption
g-Carboxy glutamic acid-
containing proteins
Matrix Gla protein May function in cartilage metabolism, may inhibit mineralization
Osteocalcin
a
May regulate activity of osteoclasts and their precursors; may mark turning point between
bone formation and resorption; regulates mineral maturation
Protein S Protein S-deficiency may result in osteopenia
Glycosaminoglycan-containing
Veriscan May ‘capture’ space that is destined to become bone,
Decorin Binds to collagen and may regulate fibril diameter; binds to TGF-b; inhibits cell
attachment to fibronectin
Biglycan May bind to TGF-b
Fibromodulin Binds to collagen; may regulate fibril formation; binds to TGF-b
Osteoadherin May mediate cell attachment
Hyaluroan May work with versican-like molecule to capture space destined to become bone
a
Their plasma or serum concentrations are used chemically to assess bone metabolism.
RGD, Arg-Gly-Asn; TGF-b, transforming growth factor-b.
Adapted from Lian JB, Stein GS, Canalis E, Robey PG and Boskey AL (1999) Bone formation: osteoblast lineage cells, growth factors, matrix proteins, and
the mineralization process. In: Murray FJ (ed.) Primer on the Metabolic Bone Diseases of Mineral Metabolism, 4th edn, pp. 14–30. Philadelphia, USA:
Lippincott/Williams & Wilkins.
BONE 559

part-achieved by modeling. Bone remodeling involves
resorption and formation of bone and takes place at
the same site (known as the bone-remodeling unit),
leading to no change in bone shape, but allowing
bone rejuvenation, adaptation to stress and strains,
the repair of microfractures and therefore the main-
tenance of mechanical integrity. In addition, bone
remodeling has an important role in the maintenance
of calcium homeostasis. This bone cell-mediated pro-
cess replaces 2–10% of the adult skeleton per annum.
0008 The principal cells that mediate the bone-forming
processes of the mammalian skeleton are the osteo-
blasts that synthesize the bone matrix on bone-
forming surfaces; osteocytes, organized throughout
the mineralized bone matrix, that supports bone
structure; and the protective bone-surface lining
cells. The principal cells that mediate the bone-
resorbing process are the multinucleated giant cells,
known as osteoclasts.
Osteoblasts
0009 Osteoblasts are the cells responsible for the synthesis
and mineralization of type I collagen. They originate
from pluripotent mesenchymal stem cells which, by
following a developmental pathway, they initially
differentiate into preosteosblasts, and then to mature
osteoblasts. A mature osteoblast which is actively
synthesizing bone matrix has many distinctive ultra-
structural characteristics, including a large nucleus,
enlarged Golgi apparatus, and extensive endoplasmic
reticulum. It also has unique biochemical characteris-
tics, including high levels of type I collagen, alkaline
phosphatase, and osteocalcin (Table 1). These cells
form an epithelial-like structure at the surface of
bone where they unidirectionally secrete the osteoid.
In a second step, this matrix than calcifies extracellu-
larly; the role of the osteoblast in this process is still
unclear.
Osteocytes
0010 The osteocytes are the most abundant cells in the
bone matrix, with about 25 000 cells per mm
3
bone.
However, despite their abundance, their function is
still poorly understood. They originate from mature
osteoblasts which become embedded in the bone
matrix after it becomes mineralized. Osteocytes are
physically different from osteoblasts and have long
protoplasmic extensions which permeate the bone
matrix and allow them to interconnect and communi-
cate with other osteocytes and osteoblasts. Recent
research has shown that osteocytes may act as sensors
for stresses and strains within the bone matrix and
thus may be important in the adaptation of bone to
weight-bearing exercise.
Bone-Lining Cells
0011Bone-lining cells are osteoblasts which are not
actively synthesizing bone and thus are known as
resting osteoblasts or surface osteocytes. They occupy
about 80% of the skeletal surface where they act as a
kind of blood–bone barrier. It is thought that they are
essential for the maintenance of blood calcium levels,
perhaps by actively pumping calcium ions from the
bone fluid compartment to the extracellular fluid
compartment.
Osteoclasts
0012Osteoclasts originate from a lineage different from
that of other bone cells, namely the hemopoietic
system, more specifically from the granulocyte–
macrophage colony-forming unit for granulocytes
and macrophages (CFU-GM). Before developing
into mature resorbing osteoclasts the CFU-GM cells
undergo proliferation and further differentiation,
after which they migrate to the bone surface where
they fuse to become large multinucleated cells. These
large, motile, multinucleated osteoclasts, situated
either on the surface of cortical or trabecular bone,
or within the cortical bone, where they are located at
the tip of the remodeling units, are responsible for the
resorption of bone at these sites.
0013Osteoclast differentiation and activation appear to
be primarily regulated by the osteoblast and its pre-
cursors. Active osteoclasts resorb bone in a closed,
sealed-off microenvironment. This is formed by
attachment of the osteoclast to the mineralized sur-
face by a marginal rim of contractile proteins, called
clear zone or sealing zone. This attachment involves
cell membrane receptors, known as integrins, which
recognize specific peptide sequences in the matrix.
The osteoclast surrounds a chamber lined with
membrane folds (the ruffled border) and secretes
self-generated protons (via a proton adenosine tri-
phosphatase) which then dissolve bone mineral.
Proteolytic enzymes, especially cathepsins (cathepsin
K) and collagenases, are also synthesized by the osteo-
clast and are secreted through the ruffled border into
the extracellular bone-resorbing compartment where
they digest collagen. Recent evidence suggests that
the osteoclast undergoes apoptosis (programmed cell
death) after a cycle of resorption.
Integrated Activity of Bone Cells
(Bone-Remodeling Unit)
0014The bone-remodeling process represents the coordin-
ated actions of osteoclasts and osteoblasts on trab-
ecular bone surface and in Haversian systems. Bone
remodeling occurs in the bone-remodeling units,
560 BONE
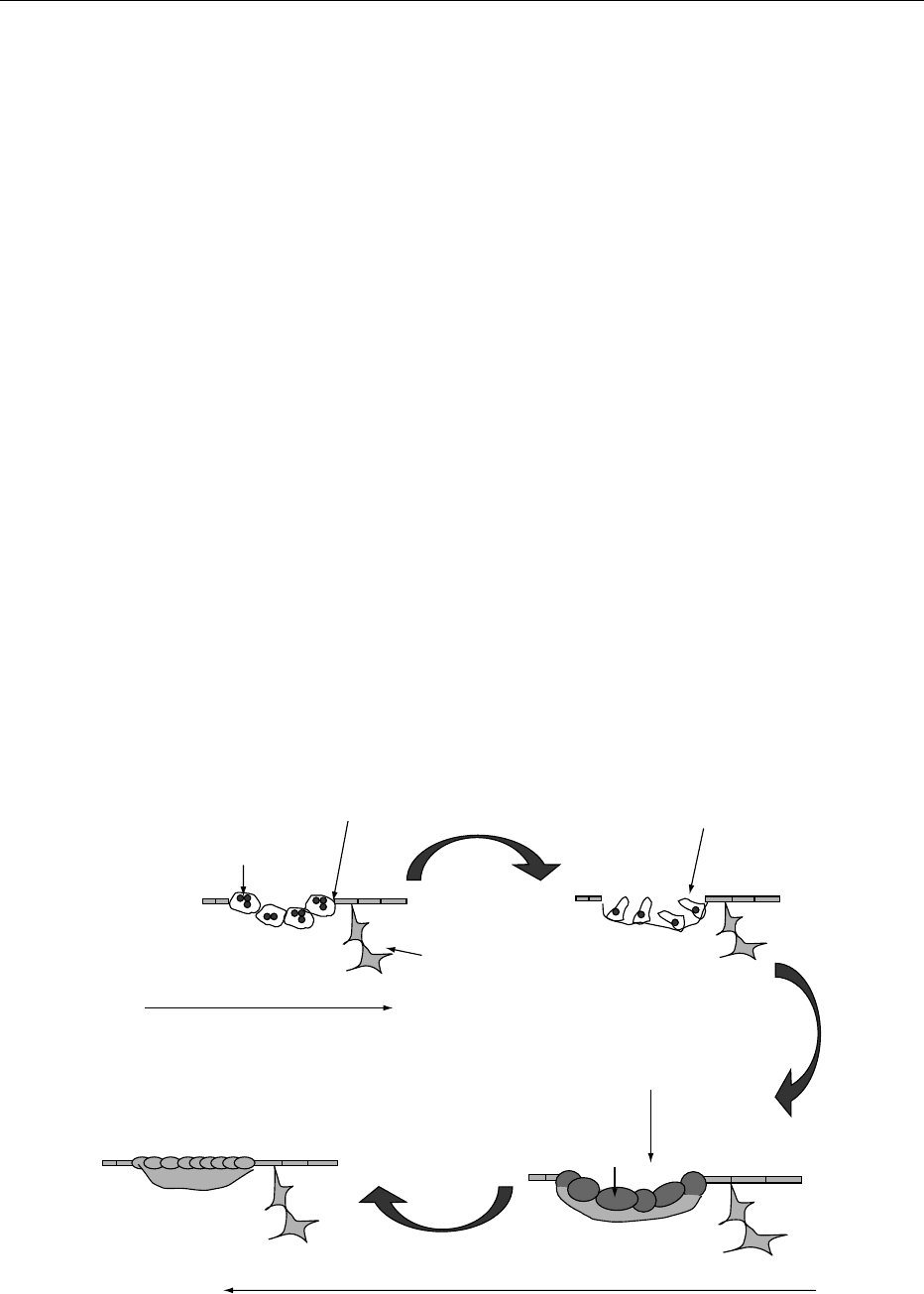
which are focal and discrete packets of bone through-
out the skeleton. The remodeling of each packet takes
a finite period (taking up to 6 months but differing in
cortical and cancellous bone, and probably longer
in cancellous bone). The sequence of cellular activity
– the bone-remodeling cycle – within every bone-
remodeling unit is always identical: (1) activation of
osteoclast precursors (possibly by a member of the
tumor necrosis factor (TNF) ligand family, known
as RANK-L (receptor activator for NF-kB ligand)
released by the osteoblast); and then (2) osteoclastic
bone resorption (over a period of 20 days), followed
by: (3) osteoblastic bone formation to repair the
defect (Figure 2). However, the remodeling that
occurs in each packet is geographically and chrono-
logically separated from other packets of remodeling.
It is believed that this can occur because the acti-
vation of the sequence of cellular events respon-
sible for remodeling is locally controlled, possibly
by local mechanisms in the bone microenviron-
ment. In a healthy adult, it has been estimated that
12 new bone-remodeling units are started every
minute in trabecular bone and 3 per minute for
cortical bone.
Coupling of Bone Formation and Bone Resorption
0015 In most circumstances, the coupling of bone forma-
tion to previous bone resorption occurs faithfully.
Packets of bone removed during resorption are
replaced during formation. The cellular and humoral
mechanisms responsible for mediating this coupling
process (or disrupting it, as in some bone diseases)
are still not clear but may involve an osteoblast-
stimulating factor (such as insulin-like growth factor
(IGF)-I, IGF-II, or transforming growth factor-b).
Remodeling Process: Aging and Disease
0016With aging there is a bone-remodeling imbalance,
with the rate of resorption exceeding that of forma-
tion. Bone formation rates have been shown to be
dramatically decreased with age and the percentage
of bone surface with no activity increases and osteo-
blasts disappear. In cortical bone, the ‘physiologic’
imbalance between the two processes leads to an
increased porosity which, in turn, is followed by
mobilization of bone mineral and a decrease in bone
mass. In trabecular bone there are normal or deeper
resorption cavities that are incompletely filled with
new bone by the osteoblasts, leading to a thinning
of the trabeculae, and, in some cases, a perforation of
the trabeculae. This will remove the structural basis
for the following formative period and the final
result will be a hole in the trabecular network, a loss
of bone and bone mineral, and a reduced strength of
the remaining bone. Therefore, an imbalance of the
remodeling process reduces bone mass and causes
detrimental architectural changes and increases
fracture risk.
0017Therefore, the balance between bone formation
and bone resorption, as effected through cellular
Bone-remodeling unit
Resting phase
Collagen formation and mineralization
Formation
Osteoblasts
Bone formation 3−6 months
Osteocytes
Activation
Reversal
Osteoclast apoptosis
Osteoclasts
Bone resorption 10−20 days
fig0002 Figure 2 Bone-remodeling cycle.
BONE 561

events, is an important determinant of bone mass
of an individual at any stage of the life cycle. In
addition, all of the diseases of bone are superimposed
on this normal cellular-remodeling sequence. In dis-
eases such as primary hyperparathyroidism, hyper-
thyroidism, and Paget’s disease, in which osteoclasts
are activated, there is a compensatory and relatively
balanced increase in the formation of new bone. In
elderly patients with osteoporosis, there is a decrease
in mean wall thickness, presumably reflecting
the inability of osteoblasts to repair adequately the
resorptive defects made during normal osteoclastic
resorption.
Regulation of Bone Cell Activity
0018Bone remodeling is regulated by a variety of systemic
hormones and by local regulatory factors (Table 2)
that affect cells of the osteoclast and osteoblast lin-
eage and exert their effects on: (1) the replication of
undifferentiated cell; (2) the recruitment of cells; and
(3) the differentiated function of cells.
tbl0002 Table 2 Systemic hormonal and local regulation of bone remodeling
Typeof regulatory factor/name Effect(s)
Systemic hormones
Parathyroid hormone (PTH) Stimulates differentiation of committed progenitors to form mature
osteoclasts; activates preformed osteoclasts to resorb bone
Parathyroid hormone-related protein (PTHrP) Effects identical to those of PTH on osteoclasts
1,25 (OH)
2
D
3
Potent stimulator of osteoclastic bone resorption
Calcitonin (CT) Potent inhibitor of osteoclastic bone resorption (transient effect); causes
cytoplasmic contraction of the osteoclast cell membrane; causes
dissolution of mature osteoclasts into mononuclear cells; it can also
inhibit osteoclast formation, inhibiting both proliferation of the
progenitors and differentiation of the committed precursors
Insulin Stimulates bone matrix synthesis and cartilage formation; necessary for
normal bone mineralization
Growth hormone (GH) May regulate bone formation
Glucocorticoids May regulate bone resorption
Sex steroids Estrogen deficiency is associated with increased osteoclastic bone
resorption (either via a direct or indirect mechanism)
Thyroid hormones May stimulate osteoclastic bone resorption
Local factors
Interleukin-1 (a, b) Potent stimulators of osteoclasts; stimulates the proliferation of the
progenitors and differentiation of committed precursors into mature
cells; activation of mature multinucleated osteoclasts
Tumor necrosis factor (TNF) Stimulates the proliferation of osteoclast progenitors, causes fusion of
committed precursors to form multinucleated cells, and activates
multinucleated cells to resorb bone.
Colony-stimulating factor 1 Mediates osteoclast formation
Interleukin-18 Inhibitor of osteoclast formation
Osteoclastogenesis-inducing
differentiation factor (ODIF)
Mediator of osteoclastic bone resorption
Interleukin-6 Weak stimulator of osteoclast formation
Interferon-g Inhibitor of osteoclastic bone resorption; inhibits differentiation of
committed precursors to mature cells
Transforming growth factor-b Inhibits osteoclast formation by inhibiting both the proliferation and
differentiation of osteoclast precursors; directly inhibits the activity of
mature osteoclasts; stimulates osteoblast proliferation and synthesis
of differentiated proteins; increased mineralized bone formation
Insulin-like growth factors (I, II) May enhance bone collagen and matrix synthesis and stimulate the
replication of cells of the osteoblast lineage
Fibroblast growth factors (1, 2) Increases bone cell population capable of synthesizing bone collagen
Platelet-derived growth factors Stimulates bone collagen synthesis
Retinoids Stimulatory effect on osteoclasts
Prostaglandins Complex and multiple effects on osteoclasts; prostaglandins of the E
series may stimulate osteoclastic bone resorption; may act as
mediators of pro-bone-resorptive growth factors
Leukotrienes Linked to osteoclastic bone resorption
Adapted from Lian JB, Stein GS, Canalis E, Robey PG, and Boskey AL (1999) Bone formation: osteoblast lineage cells, growth factors, matrix proteins,
and the mineralization process. In: Murray FJ (ed.) Primer on the Metabolic Bone Diseases of Mineral Metabolism, 4th edn, pp. 14–30. Philadelphia, USA:
Lippincott/Williams & Wilkins.
562 BONE

0019 Systemic regulation of bone turnover is controlled
by numerous hormones, including the mineral homeo-
stasis hormones (parathyroid hormone, calcitonin
and 1,25-dihydroxvitamin D; (See Calcium: Physi-
ology)), estrogen, growth hormone, thyroid hormone,
and others (Table 2). The mineral homeostasis hor-
mones ensure that the skeleton fulfills its metabolic
function as a source of ions, by regulating osteoblast
and osteoclast maturation and activity and thus en-
suring that ions are released or retained by the skel-
eton as necessary. Circulating hormones may act on
skeletal cells either directly or indirectly, modulating
the synthesis, activation, receptor-binding, and bind-
ing proteins of a local growth factor, which in turn
stimulates or inhibits bone formation and bone re-
sorption. It is likely that hormones are important in
the targeting of growth factors to tissue expressing
specific hormonal receptors.
0020 Growth factors may play a critical role in the
coupling of bone formation to bone resorption, and
possibly in the pathophysiology of bone disorders.
The local factors are synthesized by skeletal cells
and include growth factors, cytokines, and prosta-
glandins (Table 2). Growth factors are polypeptides
that regulate the replication and differentiated
function of cells. Growth factors have effects on
cells of the same class (autocrine factors) or on cells
of another class within the tissue (paracrine factors).
Bone Development and Growth
0021 Bone is often described as developing by two different
methods: intramembranous (in membrane) and endo-
chrondral (in cartilage) ossification. The fundamental
process is, however, similar. The osteoid is laid down
by osteoblasts, and this then becomes calcified. The
flat bones (such as the bones of the calvarium of the
skull) are formed by intramembranous ossification,
whereas the basal bones of the skull and the majority
of bones of the skeleton, including the long bones, are
formed by endochrondral ossification. The main
difference between the processes is the presence of a
cartilaginous phase in the latter.
Endochondral Ossification (Development of Long
Bones)
0022 Formation of a cartilage model Long bones begin as
cartilaginous regions in the early embryo. Mesenchy-
mal cells proliferate and differentiate into prochon-
droblasts and then into chrondroblasts. These cells
secrete the cartilaginous matrix. Like the osteoblasts,
the chrondoblasts become progressively embedded
within their own matrix, where they lie within the
lacunae; they are then called chrondocytes. Unlike the
osteocytes, they continue to proliferate for some time,
this being allowed in part by the gel-like consistency
of cartilage. At the periphery of this cartilage (the
perichrondium), the mesenchymal cells continue to
proliferate and differentiate. They lay down a layer
of osteoid, which immediately calcifies, so becoming
a collar of periosteal bone directly in contact with
the cartilaginous model. This is called appositional
growth. Later on, the chrondocytes enlarge progres-
sively, become hypertrophic, and undergo apoptosis.
0023Longitudinal growth, growth in diameter, and shape
modification The embryonic cartilage is avascular.
During its early development, a ring of woven bone is
formed by intramembranous ossification in the future
midshaft area under the perichondrium (which is then
the periosteum). Just after the calcification of this
woven bone, blood vessels (preceded by osteoclasts)
penetrate the cartilage, bringing the blood supply that
will form the hematopoietic bone marrow.
0024The growth plate in a growing long bone has a
proliferative zone at the top, where chondroblasts
divide actively, while their more mature descendants
synthesize the matrix and enlarge, thereby producing
longitudinal growth. Ultimately, the chondrocytes
hypertrophy and calcify their matrix. Osteoclasts pre-
sent in the marrow cavity invade this calcified cartil-
age, destroying the horizontal septa separating the
chondrocytes. After osteoclastic resorption, osteo-
blasts differentiate and form a layer of woven bone
on top of the cartilaginous remnants of the septa. This
is the first remodeling sequence, and cartilage is re-
placed by woven bone. Still lower in the growth plate,
this woven bone is subjected to further remodeling, in
which the woven bone and the cartilaginous rem-
nants are replaced with lamellar bone, representative
of mature trabecular bone. Complete calcification of
the growth plate at the end of puberty marks the end
of longitudinal growth.
0025Growth in diameter of the shaft is the result of a
deposition of new intramembranous bone beneath
the periosteum that will continue throughout life.
The midshaft is narrower than the metaphysis,
and the growth of a long bone progressively destroys
the lower part of the metaphysis and transforms it
into a diaphysis, accomplished by continuous resorp-
tion by osteoclasts beneath the periosteum.
Intramembranous Ossification (Development of
Flat Bones)
0026In intramembranous ossification, a group of
mesenchymal cells within a highly vascularized area
of the embryonic connective tissue proliferates and
differentiates directly into preosteoblasts and then
into osteoblasts. These cells synthesize and secrete
osteoid which is calcified to become woven bone.
BONE 563
Blood vessels incorporated between the woven bone
trabeculae will form the future hematopoietic bone
marrow. Later, the woven bone is remodeled and is
progressively replaced by mature lamellar bone. In
early human fetal life, resorption and apposition
begin to take place so that the cancellous bone occu-
pies the center of the mass while a layer of cortical
bone is formed on each surface by the continuous
addition of new sheets of bone by active osteoblasts.
Osteoclasts resorb bone from the inner surface to
maintain proportional thickness and shape.
Age-Related Changes in Bone Mass
0027 Bone mass in later life depends on the peak bone mass
achieved during growth and the rate of subsequent
age-related bone loss. Therefore, development of
maximal bone mass during growth and reduction
of loss of bone later in life are the two main strategies
of preventing osteoporosis. Several factors are
thought to influence bone mass. These can be broadly
grouped into factors that cannot be modified, such as
gender, age, body (frame) size, genetics, and ethnicity,
and those factors that can be modified, such as
hormonal status (especially sex and calciotropic hor-
mone status), lifestyle factors, including physical
activity levels, smoking and alcohol consumption pat-
terns, and diet. The interaction of these genetic,
hormonal, environmental, and nutritional factors in-
fluences both the development of bone to peak bone
mass at maturity and its subsequent loss.
0028 After the initial formation and development of
bone, and during childhood and adolescence, there
is a rapid linear and appositional skeletal growth, the
former reaching a maximum between ages 15 and 20
years. Bone mass then continues to increase by appos-
itional growth and the peak bone mass is probably
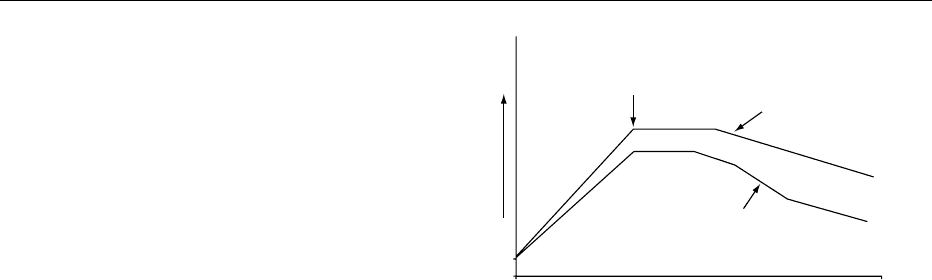
attained during the third decade of life (Figure 3),
although the exact timing is not certain and may
vary between different regions of the skeleton. Peak
bone mass and the subsequent rate of loss of bone are
important determinants of risk of osteoporosis in
later life. The regulation of peak bone mass is not
fully understood but a number of factors have been
identified. Of these, the most important are genetic
influences; other determinants, which are potentially
modifiable, include physical activity, nutritional
factors, and hormonal status:
.
0029 Genetic factors – studies in twins show that 60–
80% of peak bone mass is genetically determined;
there is also some evidence that some aspects of
bone architecture and geometry relevant to bone
strength are inherited. The heritability of peak bone
mass is believed to be polygenic (i.e., influenced by
numerous genes) and there is currently a lot of
interest in identifying these genes.
.
0030Nutritional factors adequate intakes and status of a
number of dietary components and nutrients are
thought to be important in achieving optimal
peak bone mass. These include calcium, phos-
phorus, magnesium, while high salt intakes may
be detrimental.
.
0031Physical activity – physical activity has important
effects on bone growth and architecture during
childhood and adolescence.
.
0032Hormonal status – primary hypogonadism in either
sex is associated with low bone mass, while sec-
ondary amenorrhea in women (due to anorexia
nervosa, excessive exercise, or disease) results in
low peak bone mass.
Age-Related Bone Loss
0033After peak bone mass has been attained, there is a
period of consolidation in which the transverse
diameter of the long bones and vertebrae continues
to increase by subperiosteal appositional growth. The
age at which bone loss commences is uncertain but is
believed to be around the age of 40 years in both men
and women. Bone loss then continues throughout life,
affecting both cortical and cancellous bone through-
out the skeleton. In men, bone loss averages between
0.5 and 1.0% per annum. In women, there is an
acceleration in the rate of bone loss around the time
of the menopause to about 2%, although rates of
bone loss vary widely, from less than 1% to 6% per
annum. In the early postmenopausal years, bone loss
from the spine exceeds that at other sites and overall
it is estimated that, in women, approximately 35%
and 50% of cortical and cancellous bone respectively
are lost from the skeleton over the course of a life-
time.
020
Age (years)
Bone mass
Peak bone mass
Age-related bone loss
Men
Menopausal
bone loss
Women
40 60 80
fig0003Figure 3 Age-related changes in bone mass in men and
women.
564 BONE
