Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


so these methods are only applicable for analyses of
the biotin content of vitamin preparations, liquid
infant formula, etc.
0048 A maximal intensification of the chemolumimetric
biotin detection can be achieved by using the biolu-
minescent protein aequorin as a label in a competitive
binding assay. Aequorin is a photoprotein from the
jellyfish Aequorea victoria. Aequorin associates non-
covalently with its chromophore coelenterazine,
which is oxidized into a metastable excited state,
resulting in the emission of photons at 469 nm.
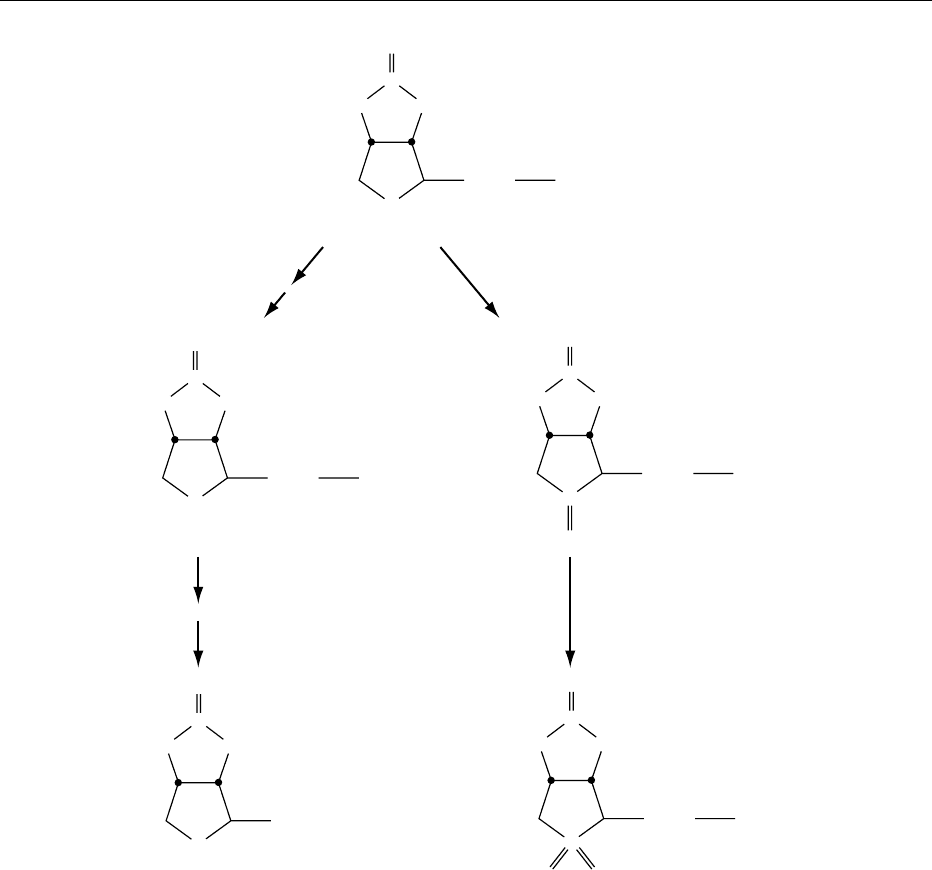
0049 In a homogenous assay, the aequorin–biotin conju-
gate competes in a one-step procedure with free biotin
for binding to avidin in solution. Binding of the
conjugate to avidin results in partial quenching of
the bioluminescence signal, and so the decrease in
luminescence can be related to the concentration of
biotin in a sample. The detection limit is reported to
be around 10
14
M biotin (¼ 10 fM).
0050 The competition assay can also be performed in a
heterogenous system by using immobilized avidin
(agarose, polyacrylamide). Both methods need special
equipment (luminometer operating in a photon-
counting mode). The amounts of avidin and biotinyl-
ated aequorin must be optimized beforehand, as the
relative amounts of avidin and biotinylated aequorin
influence the response characteristics of the assay
(e.g., detection limits and sensitivity).
Chromatographic Analysis
0051 Earlier methods attempted to separate and quantify
biotin from vitamin mixtures or feed supplements.
After dissolution of the lyophilized preparation and
addition of the internal standard (2-imidazolidone),
the sample was applied on a TLC plate and eluted
with chloroform/methanol/formic acid. Biotin was
visualized by spraying with p-dimethylaminocinna-
maldehyde and determined in situ by reflectance
measurements. Spraying with paraffin after the
coloring procedure increased the sensitivity to a
detection limit of 10 ng of biotin per spot.
0052 Quantitation by HPLC requires derivatization
of the molecule, because biotin does not absorb in
the visible or UV region. For the derivatization
9-anthryldiazomethane was used to produce fluores-
cent biotin-9-anthrylmethylester followed by separ-
ation on C 18 bonded columns with acetonitrile/
water as the mobile phase and fluorimetric detection
(excitation ¼365 nm, emission ¼425 nm). Alterna-
tively, p-bromophenacyl bromide esters and 4-
bromomethylmethoxycoumarin derivatives of biotin
were produced for UV and fluorescence detection,
respectively, after separation on RP 18 columns with
methanol/water or tetrahydrofuran/water as eluents.
0053Standards of biotin and 13 metabolites and analogs
can be separated by HPLC on a C 18 RP phase using
gradient elution with trifluoroacetic acid/acetonitril
and UV detection at 220 nm. An HPLC separation of
six vitamins including biotin from almonds has been
performed on an LC-8-DB column with hexanesulfo-
nic acid sodium salt/methanol as the eluent and de-
tection at 200 nm. The limit of biotin detection has
been found to be as low as 0.9 mg per 100 g.
0054When biotin is derivatized with panacyl bromide in
the presence of crown ether, a fluorescence compound
results with fluorescence maxima at 380 nm (excita-
tion) and 470 nm (emission). After extraction from
biological tissue with trichloroacetic acid and purifi-
cation by solid-phase extraction combined with ion-
exchange chromatography on DOWEX and TLC,
biotin is derivatized with panacyl bromide in the
presence of crown ether. The resulting panacylester
can be separated on normal-phase HPLC using
isocratic elution with methanol/dichloromethane as
well as on RP phase HPLC using gradient elution
(water/methanol). Dethiobiotin is taken as an internal
standard.
0055With this method, the biotin content in rat small
intestine has been assessed. The detection limits are
reported to be 10 pmol of biotin (normal phase
HPLC) or 100 pmol of biotin (RP-HPLC), which is
less sensitive than with protein-binding assays. Criti-
cism results from the facts, that the clean-up required
for biological sample extracts prior to derivatization
is very extensive, the derivatization should not only
include biotin, and the cross-reactivity to biotin
analogs has to be examined.
See also: Analysis of Food; Chromatography: High-
performance Liquid Chromatography; Enzymes: Uses in
Analysis; Food Composition Tables; Immunoassays:
Principles; Radioimmunoassay and Enzyme
Immunoassay; Lactic Acid Bacteria; Microbiology:
Classification of Microorganisms; Spectroscopy:
Fluorescence; Vitamins: Determination
Further Reading
Baker H, Frank O, Matovitch VB et al. (1962) A new assay
method for biotin in blood, serum, urine and tissue.
Analytical Biochemistry 3: 31–39.
Bitsch R, Salz I and Hoetzel D (1986) Biotin assessment in
foods and body fluids by a protein binding assay. Inter-
national Journal for Vitamin and Nutrition Research 59:
59–64.
Bonjour JP (1991) Biotin. In: Machlin LJ (ed.) Handbook of
Vitamins, pp. 393–427. New York: Marcel Dekker.
Eitenmiller RR and Landen WO, Jr. (1999) Vitamin
Analysis for the Health and Food Sciences. Boca
Raton, FL: CRC Press.
BIOTIN/Properties and Determination 515

Fidanza F (1991) Nutritional Status Assessment. London:
Chapman & Hall.
Green NM (1970) Spectrophotometric determination of
avidin and biotin. Methods in Enzymology 18: 418–424.
Guilarte T (1985) Measurement of biotin levels in human
plasma using a radiometric-microbiological assay.
Nutrition Reports International 31: 1155–1163.
Hentz NG and Bachas LG (1997) Fluorophore-linked
assays for high-performance liquid chromatography
postcolumn reaction detection of biotin and biocytin.
Methods in Enzymology 279: 275–286.
Lizano S, Ramanathan S, Feltus A, Witkowski A and
Dannert S (1997) Bioluminescence competitive binding
assays for biotin based on photoprotein aequorin.
Methods in Enzymology 279: 296–303.
Schroeder HR, Vogelhut PO, Carrilco RJ, Boguslaski RC
and Buckler RT (1976) Competitive protein binding
assay for biotin monitored by chemoluminescence.
Analytical Chemistry 48: 1933–1937.
Physiology
J Zempleni, University of Nebraska-Lincoln, Lincoln,
NE, USA
D M Mock, University of Arkansas for Medical
Sciences, Little Rock, AR, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Biotin is a water-soluble vitamin that serves as essen-
tial coenzyme for four mammalian carboxylases.
Biotin may also play a role in the replication and
transcription of DNA, possibly by an effect on bioti-
nylation of histones. Frank biotin deficiency causes
clinical findings such as hair loss and skin rash. Mar-
ginal biotin deficiency is frequently seen in pregnant
women and theoretically could have teratogenic
effects. Biotin status may be assessed using urinary
excretion of biotin and its metabolites, and certain
organic acids that are byproducts of deficiency of
biotin-dependent carboxylases. Direct measurement
of biotin-dependent carboxylases and biotin in
lymphocytes and fibroblasts also show promise as
indicators of biotin status. Inborn errors of biotin
metabolism (e.g., biotinidase deficiency and holocar-
boxylase synthetase deficiency to a variable degree)
can produce symptoms similar to biotin deficiency.
For adults, the safe and adequate daily dietary intake
of biotin is 30 mg. Available data suggest that pure
biotin administered orally is absorbed completely.
Uptake of biotin into cells of the intestine and periph-
eral tissues is mediated by a sodium-dependent trans-
porter that requires metabolic energy. Mammals
catabolize biotin by b-oxidation of the valeric acid
side chain and by sulfur oxidation in the thiophane
ring. Biotin, bisnorbiotin, and biotin, d,l-sulfoxide
are the most abundant biotinyl compounds in urine
and plasma.
Biochemical Function
0002In mammals, holocarboxylase synthetase (EC
6.3.4.10) catalyzes the covalent binding of biotin
to the E-amino group of lysine in four different apo-
carboxylases to form the active holocarboxylases
(Figure 1). For acetyl-CoA carboxylase (EC 6.4.1.2),
a cytosolic (acetyl-CoA carboxylase a) and a mito-
chondrial form (acetyl-CoA carboxylase b) have been
identified. Both the a and b forms catalyze the bind-
ing of bicarbonate to acetyl-CoA to form malonyl-
CoA; the latter is a substrate for fatty acid synthesis.
Although the a and b forms of acetyl-CoA carboxyl-
ase catalyze the same reaction, they appear to have
different roles in intermediary metabolism. Acetyl-
CoA carboxylase a controls fatty acid synthesis in
the cytosol by providing the substrate malonyl-CoA;
acetyl-CoA carboxylase b controls fatty acid oxida-
tion inside the mitochondria via malonyl-CoA inhib-
ition of fatty acid transport into mitochondria. (See
Fatty Acids: Metabolism.)
0003The three other mammalian biotin-dependent car-
boxylases are located exclusively in mitochondria:
pyruvate carboxylase (EC 6.4.1.1), propionyl-CoA
carboxylase (EC 6.4.1.3), and b-methylcrotonyl-
CoA carboxylase (EC 6.4.1.4). By synthesizing oxalo-
acetate, pyruvate carboxylase provides a tricarboxylic
acid cycle intermediate and catalyzes a step in gluco-
neogenesis. Propionyl-CoA carboxylase catalyzes an
essential step in the metabolism of isoleucine, valine,
the cholesterol side chain, and products of dietary
carbohydrate breakdown by intestinal microorgan-
isms. b-Methylcrotonyl-CoA carboxylase catalyzes
an essential step in leucine metabolism.
0004Biotin deficiency causes reduced carboxylase activ-
ities; substrates are shunted to alternative pathways
(Figure 1). For example, reduced activity of propio-
nyl-CoA carboxylase results in increased formation
of 3-hydroxypropionic acid and 2-methylcitric acid;
reduced activity of b-methylcrotonyl-CoA carboxyl-
ase results in increased formation of 3-hydroxyisova-
leric acid and 3-methylcrotonyl glycine. Increased
urinary excretion of these organic acids has been
used to diagnose biotin deficiency, as discussed below.
0005Biotin may also play a role in the regulation of
DNA transcription and replication on the basis of
the following observations:
1.
0006Biotinidase (EC 3.5.1.12) specifically biotinylates
histones. Histones are DNA-binding proteins that
516 BIOTIN/Physiology
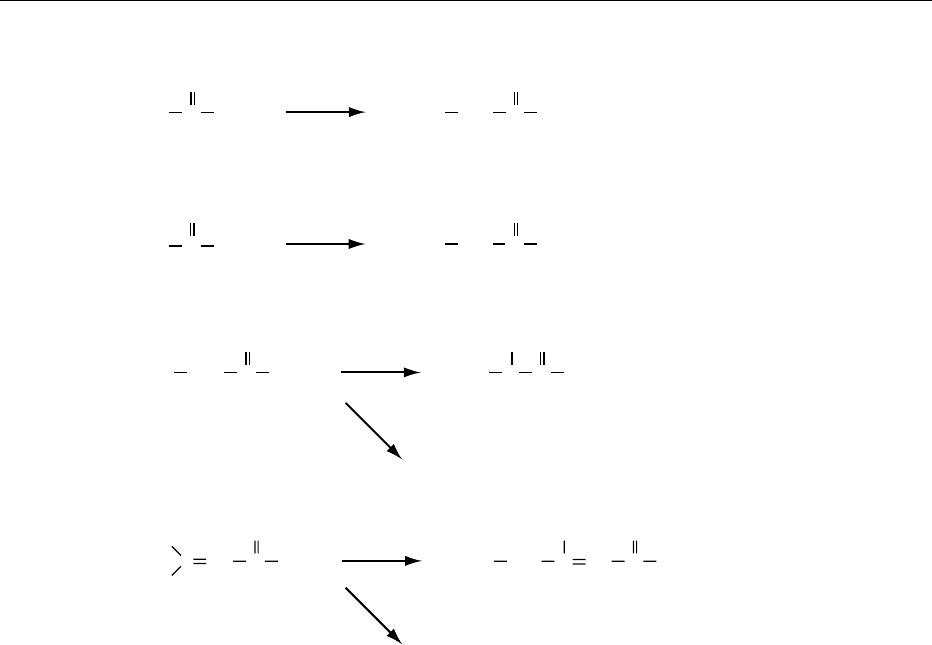
regulate transcription and replication of DNA.
Biotinylation of histones might play some role in
DNA packaging in analogy to other covalent
modifications such as acetylation, methylation,
phosphorylation, or adenosine diphosphate (ADP)-
ribosylation of histones. Biotinidase is ubiquitous
in mammalian cells, and 25% of the cellular bio-
tinidase activity is located in the nucleus. Indeed,
the following observations are also consistent with
a role for biotin in modifying histones and in turn
affecting the packaging of DNA: first, histones
dissociate from the DNA in biotin-deficient rats,
and second, biotin deficiency in rats results in
decreased phosphorylation and methylation of
histones as well as increased acetylation of
histones.
2.
0007 Rates of biotin uptake are greater in proliferating
than in nonproliferating lymphocytes. This in-
crease is mediated by an increased number of
biotin transporters on the cell surface. Cell
proliferation requires a substantial increase of
both replication and transcription. Theoretically,
the observed increased uptake of biotin by
proliferating cells may be in response to increased
demand for biotin to biotinylate histones. This is a
potential mechanism by which biotin exerts an
effect on cell replication.
3.
0008Biotin status affects gene expression. Biotin defi-
ciency causes a 40–45% reduction of rat liver
glucokinase activity; the activity is restored by
biotin administration. Injection of biotin into rats
causes a 4–19-fold increase of mRNA encoding
glucokinase.
0009In addition to this effect on mRNA synthesis,
biotin may affect the synthesis of some proteins at
the posttranscriptional level. When human-derived
liver (HepG2) cells were cultured in biotin-deficient
medium, expression of the asialoglycoprotein recep-
tor was reduced despite normal protein synthesis,
total cellular protein content, and mRNA coding
for asialoglycoprotein receptor; addition of biotin or
biocytin restored receptor expression. These observa-
tions suggest that a biotin-dependent posttranscrip-
tional event can affect the ultimate expression of
asialoglycoprotein receptor.
Cytosol and mitochondria
Mitochondria
H
3
CC
Acetyl CoA Malonyl CoA
O
ACC
SCoA
H
3
CH
2
CHOOC C COOH
O
C
H
3
C
H
3
C
H
3
C
C
CH
β-Methylcrotonyl CoA
β-Methylglutaconyl CoA
3-H
y
drox
y
isovaleric acid and 3-meth
y
lcroton
y
l
g
l
y
cine
C SCoA HOOC CH
2
C CH C SCoA
O
CH
3
O
MCC
H
3
CC
H
HOOC
C
O
SCoACH
2
C
Propionyl CoA
3-Hydroxypropionic acid and 2-methylcitric acid
Biotin-
deficient
Biotin-
deficient
Methylmalonyl CoA
SCoA
PCC
O
Pyruvic acid Oxaloacetic acid
PC
O
COOH
HOOC H
2
C C SCoA
O
fig0001 Figure 1 Biotin-dependent carboxylases. ACC, acetyl-CoA carboxylase; PC, pyruvate carboxylase; PCC, propionyl-CoA
carboxylase; MCC, b-methylcrotonyl-CoA carboxylase.
BIOTIN/Physiology 517

Storage, Metabolism, and Excretion
0010 Depletion and repletion experiments of biotin-
dependent carboxylases in rat liver provided evidence
that mitochondrial acetyl-CoA carboxylase may serve
as a reservoir for biotin. Neither cytosolic acetyl-
CoA carboxylase nor the mitochondrial pyruvate
carboxylase, propionyl-CoA carboxylase, or b-
methylcrotonyl-CoA carboxylase seem to serve as
biotin reservoirs.
0011 The liver accumulates a significant percentage of
ingested biotin. For example, rat liver accumulates
approximately 4% of [
14
C]biotin within 1 h of intra-
venous administration. Once steady-state conditions
are attained, biotin is located mainly in liver mito-
chondria (30% of total [
14
C]biotin) and cytosol
(59%); smaller amounts can be found in microsomes
and nuclei. Greater than 80% of the [
14
C]biotin
present in the cytosolic fraction is acid-precipitable,
suggesting that biotin has become covalently bound.
0012 During the normal intracellular turnover of pro-
teins, holocarboxylases are degraded to biotin linked
to lysine (biocytin) or biotin linked to an oligopeptide
containing at most a few amino acid residues. Bio-
tinidase catalyzes the hydrolysis of the amide bond
between biotin and lysine. Biotinidase likely serves in
both absorption of biotin (cleavage of protein-bound
dietary biotin) and recycling of biotin (cleavage of
biocytin). Biotinidase also catalyzes a specific bioti-
nylation of histones, as noted above. (See Protein:
Synthesis and Turnover.)
0013 A significant proportion of biotin undergoes cata-
bolism before excretion (Figure 2). Two principal
pathways of biotin catabolism have been identified
in mammals. In the first pathway, the valeric acid
side chain of biotin is degraded by b-oxidation. b-
Oxidation of biotin leads to the formation of bisnor-
biotin, tetranorbiotin, and related intermediates that
are known to result from b-oxidation of fatty acids.
The cellular site of this b-oxidation of biotin is uncer-
tain. Spontaneous (nonenzymatic) decarboxylation
of the unstable b-keto acids (b-keto-biotin and b-
keto-bisnorbiotin) leads to formation of bisnorbiotin
methyl ketone and tetranorbiotin methyl ketone;
these catabolites appear in urine.
0014 In the second pathway, the sulfur in the thiophane
ring of biotin is oxidized, leading to the formation
of biotin l-sulfoxide, biotin d-sulfoxide, and biotin
sulfone. Sulfur oxidation may be catalyzed by a
NADPH-dependent process in the smooth endoplas-
mic reticulum. Combined oxidation of the ring sulfur
and b-oxidation of the side chain lead to metabolites
such as bisnorbiotin sulfone. In mammals, degrad-
ation of the biotin ring to release carbon dioxide
and urea is quantitatively minor.
0015On a molar basis, biotin accounts for approximately
half of the total avidin-binding substances in human
serum and urine (Table 1). Bisnorbiotin, bisnorbiotin
methyl ketone, biotin d,l-sulfoxide, and biotin sul-
fone account for most of the balance. Using thin-layer
chromatography and staining with p-dimethyl-
aminocinnamaldehyde, tetranorbiotin l-sulfoxide
was also identified in human urine. However, avidin
binding of this metabolite was too weak to allow
quantitation.
0016The biliary route of biotin excretion is quantita-
tively minor. For example, in rats, approximately 2%
of intravenously administered [
14
C]biotin was ex-
creted in bile but approximately 61% was excreted
in urine.
0017The relationship of metabolite profile to biotin
nutritional status has not been fully elucidated. In
human and rat urine, the percent excretion of biotin
increases when the biotin intake is increased from
physiologic to pharmacologic amounts. This may
reflect saturation of renal reabsorption, metabolic
pathways, or both.
Deficiency
Clinical Symptoms of Frank Biotin Deficiency
0018The fact that humans have a requirement for biotin
has been clearly documented in two situations: first,
parenteral nutrition without biotin supplementation
in patients with short-gut syndrome and other causes
of malabsorption; and second, prolonged consump-
tion of raw egg white. The critical event in the egg
white-induced biotin deficiency is a highly specific
and very tight binding (k
b
¼10
15
mol l
1
) of biotin
by avidin, a glycoprotein found in egg white. Avidin
is resistant to intestinal proteolysis in both the free
and the biotin-bound form. Thus dietary avidin binds
and prevents the absorption of biotin. Cooking de-
natures avidin, rendering it susceptible to digestion
and hence unable to interfere with absorption of
biotin. (See Eggs: Dietary Importance.)
0019Biotin deficiency has also been reported or inferred
in several other circumstances, including pregnancy,
individuals undergoing dialysis, individuals suffering
from chronic gastrointestinal disease, Leiner’s dis-
ease, and sudden infant death syndrome.
0020The clinical findings of frank biotin deficiency in
adults and older children are similar regardless of
whether deficiency is caused by egg-white feeding or
omission of biotin from parenteral nutrition. Typi-
cally, the findings begin to appear gradually after an
interval of 6 months to 3 years of parenteral nutrition
or after 6 weeks to several years of egg-white feeding.
Thinning of hair, often with loss of hair color, was
518 BIOTIN/Physiology
reported in most patients. A skin rash described as
scaly (seborrhoeic) and red (eczematous) was present
in the majority; in several, the rash was distributed
around the eyes, nose, and mouth. Depression,
lethargy, hallucinations, and paresthesias of the
extremities were prominent neurological symptoms
in the majority of adults.
0021 In infants who developed biotin deficiency, the
signs of deficiency began to appear within 3–6
months of initiation of total parenteral nutrition.
The rash initially appeared around the eyes, nose,
and mouth; ultimately, the ears and perineal orifices
were involved. The appearance of the rash was simi-
lar to that of cutaneous candidiasis (i.e., an erythe-
matous base and crusting exudates); typically,
Candida could be cultured from the lesions. The
character and distribution of the biotin deficiency
rash are quite similar to the rash of zinc deficiency.
In some infants, hair loss can progress to total bald-
ness, including loss of eyebrows and lashes. The most
striking neurological findings in biotin-deficient
infants was hypotonia, lethargy, and developmental
delay. In these infants, a peculiar withdrawn behavior
was often noted and may have reflected the same
central nervous system dysfunction diagnosed as
depression in adult patients.
Biochemical Markers of Biotin Deficiency
0022Our studies provide evidence that marginal biotin
deficiency is much more common than frank biotin
O
C
S
Biotin
(CH
2
)
4
COOH
HN NH
O
C
S
Bisnorbiotin
(CH
2
)
2
COOH
HN NH
O
C
S
O
Biotin sulfoxide
(CH
2
)
4
COOH
HN NH
O
C
S
Tetranorbiotin
COOH
HN NH
O
C
S
OO
Biotin sulfone
(CH
2
)
4
COOH
HN NH
fig0002 Figure 2 Pathways of biotin catabolism.
BIOTIN/Physiology 519
deficiency. Diagnosis of marginal biotin deficiency
requires sensitive metabolic indicators. The best val-
idated indicators of marginal biotin deficiency are
urinary excretion of biotin and metabolites and urin-
ary excretion of organic acids such as 3-hydroxyiso-
valeric acid (see above: Figure 1). These organic acids
are synthesized and excreted in increased quantities in
biotin-deficient individuals because the normal catab-
olism of their precursors by biotin-dependent carb-
oxylases is reduced. Activities of biotin-dependent
carboxylases in lymphocytes and fibroblasts and
plasma concentrations of biotin and metabolites
have been reported to be abnormally low in individ-
uals who developed frank biotin deficiency after
1 month of biotin-free intravenous feeding.
Teratogenic Effects of Biotin Deficiency
0023 Biotin deficiency is teratogenic in several animal
species at degrees of deficiency that produce no obvi-
ous findings in the pregnant animal. Hens with
marginal biotin deficiency produce eggs with higher
embryonic mortality, reduced hatchability, chrono-
dystrophy (‘parrot beak’ deformity), perosis (an ab-
normality of bone tendon formation that results in
a deformity analogous to ‘club foot’), micromelia,
and syndactyly. Effects on hatchability and viability
have been reported in turkey poults. In some strains
of mice, asymptomatic biotin deficiency during
pregnancy causes substantial increases in fetal
malformations and mortality. Ninety-four percent of
pups from biotin-deficient dams had cleft palate,
85% had micrognathia, and 41% had micromelia;
multiple malformations were common.
0024 Biotin status may be reduced during human preg-
nancy. In the majority of pregnant women, excretion
of 3-hydroxyisovaleric acid is significantly greater
than the upper limit of normal. The serum concen-
tration of biotin commonly decreases significantly
from early to late pregnancy and reaches values that
are below the lower limit of normal in some individ-
uals. Likewise, the urinary excretion rates of biotin
and bisnorbiotin are often significantly less in late
pregnancy than in early pregnancy.
Inborn Errors of Metabolism
0025Two important classes of inborn errors of biotin
metabolism have been identified:
1.
0026Biotinidase deficiency is an inborn error of metab-
olism that is characterized by partial (70–90%)
or profound (> 90%) reduction of biotinidase
activity. The combined incidence of partial plus
profound biotinidase deficiency is 1: 60 000 new-
borns. Clinical findings of biotinidase deficiency
largely mimic biotin deficiency and include devel-
opmental delay, seizures, skin infection, hepato-
megaly, and splenomegaly. These symptoms are
thought to be a consequence of one or more im-
pairments leading to reduced biotin status: (i) de-
ficient salvage of covalently bound biotin during
holocarboxylase turnover may result from in-
complete release of biotin from biocytin; (ii) sub-
stantial amounts of biocytin are lost in urine;
(iii) release of covalently bound dietary biotin
may be impaired, leading to malabsorption.
Biotinidase-deficient patients respond well to
physiologic or pharmacologic doses of biotin.
2.
0027Multiple carboxylase deficiency is an inborn error
of metabolism in humans that is caused by defi-
ciency of holocarboxylase synthetase, leading to
less efficient biotinylation of apocarboxylases.
Isolated carboxylase deficiencies are caused by
mutations of genes encoding the mammalian
apocarboxylases.
(See Inborn Errors of Metabolism: Overview.)
Intestinal Absorption
0028Studies in rats provided evidence that intestinal
uptake of biotin occurs by two distinct mechanisms.
The first is a saturable, structurally specific trans-
porter located in the brush border membrane. This
transporter is dependent on sodium and energy, and is
electroneutral. As judged by the maximal transport
rate (V
max
) of the transport process, the transporter is
appropriately up- and downregulated by biotin defi-
ciency and biotin excess, respectively. In the rat,
biotin transport is most active in the upper small
bowel; activity of biotin transport declines proceed-
ing from the jejunum to the ileum to the colon. How-
ever, activity in the colon is still 3% of jejunal values,
leaving open the possibility that biotin synthesized by
tbl0001 Table 1 Biotin and metabolites in human serum and urine
Compound Serum (pmoll
1
)Urine(nmol24h
1
)
Biotin 244 + 61 35 + 14
Bisnorbiotin 189 + 135 68 + 48
Biotin d,l-sulfoxide 15 + 33 5 + 6
Bisnorbiotin methyl ketone ND
a
9 + 9
Biotin sulfone ND
a
5 + 5
Total biotinyl compounds 464 + 178
b
122 + 66
Means + SD are reported (n ¼15 for serum; n ¼6 for urine).
a
ND, not determined. Bisnorbiotin methyl ketone and biotin sulfone had
not been identified at the time when this study of serum was conducted and
hence these unknowns were not quantitated against authentic standards.
b
Including three unidentified biotin metabolities.
From Zempleni J and Mock DM (1999) Biotin biochemistry and human
requirements. Journal of Nutrition and Biochemistry 10: 128–138, with
permission.
520 BIOTIN/Physiology

enteric flora might contribute to absorbed biotin. In
the rat, activity of the biotin transporter increases
with age from suckling to weanling to young adult
(aged 3 months). As with the other changes observed
in the biotin transporter, the mechanism leading to
increased transport appears to be an increase in the
number or activity of the carriers, rather than changes
in the affinity of the carrier.
0029 The second mechanism of intestinal biotin absorp-
tion is passive diffusion. This process predominates at
large luminal biotin concentrations; at that point, the
biotin transporter is likely saturated.
0030 The biotin transporter in human intestine is similar
to that identified in rats. The human transporter is
saturable, electroneutral, sodium-dependent, and
capable of accumulating biotin against a concentra-
tion gradient. The anatomical distribution along the
human gastrointestinal tract is similar to that ob-
served in the rat. Changes in transport activity appear
to be mediated primarily by an increase in the number
or activity of transporters, rather than by changes in
the affinity of the transporter. The exit of biotin from
the enterocyte (i.e., transport across the basolateral
membrane) is also carrier-mediated; however, biotin
export is independent of sodium, is electrogenic, and
cannot accumulate biotin against a concentration
gradient.
0031 Recently, a biotin transporter in human tissues has
been cloned, sequenced, and expressed in mammalian
cells. This multivitamin transporter binds and trans-
ports not only biotin but also pantothenic acid and
lipoic acid. Binding affinities are similar for these
nutrients.
0032 Clinical studies have also provided evidence that
biotin is absorbed from the human colon. When biotin
is instilled directly into the lumen of the colon, the
plasma concentration of biotin increases; however,
when the same dose of biotin is given orally, the
increase in plasma concentration is greater.
Bioavailability
0033 Recent reports provide evidence that biotin is
absorbed almost completely even if large doses of
biotin are ingested. However in these studies, biotin
was administered in aqueous solution rather than
being endogenous to the diet. A large percentage of
biotin in food may be bound to protein and therefore
is likely to require cleavage from the protein in order
to be transported into enterocytes by the biotin trans-
porter. Biotinidase is thought to be responsible for
release of biotin from protein. The release of biotin
might occur in or near the intestine mucosa via the
action of mucosal biotinidase; biotinidase in pancre-
atic juice might release biotin during the luminal
phase of proteolysis. Theoretically, biotinyl oligopep-
tides might also be absorbed directly, either by a
specific biotin transporter or by a peptide transporter.
However, the mechanism remains to be elucidated.
Biotinidase is present in pancreatic juice and in intes-
tinal mucosa, but biotinidase activity is not enriched
in intestinal brush border membranes. Currently, only
limited data are available regarding the bioavailabil-
ity of protein-bound biotin in food. (See Bioavail-
ability of Nutrients.)
Transport of Biotin from the Intestine to
Peripheral Tissues
0034Biotinidase has been proposed to serve as a biotin-
binding protein in plasma and as a carrier protein
for the transport of biotin into the cell. Two biotin-
binding sites on biotinidase have been identified. One
had an equilibrium dissociation constant (k
d
)of
3 nmol l
1
and a maximum binding capacity (B
max
)
of 0.065 mol of biotin per mol of biotinidase;
the other had a k
d
of 59 nmol l
1
and a B
max
of
0.79 mol of biotin per mol of biotinidase. However,
empirical studies with [
3
H]biotin provide evidence
that biotin binds primarily to albumin. Of the total
biotin in human plasma, 81% is free, 12% is cova-
lently bound, and 7% is reversibly bound.
Transport of Biotin in Liver and Peripheral
Tissues
Biotin Uptake into Liver
0035Rat liver cells accumulate biotin by a transporter-
mediated process that depends on metabolic energy;
uptake is sodium-dependent and Na-K-ATPase-
dependent. The ureido portion of the biotin molecule
plays a role in binding to the transporter. Organic
anions such as bilirubin and cholic acid compete
with biotin uptake by hepatocytes.
0036Biotin uptake into HepG2 cells is also transporter-
mediated, energy-dependent, sodium-dependent, and
Na-K-ATPase-dependent; a free carboxyl group on
the valeric acid side chain of biotin is essential for
recognition by this transporter. Apparent Michaelis
constant (K
m
) and V
max
in HepG2 cells are
19.2 mmol l
1
and 6.8 nmol mg protein
1
min
1
,
respectively.
Biotin Uptake into Lymphocytes
0037In analogy to liver cells, human lymphocytes accumu-
late biotin by a transporter that requires metabolic
energy and is dependent on Na-K-ATPase. Apparent
K
m
and V
max
values of this biotin transporter are
BIOTIN/Physiology 521
2.6 nmol l
1
and 2.9 fmol 10
6
cells
1
30 min
1
, re-
spectively. In lymphocytes, the thiophane portion of
the biotin molecule is more important than the ureido
portion for binding to the transporter. The biotin
transporter in lymphocytes is very specific for biotin;
inhibition of biotin transport by hexanoic acid, lipoic
acid, bilirubin, and pantothenic acid is minor. The
same transporter that mediates biotin uptake into
lymphocytes also accounts for biotin efflux from
these cells as judged by countertransport experiments.
Biotin Uptake into the Central Nervous System
0038 Biotin enters the central nervous system by a satur-
able transport system. A free carboxylic acid group is
important for transport across cerebral capillaries;
pantothenic acid and nonanoic acid compete for
uptake, suggesting that uptake is mediated by an
organic anion carrier. In the brain, biotin is covalently
bound to proteins, presumably biotin-dependent
carboxylases.
Placental Transport of Biotin
0039 A biotin transporter is present in the placental brush
border membrane. In mammals, a single protein
termed the multivitamin transporter can transport
biotin, pantothenic acid, and lipoic acid across the
placenta; this protein has been cloned and function-
ally expressed. A recent study of fetal and maternal
plasma concentrations of biotin at 18–24 weeks’ ges-
tation of normal human pregnancies reported a fetal-
to-maternal biotin ratio ranging from 3 to 17:1,
consistent with active transport across the placenta.
Requirements and Adequate Intakes
Adequate Intakes
0040 For biotin, scientific knowledge is currently insuffi-
cient to estimate average requirements and formulate
recommended dietary allowances. Notwithstanding,
the Food and Nutrition Board of the National Re-
search Council has published recommendations for
adequate intake of biotin (Table 2). These data are
based on empirical biotin intakes in a group of
healthy people.
Factors that Affect Biotin Requirements
0041 Pregnancy (see above) and lactation appear to pro-
duce an increased demand for biotin. Biotin secretion
into breast milk accounts for the higher recommen-
dations for adequate intake of biotin in lactating
compared to normal women (Table 2). At 8 days
postpartum, biotin in human milk is approximately
8 nmol l
1
and accounts for 44% of biotin plus
measured catabolites; bisnorbiotin and biotin d,l-
sulfoxide account for 48% and 8%, respectively. By
6 weeks postpartum, the biotin concentration in-
creases to approximately 30 nmol l
1
and accounts
for about 70% of biotin plus catabolites; bisnorbiotin
and biotin d,l-sulfoxides account for about 20% and
less than 10%, respectively.
0042Accumulating data provide evidence that long-
term anticonvulsant therapy in adults and children
can lead to biotin depletion severe enough to interfere
with amino acid metabolism. This conclusion was
originally based on decreased plasma concentrations
of biotin as determined by the Lactobacillus plan-
tarum bioassay; the incidence of biotin concentra-
tions below the normal range was approximately
75% in a cumulative study group of 274 adults under-
going long-term therapy with a variety of anticonvul-
sant drugs. The diagnosis of biotin deficiency was
strengthened by the demonstration of increased
urinary excretion of the characteristic organic acids
(e.g., 3-hydroxyisovaleric acid) in adults and children
receiving long-term anticonvulsant therapy. (See
Drug–Nutrient Interactions.)
0043The mechanism of biotin depletion during anticon-
vulsant therapy is not known. The anticonvulsant
drugs implicated include phenobarbital, phenytoin,
carbamazepine, and primidone. These drugs each
have a carbamide (-NH-CO-) moiety in their struc-
ture, as does biotin; in some cases they incorporate a
full ureido group (-NH-CO-NH-). Physiological con-
centrations of primidone and carbamazepine specif-
ically and directly inhibit biotin uptake by brush
border membrane vesicles from human intestine.
This finding suggests that impaired intestinal absorp-
tion of biotin may contribute to biotin deficiency. In
addition, phenobarbital, phenytoin, and carbamaze-
pine displace biotin from biotinidase and thus
tbl0002Table 2 Adequate intake of biotin
Life-stage group Adequateintake (mgday
1
)
Infants
0–6 months 5
7–12 months 6
Children
1–3 years 8
4–8 years 12
Males and females
9–13 years 20
14–18 years 25
19 years 30
Pregnancy 30
Lactation 35
From Yates AA, Schlicker SA and Suitor CW (1998) Dietary reference
intakes: the new basis for recommendations for calcium and related
nutrients, B vitamins, and choline. Journal of the American Dietary
Association 98: 699–706, with permission.
522 BIOTIN/Physiology

conceivably could have an effect on plasma transport
of biotin, renal handling of biotin, or cellular uptake
of biotin. Recently, increased urinary excretion rates of
biotin d,l-sulfoxides and bisnorbiotin have been dem-
onstrated in adults and children receiving long-term
anticonvulsant therapy, raising the possibility that
accelerated catabolism of biotin contributes to the
biotin deficiency associated with long-term anticon-
vulsant therapy.
Intake
0044 Biotin is widely distributed in foodstuffs, but the
content of even the richest sources is low when com-
pared to the content of most other water-soluble vita-
mins. Foods relatively rich in biotin include egg yolk,
liver, and some vegetables. The average dietary biotin
intake has been estimated to be 70 mg per day for the
Swiss population. These data are in reasonable agree-
ment with the estimated dietary intake of biotin in a
composite Canadian diet (62 mg per day). Calculated
intake of biotin for the British population was 35 mg
per day.
0045 Infants who ingest 800 ml of mature breast milk
per day ingest approximately 6 mg (24 nmol) of biotin.
It remains unclear whether biotin synthesis by gut
microorganisms contributes importantly to the total
biotin absorbed. However, an infant developed biotin
deficiency while consuming a biotin-free elemental
formula.
See also: Bioavailability of Nutrients; Drug–Nutrient
Interactions; Eggs: Dietary Importance; Fatty Acids:
Metabolism; Inborn Errors of Metabolism: Overview;
Protein: Synthesis and Turnover
Further Reading
Hymes J and Wolf B (1999) Human biotinidase isn’t just for
recycling biotin. Journal of Nutrition 129: 485S–489S.
Kim K-H (1997) Regulation of mammalian acetyl-
coenzyme A carboxylase. In: McCormick DB, Bier DM
and Goodridge AG (eds) Annual Review of Nutrition,
pp. 77–99. Palo Alto, CA: Annual Reviews.
Knowles JR (1989) The mechanism of biotin-dependent
enzymes. Annual Review of Biochemistry 58: 195.
Mock DM (1996) Biotin. In: Ziegler EE and Filer LJ Jr (eds)
Present Knowledge in Nutrition, 7th edn, pp. 220–235.
Washington, DC: International Life Sciences Institutes,
Nutrition Foundation.
Wang H, Huang W, Fei Y-J et al. (1999) Human placental
Na
þ
-dependent multivitamin transporter. Journal of
Biological Chemistry 274: 14875–14883.
Wolf B (1991) Worldwide survey of neonatal screening for
biotinidase deficiency. Journal of Inherited Metabolic
Disease 14: 923–927.
Wolf B and Heard GS (1991) Biotinidase deficiency. In:
Barness L and Oski F (eds) Advances in Pediatrics, pp.
1–21. Chicago: Book Medical Publishers.
Wood HG and Barden RE (1977) Biotin enzymes. Annual
Review of Biochemistry 46: 385–413.
Yates AA, Schlicker SA and Suitor CW (1998) Dietary
reference intakes: the new basis for recommendations
for calcium and related nutrients, B vitamins, and
choline. Journal of the American Dietary Association
98: 699–706.
Zempleni J and Mock DM (1999) Biotin biochemistry and
human requirements. Journal of Nutrition and Biochem-
istry 10: 128–138.
Zempleni J and Mock DM (2000) Marginal biotin defi-
ciency is teratogenic. Proceedings of the Society for
Experimental and Biological Medicine 223: 14–21.
BIOTIN/Physiology 523

BISCUITS, COOKIES, AND CRACKERS
Contents
Nature of the Products
Methods of Manufacture
Chemistry of Biscuit Making
Wafers
Nature of the Products
S Zydenbos and V Humphrey-Taylor,
New Zealand Institute for Crop & Food Research
Limited, Christchurch, New Zealand
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Biscuits, cookies, and crackers are enjoyed the world
over by consumers who select their favorites based on
flavor and appearance, but to bakers, distinguishing
between these baked products can be a complex task.
The basic characteristic that separates a biscuit,
cookie, or cracker from other baked products, such
as bread or cake, is a moisture content below 5%.
Biscuits, cookies, and crackers have a cereal base of at
least 60%. This is usually wheat but is sometimes oat,
barley, rye, or rice. Other major ingredients are fat or
shortening, and sugar. While water is also added to a
biscuit, cookie, or cracker dough, this is not a major
component of the final product since it evaporates
during baking.
0002 In this article, the history and usage of biscuits,
cookies, and crackers are examined. This is followed
by a discussion of the classification of biscuits,
cookies, and crackers, the definitions of which over-
lap and are geographically dependent. Finally, some
features of the different types of biscuits, cookies, and
crackers are considered.
History and Usage
0003 The origin of the name biscuit comes from Latin,
where bis coctus means twice-baked. There is also
an Old French word, bescoit, that has a similar mean-
ing. It is thought that these products have been baked
for thousands of years. The original process consisted
of baking the biscuits in a hot oven and subsequently
drying them in a cool oven. It is very rare to find this
double baking technique in modern biscuit produc-
tion. However, a special type of microwave oven is
often connected after a gas-fired oven in factories.
Cookie is derived from a Dutch word, koekje, which
means little cake, while the sound of a cracker being
eaten probably led to use of that name.
0004Biscuit, as defined above, is a term used in the UK
(and in New Zealand, Australia, and South Africa).
The word biscuit is also used for a baked product in
the USA. This is a leavened bread-like product that is
similar to the UK scone and is not discussed in this
article. In the USA, the word cookie describes the
same type of products that are called biscuits in
the UK. Cracker is a generic term used throughout
the world and refers to products with very low sugar
and fat contents.
0005The low moisture content of biscuits, cookies, and
crackers means that these products have a long shelf-
life and a relatively low risk of spoilage by micro-
organisms. This has led to a history of usage in epic
journeys, such as the flight of the Israelites from
Egyptian slavery and the sea voyages of the fifteenth-
century explorers. British and European traditions
usually served biscuits in a semiformal situation
with tea or coffee between main meals, especially in
the afternoon. Small biscuits were usually made so
that a range of appearances and flavors could be
eaten without a large intake of food. Modern biscuits,
cookies, and crackers are often used as a casual snack
food, especially in the USA. Many of these are much
larger than traditional biscuits, as they are sometimes
eaten to replace meals by consumers who have busy
lifestyles.
Classification
0006Biscuit classification varies according to geographic
location. Different and quite specific systems are used
in the UK and USA, while in other parts of the world,
the distinction between biscuits or cookies and cakes
is not as clear; these products are often classified as
small confectionery products. For example, biscuits
in France are often classified under the title petits
fours sec. These words literally translate as small
oven dry, giving the implication that these are small,
dry baked goods. However, petits fours secs may also
encompass other products that are outside the biscuit
524 BISCUITS, COOKIES, AND CRACKERS/Nature of the Products
