Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

flavors, and create an attractive finish. Sweeteners
also increase tenderness, crust color, volume, and
moisture retention, while maintaining the proper bal-
ance between liquids and solids responsible for prod-
uct contour/shape. Machining properties and baking
characteristics are also closely related to sugar. Sweet-
eners tenderize the finished product by interfering
with gluten hydration and starch gelatinization. (See
Sweeteners: Intensive.)
0012 High-fructose corn (maize) syrups are becoming
increasingly important, but sucrose from sugar cane
or beet is still the major sweetener. Commercial
sugars are often categorized as granulated or
powdered. Granulated sugars range from coating
sugar (extremely fine grain) to coarse. Powdered
sugars are made by grinding granulated sugars and
screening through fine bolting cloths. Generally, as
the size of the sugar crystal increases, the size and
symmetry of the biscuit decrease, while the thickness
and color increase. Often anticaking agents, e.g., corn
starch, are added to insure proper flow of the sugar
during raw material handling. Where pumpability
may offer a processing benefit, granular sucrose can
be combined with water to form ‘liquid sugar,’ usu-
ally at 67% sugar solids or 67
Brix.
0013 Sucrose may be hydrolyzed (inverted) to glucose
(dextrose) and fructose (laevulose) by heating it in
the presence of a dilute weak acid or mixing it with
invertase enzyme. Sucrose is the standard for sweet-
ness, with an arbitrary rating of 100. Fructose and
glusose are rated at 170 and 74, respectively. Their
combined sweetness in a completely inverted sugar
is 127, sweeter than the starting sucrose. Inverted
sugars are used primarily for their hygroscopicity
and browning reactions, which contribute moisture
retention and color development in the biscuits. (See
Carbohydrates: Sensory Properties.)
Shortenings and Emulsifiers
0014Fats are the third major component used in biscuit
making, but are considerably more expensive than
flour or sugar. Besides being used in the doughs, fats
or oils are used as surface sprays, in cream fillings and
coatings (such as chocolate), and as release agents. In
dough, they tenderize (impart shortness to) the crumb
by being dispersed in films and globules during
mixing, which interferes with gluten development.
Shortening also aids dough aeration during the
creaming step. The overall effect improves palatabil-
ity, extends shelf-life, improves flavor and, of course,
adds caloric energy. (See Fats: Uses in the Food
Industry.)
0015Animal fats, primarily lard, were originally used by
bakers. Compound (part animal and part vegetable
source) shortenings and all-vegetable shortenings
were then developed. Soya bean, cottonseed, palm,
coconut, and peanut oils are the primary vegetable
sources used in shortening production. Continued
advancements in purification and hydrogenation de-
veloped vegetable oils that could replace animal fats
with equal or better flavors, melting points, consist-
ency, and availability. Because of current health con-
cerns, most bakeries have switched to fats of plant
origin. (See Vegetable Oils: Types and Properties.)
0016Surfactants (surface-active agents) are given many
names by bakers: crumb softeners, emulsifiers, anti-
staling agents, or dough conditioners. Examples
include lecithin, mono- and diglycerides, diacetyl
tbl0002 Table 2 Representative formulae for various cracker types
Ingredient Snack cracker % Soda (saltine) % Graham %
Strong flour 66.37 69.60 25.40
Soft flour NA NA 25.40
Graham flour NA NA 12.60
Water 18.10 21.58 16.40
Granular sugar 3.85 NA 7.62
Shortening 3.85 6.61 5.08
Ammonia bicarbonate 2.05 NA 0.38
Corn syrup 1.90 NA NA
Malt syrup 1.28 0.64 0.44
Brown sugar or honey NA NA 4.64
Meal 0.96 NA NA
Sodium bicarbonate 0.71 0.44 0.63
Salt 0.60 0.97 0.89
Monocalcium phosphate 0.30 NA 0.25
Protease 0.03 NA NA
Lecithin NA NA 0.25
Optional seasonings As desired NA 0.02
Yeast NA 0.16 NA
NA, not applicable.
BISCUITS, COOKIES, AND CRACKERS/Chemistry of Biscuit Making 535

tartaric acid esters of fatty acids, polysorbate 60 and
sodium stearloyl 2-lactylate. Surfactants at low con-
centrations act to modify the surface behaviors of
liquids. They are believed to complex with the pro-
tein–starch structure, thereby strengthening the film,
and to delay dough setting during baking. The behav-
ior of surfactants is due to their amphoteric (possess-
ing both hydrophilic and hydrophobic molecular
regions) properties. Their behavior varies according
to the charges on the molecules, their solubility, the
hydrophilic–lipophilic balance, and the type of
functional groups involved. (See Emulsifiers: Uses in
Processed Foods.)
0017 Surfactants modify dough consistency and reduce
stickiness by reacting with the gluten. The greasiness
of biscuits with high fat content is also reduced by
surfactants. Crumb softeners also complex with the
starch molecules to delay retrogradation and texture
staling. The grain pattern and volume of the finished
product are often improved, as surfactants increase
dough gas-retaining properties.
0018 Antioxidants retard the development of oxidative
rancidity during product storage. All fats are subject
to oxidative or hydrolytic rancidity which causes ob-
jectionable odors and flavors, but antioxidants delay
these reactions from occurring within the biscuits’
shelf-life. They are usually added to bulk shortenings
and are important for preserving low-moisture prod-
ucts, which are expected to remain edible for several
months. (See Antioxidants: Natural Antioxidants;
Synthetic Antioxidants.)
Is Water an Ingredient?
0019 Water is often thought of as a processing aid or cata-
lyst, rather than as an ingredient. It is incorporated at
the dough stage but driven off during baking. Water
functions in several ways, including hydrating flour
proteins and starch, dissolving sugars, salts, and vari-
ous leavening chemicals, aiding in ingredient distribu-
tion and helping control dough temperature.
0020 A dough’s consistency is directly related to its water
content, or absorption. Many factors affect dough
absorption. Approximately 46% of flour’s total
absorption is associated with the starch, 31% with
protein, and 23% with the pentosans. Acceptable
consistency can be obtained only after sufficient
water is present to hydrate the flour. This is regarded
as bound water and controls the dough’s consistency.
As bound water layers are ‘stacked up,’ some of the
water is held less and less strongly, resulting in water
that can escape (evaporation) and/or migrate as
free water. Water activity (a
w
) is an important way
to measure and monitor water’s mobility in baked
products.
Making Biscuits Lighter
0021Leavening agents aerate the dough or batter to make
it light and porous. The leavening action is respon-
sible for good volume, improved eating quality, and a
uniform cell structure. Leavening can be achieved by
various methods, including yeast fermentation, the
mechanical incorporation of air by mixing and
creaming, formation of water vapor during baking,
and the creation of carbon dioxide and/or ammonia
by chemical leaveners. However, creation of the
initial air bubbles during the mixing phase is critical
before any of the other leavening agents can take
effect. (See Leavening Agents.)
0022Small products like biscuits that bake quickly need
a fast-acting leavener that will release the gas before
the structure sets. The most widely used source of
carbon dioxide in chemically leavened systems is the
reaction of sodium bicarbonate or baking soda
(NaHCO
3
) with an acid, usually the acidic salt of
a weak mineral acid. The leavening acid promotes a
controlled and nearly complete evolution of carbon
dioxide from sodium bicarbonate in an aqueous solu-
tion. Some examples include monocalcium phosphate
monohydrate (CaH
4
PO
4
)
2
.H
2
O), sodium acid pyro-
phosphate (Na
2
H
2
P
2
O
7
) and potassium acid tartrate
(KHC
4
H
4
O
6
). When these agents combine with
water, they react to form controlled amounts of
carbon dioxide. Sodium bicarbonate also raises
dough pH.
0023Ammonium bicarbonate (NH
4
HCO
3
) generates
carbon dioxide, ammonia, and steam when heated.
It increases spread and gives a larger, more desirable
surface ‘crack’ in some types of hard, high-sugar bis-
cuits. However, it can be used only with low-moisture
biscuits that are baked sufficiently to drive off all
residual ammonia.
Other Ingredients
0024Milk products, eggs, and salt are added for variety.
Milk and eggs are viewed as wholesome ingredients
by consumers; however, they are among the most
expensive.
0025Milk and whey are good sources of protein and
lactose, which aid in shape retention and browning
reactions. They add flavor and nutrients, improve
texture, crust color, moisture retention, and control
spread. They are usually added in dried form. (See
Whey and Whey Powders: Production and Uses.)
0026Eggs contribute color, structure, nutritional value,
and some flavor. They affect texture as a result of
their emulsifying, tenderizing, leavening, and binding
actions. The form of eggs used can be fortified or
whole components (yolks and whites) in the liquid,
frozen or dried state, or combined with sugar.
536 BISCUITS, COOKIES, AND CRACKERS/Chemistry of Biscuit Making
0027 Salt performs two principal functions in biscuit
doughs. The first is flavor. It accentuates or potenti-
ates the flavor of other ingredients (e.g., the sweetness
of sugar is emphasized), and it removes the flatness or
lack of flavor in other foods. Moreover, salt has a
slight effect on the consistency of hard doughs,
because it has a strengthening effect on gluten. Salt
also controls fermentation and aids in suppressing
undesirable bacteria.
0028 Minor ingredients include malt, proteases, mold
inhibitors, spices and flavorings. Though used in
relatively small amounts, these ingredients have
quite important effects on the sensory and physical
qualities of biscuits.
0029 Malt is prepared from barley by sprouting it, then
drying it at controlled temperatures. Diastatic malt
provides enzymes that break starch into simple sugars
and add flavor and color. If the malt is heated suffi-
ciently to inactivate the (amylase) enzymes, for use as
a flavorant, it is called ‘nondiastatic.’ (See Malt:
Chemistry of Malting.)
0030 Proteases are important in crackers (low sugar).
They are often added to modify the gluten frame-
work. The effect of a protease is to make the dough
less elastic, so that shrinkage does not occur during
sheeting and cutting. Proteases may occasionally be
used in cookie production. A dough containing gluten
that is too strong will decrease biscuit spread, so
proteases can improve the spread ratio. (See Enzymes:
Uses in Food Processing.)
0031 Mold inhibitors are not generally used in low-
moisture cookies and crackers, but some types
which are higher in moisture may benefit from their
inclusion. Other food products used in biscuits (i.e.,
fillings, toppings, and creams) may require an
inhibitor. Some examples include sodium diacetate
(Na
2
C
2
H
3
O
2
.HC
2
H
3
O
2
. ½H
2
O), calcium propion-
ate (Ca(CH
3
CH
2
COO)
2
) and sodium propionate
(CH
3
CH
2
COONa).
Added Flavors
0032 Our choice of food is largely influenced by taste and
flavor. Smell, taste, touch, and sight are all influenced
by the chemical and physical properties of the food.
Sweet bakery foods are often selected for this reason.
Therefore, selecting the correct flavor is extremely
critical. Selection of the best flavor is most often
achieved through experience and trial and error. (See
Flavor (Flavour) Compounds: Structures and Charac-
teristics.)
0033 Biscuits may be flavored in one or more of three
ways: adding the flavoring to the dough before
baking; dusting or spraying the flavor on after baking;
or by flavoring a nonbaked portion such as cream
filling, icing, or jam that is applied after baking.
0034Spices are aromatic vegetable products (tree bark,
seeds, fruits, and roots), and are usually finely
ground. They improve quality through smells and
tastes. The most commonly used spices are cinnamon,
mace, nutmeg, caraway, anise, allspice, poppy seed,
coriander, ginger, cloves, and fennel.
0035Flavorings are alcohol extracts from fruits or
beans. Vanilla is the most common because it blends
with and enhances other flavors. Flavorings, such as
vanilla or almond, can be either natural or synthetic.
Because flavorings are volatile, much of them may be
lost during baking.
Added Colors
0036Color additives are used in biscuits and in fillings,
icings, and coatings to create a perception of quality
and richness. (See Colorants (Colourants): Properties
and Determination of Natural Pigments; Properties
and Determinants of Synthetic Pigments.)
Chemical Changes During Baking
0037Biscuits are usually baked in a tunnel oven. The
dough pieces are placed on either a flexible metal or
wire mesh band that travels continuously through the
oven’s length, which may reach 100 m. Baking is
controlled by varying the temperatures in the individ-
ual zones within the oven. It is not uncommon for the
drier to consist of 5–7 zones. Baking time is regulated
by the band speed. Crackers and some biscuits are
docked by pressing blunt pins into the dough sheet.
Docking dough pieces before baking creates air pas-
sages through the crust and seals the top to the
bottom, reducing big blisters.
0038Dough undergoes several changes during baking
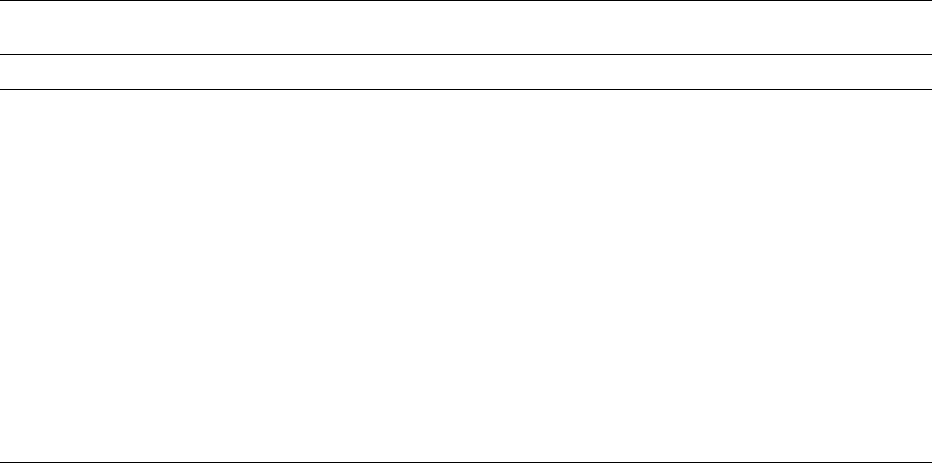
(Figure 2). Changes in dimension and texture, loss
of moisture, and color and flavor development are
the most important. Baking is divided into three
phases. The first involves dough expansion and the
start of moisture loss. Dough expansion and water
Baking time
Property
fig0002Figure 2 Physical changes in biscuits during baking. Key:
—
.
—, color; —, thickness;
...
, weight.
BISCUITS, COOKIES, AND CRACKERS/Chemistry of Biscuit Making 537

loss reach maximum rates and colour development
starts during the second phase. The third phase con-
cludes baking with a lower rate of moisture loss,
thinning of the biscuit, and increasing surface color.
Dimension and Texture Changes
0039 As dough pieces enter the oven, the biscuit’s internal
temperature rises, and the sugars and fats melt. The
complex matrix contains liquified fat and dissolved
sugars. Water evaporation causes the solution to
become more concentrated, and the dough tempera-
ture continues to increase. Leaveners and steam cause
the dough volume to expand. With sufficient heat,
limited protein denaturation and starch swelling
occur, helping to create some structure. However,
the high sugar and fat content inhibit full starch
gelatinization.
0040 Doughs rich in fat and sugar, but containing little
water, often have unhydrated proteins and ungelati-
nized starch when baked. With this formulation, a
rigid structure cannot be achieved, and is therefore
replaced by a soft, sugary matrix. During baking, the
dough expands greatly via steam and leavening, but
does not set properly, resulting in collapse of the
biscuit upon removal from the oven. This expan-
sion–collapse effect is responsible for the characteris-
tic cracked surface of some biscuits.
Moisture Loss
0041 Dough moistures average 11–30% before baking and
1–5% after. Moisture can be lost only from the biscuit
surface. It migrates from the center to the surface of
the biscuit by capillary action and diffusion. The free
water is evaporated very readily, but part of the
bound water will also be liberated, in association
with color development. Moisture content and
water activity are critical parameters, as both directly
influence storage life.
Color Changes
0042 One of the most important color reactions is the
Maillard reaction. It involves the interaction of redu-
cing sugars with amino groups in the proteins, mainly
from lysine, and produces an attractive reddish-
brown hue. It is also associated with the dextriniza-
tion of starch and the caramelization of sugars. These
reactions require very high temperatures, which are
reached only at the biscuit surface. (See Browning:
Nonenzymatic.)
Storage and Staling
0043 Most freshly baked biscuits are cooled before pack-
aging or secondary processing, e.g., icing or sand-
wiching with a cream filling. Snack crackers are
sprayed with oil and salted/seasoned prior to cooling.
The cooling period is usually 15–2 times longer than
the baking period. During cooling and storage, the
biscuit continues to undergo texture changes. Hard
dough biscuits are rigid and crisp immediately after
baking. Soft dough products are still flexible at the
end of baking, but become firm and crisp after
cooling, as a result of sugar recrystallization and
glass transition temperature.
Changes in Moisture Distribution
0044The residual moisture is not uniformly distributed as
a biscuit leaves the oven. Most of the moisture lies in a
lamella near the center, leaving the surface and the
outer periphery almost dry.
0045‘Checking’ is a change associated with uneven
moisture distribution. Dimensional changes within
the biscuit after baking cause it to crack. The center
shrinks as it loses moisture, but the rim expands as it
absorbs moisture. Checking can happen in almost any
type of biscuit or cracker, but is most commonly
found in semisweet products.
See also: Bread: Chemistry of Baking; Browning:
Nonenzymatic; Carbohydrates: Sensory Properties;
Colorants (Colourants): Properties and Determination of
Natural Pigments; Properties and Determinants of
Synthetic Pigments; Emulsifiers: Uses in Processed
Foods; Enzymes: Uses in Food Processing; Fats: Uses in
the Food Industry; Flavor (Flavour) Compounds:
Structures and Characteristics; Flour: Roller Milling
Operations; Analysis of Wheat Flours; Dietary
Importance; Malt: Chemistry of Malting; Vegetable Oils:
Types and Properties; Whey and Whey Powders:
Production and Uses
Further Reading
Blanshard JMV, Frazier PF and Gailliard T (1986) Chemis-
try and Physics of Baking. London: Royal Society of
Chemistry.
Fennema OR (1976) Principles of Food Science. New York:
Marcel Dekker.
Manley DJR (1983) Technology of Biscuits, Crackers and
Cookies. Chichester: Ellis Horwood.
Matz SA (1991) The Chemistry and Technology of Cereals
as Food and Feed, 2nd edn. New York: Van Nostrand
Reinhold/AVI.
Matz SA (1992) Cookie and Cracker Technology, 3rd edn.
McAllen, TX: Pan-Tech International
Pomeranz Y (1978) Wheat Chemistry and Technology, 2nd
edn. St Paul, MN: American Association of Cereal
Chemists.
Pyler EJ (1988) Baking Science and Technology, 3rd edn.
Kansas City, MO: Sosland.
Smith WH (1972) Biscuits, Crackers, and Cookies: Tech-
nology, Production, and Management. Barking: Applied
Science.
538 BISCUITS, COOKIES, AND CRACKERS/Chemistry of Biscuit Making

Stauffer CE (1990) Functional Additives for Bakery Foods.
New York: Van Nostrand Reinhold/AVI.
Sultan WJ (1986) Practical Baking, 4th edn. Westport: AVI.
Wade P (1988) Biscuits, Cookies and Crackers, New York:
Elsevier.
Wafers
K Tiefenbacher, Vienna, Austria
Copyright 2003 , Elsevier Science Ltd. All Rights Reserved.
Background
0001 Wafers – thin, crisp and precisely shaped products
from cereals – are a very special part of the biscuits,
cookies, and crackers area. The name covers several
types of wafers, quite different in recipes, manu-
facturing equipment, and end use. The fundamental
steps of making flat wafers, hollow wafers, molded
cones, rolled wafer cones, and wafer sticks are out-
lined in some detail.
0002 Concepts like water activity and glass transition
are of critical importance here. Moreover, the func-
tion of wafers as a part of combined food products is
discussed. Finally, some important trends in both
products and manufacturing equipment should be
noted.
Main Wafer Features
0003 Wafers are baked as sheets, cones, and sticks or with
different fancy shapes. The characteristic features
with respect to other bakery products are:
1.
0004 Wafers are very thin biscuits; the overall thickness
is usually between less than 1 and 5 mm. They
often have a typical ‘wafer pattern’ on one surface
or on both. The surfaces are smooth and precisely
formed, with the dimensions and all the details –
engravings, logos, etc. – of the baking molds. See
Figure 1 for some examples.
2.
0005 Wafers are cereal-based low fat products made of
wheat flour, sometimes with addition of other
flours or starches. The product density is in the
range of 0.10–0.25 g cm
3
. In cross-section, the
wafer matrix is highly aerated and primarily of
gelatinized starch.
3.
0006 Wafers, by their typical delicate and crisp texture,
combine well with different fillings (cream, ice-
cream, foam) and coatings.
There is sometimes confusion in terminology between
the crisp ‘wafers’ as described here and ‘waffles,’
which are of a soft, cake-like texture but show some
kind of wafer pattern, too.
Basic Wafer Types
0007There are two basic types of wafers:
1.
0008No- or low-sugar wafers. After baking, these con-
tain from zero to a very low percentage of sucrose
or other sugars. Typical products are flat and
hollow wafer sheets, molded cones, cups, and
fancy shapes.
2.
0009Higher-sugar wafers. Well over 10% of sucrose
or other sugars are responsible for the plasticity
of the hot, freshly baked sheets. These are formed
into different shapes before sugar recrystallization
occurs. Typical products are rolled sugar cones,
rolled wafer sticks or tubes, and deep-formed
fancy shapes.
(a)
(b)
(c)
(d)
fig0001Figure 1 Wafer products.From top to bottom: (a), molded cones;
(b), flat wafers, creamed/enrobed; (c), hollow wafer pieces;
(d), wafer sticks.
BISCUITS, COOKIES, AND CRACKERS/Wafers 539
As in both wafer types, the main ingredient is flour,
wafers fit very well into current dietary recommenda-
tions to consume more cereals. They are high-carbo-
hydrate, low fat products. (See Cereals: Dietary
Importance.)
0010 The baking of ‘wafers’ between hot metal plates
has been known since medieval times, but these first
wafers were more similar to our waffles or pancakes
in their high fat and egg contents and their texture.
0011 Modern wafers are low-fat cereal products, very
similar to the altar breads for Christian churches,
and are basically made out of flour and water. The
first wafer ovens were used after World War I, but
more automatic manufacturing lines have been avail-
able since the mid-1950s.
Wafer Recipes
001 2 Before creating a wafer recipe, two main questions
have to be answered:
1.
0013 What is the end use of the wafer? If it is part of
a cream-filled, chocolate-covered biscuit, where
contributing a crisp texture element is far more
important than the taste of the wafer itself, recipes
with few components are recommended. If the
wafers are consumed directly as wafer bread or
wafer sticks, more sophisticated recipes are
chosen.
2.
0014 What kind and quality of raw materials are avail-
able? Low- to medium-protein soft wheat flours
with a low water absorption work best, especially
for no-sugar wafers. Problems with suboptimal
flours must be balanced by variations in minor
ingredients in the recipe. The use of wholemeal
flour is possible, and in some regions, other cereals
such as rice or corn are used for wafer production.
Table 1 lists the common ingredient ranges for both
types of wafer.
In-line Manufacturing of Wafer Biscuits
0015Wafer biscuits are the most important products by
volume. To explain the different steps of manufactur-
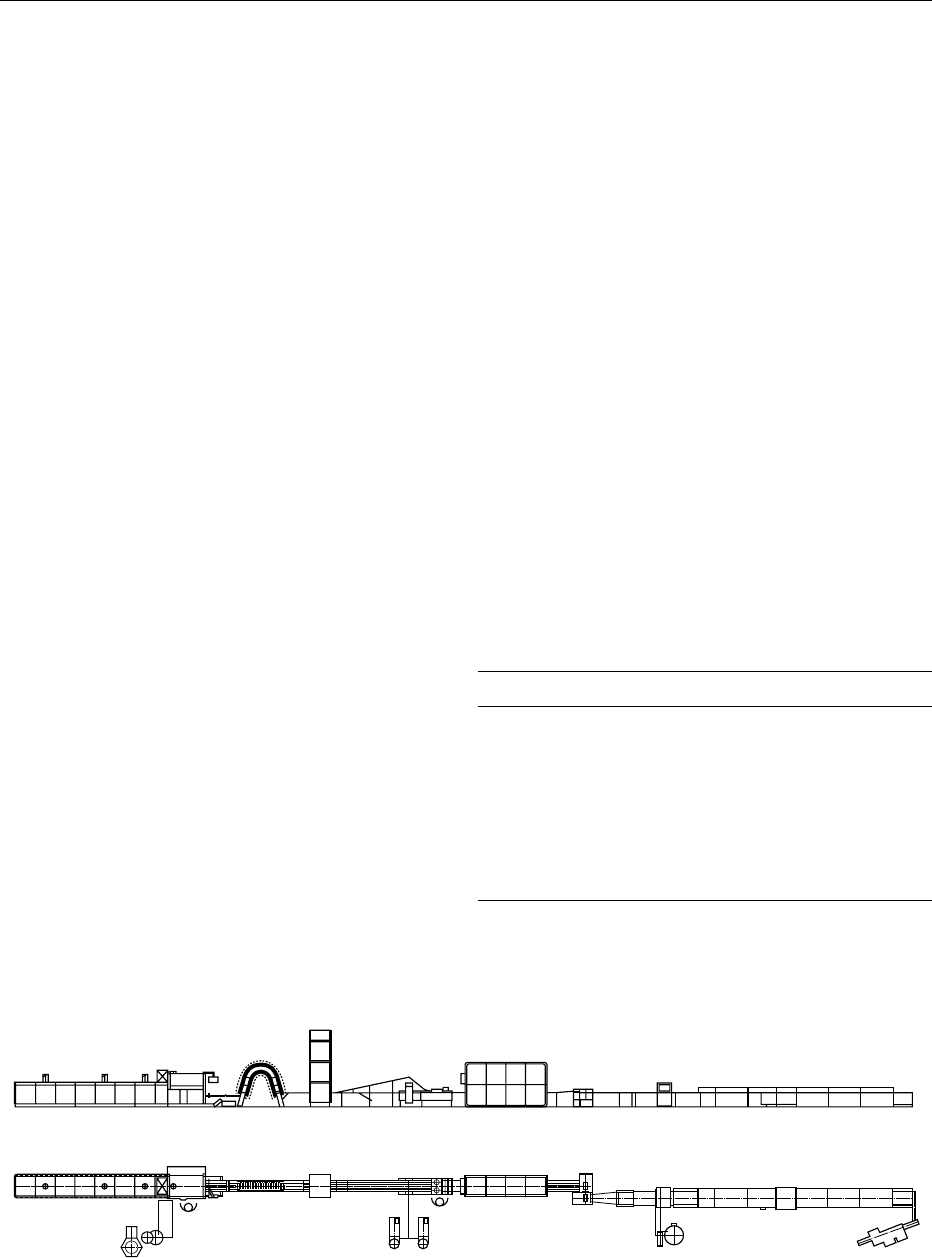
ing, we follow Figure 2.
Batter Preparation (Figure 2, process 1)
0016Wafers are made from a fluid batter with a typical
viscosity in the range of 300–2000 mPs. First, the
water-soluble components are dissolved. By adding
the farinaceous ingredients, a homogenous sus-
pension, the ‘wafer batter,’ is obtained within a few
minutes of mixing.
Batter Transport and Depositing (Figure 2,
process 2)
0017From an intermediate tank via a ring main, the batter
is pumped to the oven and spread on to the baking
molds by a depositor head.
Wafer Sheet Baking (Figure 2, process 3)
0018The baking of wafer sheets is performed in ‘tongs,’
i.e., pairs of cast-iron metal plates with a hinge and
tbl0001Table 1 Wafer batter ingredient ranges (weight parts, flour
¼100)
No (low)-sugar wafer Higher-sugar wafer
Wheat flour 100 100
Water 120–160 100–140
Starch 0–12 0–5
Sucrose 0–4 25–75
Oil/fat 0.5–2 1–6
Milk powder 0–2 0–2
Soya lecithin 0.2–1 0.2–1,5
Salt 0–0.6 0–0.6
Sodium bicarbonate 0.1–0.5 0–0.3
Optional minor ingredients: other cereal flours, soya flour, other sugars
and syrups, egg-based ingredients; whey powder, yeast, caramel color,
cocoa powder, colors, ammonium bicarbonate, enzymes.
3
2
1
45 6 7 8 9
10
fig0002 Figure 2 Manufacturing line for creamed and enrobed wafer biscuits from flat wafers. (1), batter preparation; (2), batter transport
and depositing; (3), wafer sheet baking; (4), wafer sheet cooling; (5), conditioning of sheets; (6), creaming and wafer book building;
(7), cooling and cutting of books; (8), enrobing; (9), cooling of enrobed pieces; (10), packaging.
540 BISCUITS, COOKIES, AND CRACKERS/Wafers

latch on opposite sides. Baking plate sizes up to
350 730 mm are available. The precisely machined
baking plates carry reedings or other engravings. We
call the resulting wafers ‘flat’ wafer sheets, with an
overall thickness of no more than 2–5mm.
001 9 But such baking plates can also carry special figures
(nuts, sticks, hemispheres, fancy shapes) up to a depth
of approximately 20 mm, thus yielding the so-called
‘hollow’ wafer sheets.
002 0 Modern wafer-baking plates often are surface-
plated, e.g., with chromium for easier release and
reduction of cleaning stops. The plates are edged
with metal strips to give a closed baking mold, except
for small venting channels for steam release. Wafer-
baking ovens frequently have 32–104 pairs of plates,
continuously circulating on a chain. They are mostly
gas, sometimes electrically heated and operate at
mold temperatures between 160 and 190
C.
0021 The baking process Within a few seconds after
batter deposition, the baking molds close and are
locked. At first, the batter is distributed mechanically,
but then the mold is filled completely by the steam
that evolves. A small quantity of batter is extruded as
baking waste ‘bobbles’ through the venting channels.
As gelatinization of starch starts immediately, the
pressurization of the mold by steam occurs at the
right moment, resulting in a well-aerated starch foam.
002 2 When most of the water has been driven off, the
glass temperature of the wafer matrix rises, and
the stable structure is formed. The temperature of
the wafer increases to 160–190
C, the temperature
of the baking mold. Then, from Maillard reactions,
the typical wafer color and flavor develop. (See
Browning: Nonenzymatic.)
002 3 The overall baking times are between 1.5 and 2.5
min, depending on the wafer thickness and baking
temperature.
002 4 During the wafer manufacturing process, there is
no substantial degradation of starch molecules com-
pared with other bakery products such as extruded
cereals. Therefore, wafers have two unique textural
properties:
1.
0025 Extreme crispness on biting and initial chewing.
2.
0026Good mouth feel during prolonged chewing and
swallowing owing to the absence of sticky, glutin-
ous stimuli.
Wafer Release and Cooldown (Figure 2, process 4)
0027At one end of the oven, the plates open to release the
baked sheets and to spread fresh batter, and then
reclose very quickly. The sheets are cooled to room
temperature while passing over an arch-type sheet
cooler.
Wafer Conditioning (Figure 2, process 5)
0028Next, the wafers optionally pass a conditioning unit,
where the moisture content of the sheets is carefully
increased so as to achieve some stability in both the
texture and size of the wafer.
0029Moisture sorption and wafer texture After baking,
the residual moisture is 0.8–1.5%. Looking to the
sorption isotherm, this corresponds to a water activ-
ity of around 0.1 or even lower. As both the water
activity of the air in the production area and the
water activity of filling creams or coatings are well
above that, wafers pick up moisture very easily. In
line with this sorption, the dimensions of the sheet
increase by 0.2–0.3% for every 1% of additional
moisture. This can result in cracking of the coating
in enrobed wafer biscuits.
0030To compensate for the low water activity, humidity
conditioning up to approximately 4.5% wafer mois-
ture is possible. This is recommended, especially if
enrobed or chocolate molded wafer products are
made, in order to anticipate this first dimension
increase and to avoid any cracking of the coating
during its shelf-life.
0031Moreover, with increasing water activity, the wafer
texture changes from a soft to a harder crispness,
accompanied by a higher mechanical stability, which
is good both for handling and for the final product
texture. Up to 5–6% moisture content, the wafer
sheets keep their typical crisp texture, but higher
moisture levels will result in most cases in inadequate,
tough, or even soft and soggy textures (see Table 2 for
details).
tbl0002 Table 2 Water activity, water content, and texture of wafers
Wafer condition Wafer texture Water activity, approx. Moisture (%), approx.
Freshly baked Very tender, crisp <0.1 <2
After conditioning Crisp, harder 0.3 4.5
Limit of crispness Crisp to tough 0.5/0.55 6
Wafers, foam-filled Soft to flexible 0.7 12
Collapse of structure Very soft, shrinks >0.85 >20
BISCUITS, COOKIES, AND CRACKERS/Wafers 541

Creaming and Book Building (Figure 2, process 6)
0032 The sheets then pass the creaming station, where a
layer of a sugar- and fat-based cream is applied to one
side. The type of cream flavor liked most varies re-
gionally, but chocolate, vanilla, hazelnut, milk, straw-
berry, and lemon are the most common. Creaming is
done at temperatures of 30–40
C either by contact
spreading or by depositing a preformed cream film.
For flat wafers, several creamed sheets together with
a noncreamed top sheet form a so-called ‘wafer book.’
0033 For hollow wafers, the cream is added to the hollow
parts either by spreading or by controlled single
depositing. Again, either two hollow wafer sheets or
a hollow and a flat sheet are combined to form a
‘book.’
Cooling and Cutting (Figure 2, process 7)
0034 The wafer books pass a cooling tunnel to set the
cream, after which they are wire or saw-cut into
small biscuits.
EnrobingorMoldinginChocolate,Cooling(Figure2,
processes 8 and 9)
0035 The cut biscuits may be enrobed with chocolate-type
coatings, sometimes after the application of chopped
nuts or crispies to the top wafer. Molding in chocolate
is another possibility. After a final cooling step, the
biscuits are ready for packaging.
Packaging (Figure 2, process 10)
0036 The biscuits have to be packed tightly to protect
against humidity, but also against oxygen and light
to prevent oxidative deterioration and to insure a
shelf-life of 6–9 months. Inadequate packaging-film
moisture barriers and bad sealing are the most fre-
quent reasons for later complaints by customers.
Laminated or specially coated films are used for the
typical wafer product packaging in flow packs,
boxes, or bags.
Manufacturing of Molded Wafer Cones
0037 Another type of ‘hollow’ wafers, cones, cups, and
fancy shapes with up to 185 mm in length are pro-
duced in cast-iron molds. Holes for four to six items
are provided in each of these molds, and 12–72 molds
are circulated in one oven. The lower part of the mold
is made of two symmetric halves that open to release
the baked pieces. After their reclosure, they take up a
fresh batter deposit, and finally the ‘core,’ the upper
part of the mold, closes the mold for a new baking
cycle. The baked cones are cooled down and stacked
for packaging.
0038Recipewise, there are two groups of molded cones:
1.
0039No- or low-sugar cones, generally known as ‘cake
cones,’ whose recipes are similar to those for
sheets (see Table 1).
2.
0040The so-called ‘molded sugar cones’ have an inter-
mediate sugar content, usually below 20 parts of
sucrose for 100 parts of flour.
Recent Developments in Molded Wafer Cones
(see Figure 2)
0041Whereas traditionally molded cones have flat tops
and regular, symmetric reedings now for a few years
molds for more sophisticated products can be manu-
factured. We now see cones and cups in the market
showing curvilinear tops and artfully designed out-
sides. Figure 3 shows the first of these, which comes
from a Japanese cone manufacturer.
Manufacturing of Rolled Wafer Cones
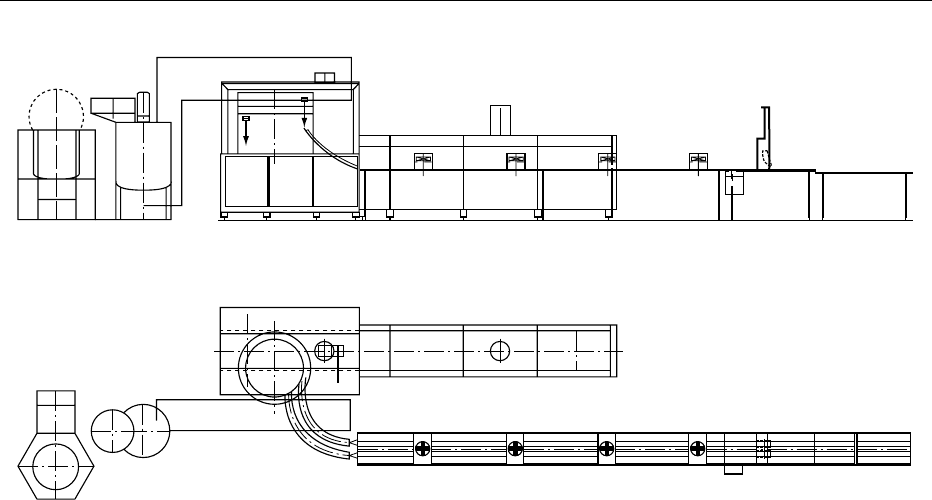
(see Figure 4)
0042‘Rolled sugar cones’ need a concentration of more
than 20% of sucrose or other sugars in the finished
product. The first three steps of rolled sugar cone
manufacturing are rather similar to no/low-sugar
wafer sheet baking with the exception of reduced
steam pressure as there are no baking ledges to build
up pressure and to extrude ‘bobbles.’ The deposit is
just to form an oval or circular sheet.
1.
0043Batter preparation (Figure 4, process 1)
2.
0044Batter transport and depositing (Figure 4,
process 2)
fig0003Figure 3 Molded wafer cone: example for new, nonflat top
design.
542 BISCUITS, COOKIES, AND CRACKERS/Wafers
3.0045 Wafer sheet baking (Figure 4, process 3): Now-
adays, equipment is available to manufacture up
to 13000 cones per hour.
4.
0046 Wafer take-off, rolling, and cone release (Figure 4,
process 4): When, after baking, the mold reopens,
the sheet is automatically stripped off the plate and
rolled immediately on tapered mandrels to form
the finished cone. A series of rolling devices
mounted on a round table operates continuously:
Sheet removal, rolling, release, etc.
Glass Transition and Formability
0047 During the rolling procedure, the wafer temperature
is still above the glass transition point. The molten
sugar acts as a plasticizer, and so any rolling or deep-
forming operation can be performed. Besides the
rolled cones, three more types of products are manu-
factured by the same principle:
1.
0048 Wafer ‘rolls’: Wafer sheets of rectangular shape
are baked, stripped off the baking plate, and
rolled into a sugar wafer ‘rod’ without a center
hole (different from wafer ‘sticks’ with a
center hole discussed later). The rod is later cut
into smaller cylindrical pieces. Typically, these
pieces are finally partially enrobed with choc-
olate-type coatings.
2.
0049 ‘Deep-formed’ wafers (cups, shells), where the
hot, flat wafer piece is introduced into a forming
tool, sometimes with an additional embossing or
stamping device, which defines the final shape.
3.
0050Rolled wafer sticks. These are discussed in a
separate chapter below.
Cone Cooling (Figure 4, process 5)
0051The fresh cones pass through a cooling section, where
ambient or cooled air is applied. Here, sugar recrys-
tallization finishes to give the final strong and brittle
texture.
Cone Sleeving and Stacking (Figure 4, process 6)
0052Now, paper sleeves are applied automatically, and
these form the packaging material for the typical end
product, industrially manufactured icecream cones.
Next, is automatic cone stacking and packaging.
0053Later in the icecream plant, the wafer cones are
sprayed inside with chocolate and filled, and finally,
the paper cone is closed.
Manufacturing of Rolled Wafer Sticks
0054Rolled wafer sticks are hollow tubes with walls con-
sisting of very thin multiple layers, similar in texture
to crunchy cereals. These layers do not carry a wafer
pattern and are approximately 0.5mm in thickness.
But from the recipe, the sticks are typical higher-sugar
wafers (see Table 1).
0055In manufacturing, a stripe of batter is applicated to
a heated drum and baked into a continuous wafer
band. The band is rolled immediately while hot into
a continuous still formable tube with an internal
1
1
2
2
4
4
56
56
3
3
fig0004 Figure 4 Manufacturing line for rolled sugar cones. 1, batter preparation; 2, batter transport and depositing; 3, wafer sheet baking;
4, take-off, rolling and cone release; 5, cooling of cones; 6, cone sleeving, stacking.
BISCUITS, COOKIES, AND CRACKERS/Wafers 543

diameter of about 6–36 mm. From here, there are
several options to create a large family of finished
products:
1.
0056 The tube is automatically cut into wafer stick
pieces and cooled. These are consumed as sweet
snacks or with icecream, for example. Other end
uses include intermediate products for confection-
ers to finish by additional filling or decorating
operations.
2.
0057 During the rolling operation, the tube is coated
inside with compound chocolate or filled with
cream, either partially or fully. Then, cutting and
cooldown, followed optionally by a coating and/
or decorating process, give the finished product.
3.
0058 The filled tube is transformed into small pillow-
like bits by a combined squeezing–cutting oper-
ation.
4.
0059 The wafer tube pieces, nonfilled, coated inside,
or partially cream-filled immediately after cutting
while still hot, pass a pressing station to form oval
or even flat pieces. Such flat pieces may form the
center of premium-type confectionery items with a
chocolate coating, including chopped nuts or
cereal crispies, for example.
5.
0060 Larger-diameter tubes are pressed flat, and by an
embossing and stamping operation, so-called ‘fan
wafers’ or other elaborate shapes can be generated.
The diameter and length of the sticks as well as the
number of very thin, ‘glassy’ sheets forming the wall
of the stick can be adjusted individually. Rolled wafer
sticks have a unique brittle wall texture without being
too hard in biting and chewing.
Savory Wafer Products
0061 Whereas many end uses of wafers in combination with
sweet fillings, coatings, etc. are well known, savory or
neutral-tasting wafer products are becoming increas-
ingly popular in some areas. Some examples:
1.
0062 Wafers as crispbread, delivering a crunchy but
softer texture than traditional hard crisp breads.
Besides wheat flour there are several options for
other cereal and noncereal flours as raw materials.
The products are either neutral in taste to be eaten
with sweet or nonsweet toppings or have spices,
cheese, and other flavorsome ingredients.
2.
0063Wafers – flat or hollow wafers – with savory fill-
ings, e.g., peanut butter or cheese creams.
3.
0064Wafer sticks, a recent development, made from
a nonsweet but still rollable material. These are
typically combined with savory cream fillings.
Trends – From the Very Wafer to a More
Sophisticated End Product
0065After discussing the different wafer types and their
traditional end uses (for an overview, see Table 3), a
sharply increasing number of new confectionery
brand products during the last decade are worth
mentioning. Here, wafers are only a smaller, but still
important, part of the deal. These are small confec-
tionery pieces, mostly in bite-size or sectioned bars,
often chocolate-covered, where the wafer has three
main functions:
1.
0066To add its typical texture and crispness, e.g., to
a soft filling cream with a piece of nut and a
chocolate cover, thus imparting a multitextured
impression.
2.
0067To make the overall product ‘lighter’ in calories
and nutritionally, as the wafer part is of an
extremely low density and cereal-based.
3.
0068To define the precise structure of the piece, e.g.,
when holding a soft filling, or, for noncoated
pieces, to keep the consumer’s hands clean if the
filling melts due to warmer conditions.
Looking to some of the current trends, we see increas-
ing interest in combining lower fat fillings such as
toffee (caramel). Another trend may be newer fat
free fruit-type fillings with water activities that are
so low that the wafers stay crisp. Even some new
examples of fillings with a higher moisture, resulting
in a ‘soft’ wafer part, can be rolled out.
tbl0003 Table 3 Which product from which wafer type?
Endproduct No (low)-sugar wafer Higher-sugar wafer
Wafer crisp bread Flat wafer sheet
Sugar wafer biscuits, creamed Flat wafer sheet
Wafer biscuits, creamed and enrobed or molded
in chocolate
Flat wafer sheet
Containers, e.g., for icecream Molded cake cone Molded sugar cone, rolled sugar cone
Confectionery, filled, enrobed, decorated Hollow wafer or hollow þflat wafer Wafer stick or sugar cone, small
Fan wafers Wafer stick, pressed, embossed
Wafer rolls, partially enrobed Rolled sheet
Wafer bowls Deep-formed sheet
544 BISCUITS, COOKIES, AND CRACKERS/Wafers
