Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


the substrate. The simplest biosensor approach is to
measure amperometrically the oxygen depletion or
hydrogen peroxide production associated with the
reactions involving oxidase enzymes. The combin-
ation of simplicity, ease of manufacture, high sensi-
tivity, availability, and low cost of instrumentation is
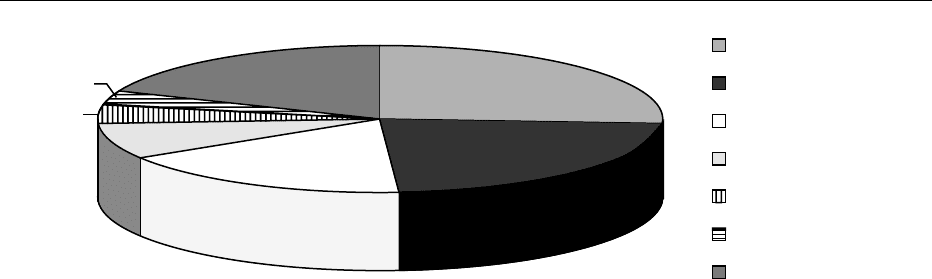
highly attractive in this type of devices. A variety of
enzymes belonging to classes of oxidoreductases, hy-
drolases, and lyases have been applied in enzyme-
based sensors. These enzymes can act on fatty acids,
sugars, amino acids, organic acids, and phenols. Bio-
sensors based on the use of redox mediators to shuttle
the electrons, resulting from the catalytic oxidation or
reduction of the substrate of interest, to the electrode
has been an area of great interest to many researchers.
Much of the work is centered on the use of redox
mediator compounds such as ferrocene, TTF, TCNQ,
hexacyanoferrate (III), methylene blue, quinones, and
their derivatives. Careful selection of the mediator
can also eliminate interference problems from other
substances in the sample.
0030 To date, there are several successful biosensor
devices on the market based on the different ampero-
metric transduction principles outlined above. These
include the MediSense mediated blood glucose ana-
lyzer for medical application and the Yellow Springs
hydrogen peroxide-based systems (YSI, Yellow
Springs, OH) developed for both medical and food
analysis and for bioprocess control. The YSI 2700
SELECT
TM
Biochemistry Analyser (Figure 5) can be
used for on-line fermentation monitoring or off-line
sample analysis for food and drink fermentation.
Nutrient and bioproducts analysis can be carried
out using the biosensor system including glucose,
l-lactate, l-glutamine, l-glutamate, ethanol, lactose,
sucrose, galactose, and methanol. Other commer-
cially available biosensors for food analysis include;
Sensomat B10, for alcohol analysis (Gonotec,
Germany); Microzym-L for lactate analysis (Kalger
GmbH, Germany) and Glu-11 for glucose analysis
(TOA Electronics, Japan). A range of single and
multianalyte biosensors based on the same principle
are being developed for a diverse range of applica-
tions in the food industry.
0031The majority of biosensors developed for food
analysis are water-based systems. Organic-phase
enzyme electrodes, however, have been developed to
work in organic solvents and have been used for the
analysis of compounds such as cholesterol, alcohols,
organic peroxides, and phenols. This technique has
the advantages of ease of sample preparation from
materials, such as fats and oils, and increased enzyme
stability. In principle, analytes soluble in fatty sub-
stances can also be measured using biosensors.
Enzyme biosensors have also been widely applied to
the detection of phenolic compounds. Contamination
by these aromatic compounds results from the pro-
duction of drugs, dyes, and antioxidants and also
from the paper and pulp industry. Enzymes used in
biosensor development for this type of compounds
are phenol oxidases (tyrosinases), laccases, and per-
oxidases.
0032Biocatalytic sensors based on enzyme inhibition are
the most commonly reported biosensors for the detec-
tion of toxic compounds and heavy metal ions. These
sensors are based on the selective inhibition of specific
enzymes by classes of compounds, or by more general
inhibition of enzyme activity. The measurement of the
enzyme activity can be used as a screening test to
evaluate the contamination by compounds such as
organophosphorous and carbamate insecticides and
the triazine herbicides and other compounds that
display similar toxicological behavior. Enzymes used
include: choline esterase, horse-radish peroxidase,
polyphenol oxidase, urease, and aldehyde dehydro-
genase.
0033Biosensors based on the use of whole cells as
the sensing layer generally respond to more than
one analyte, but can offer increased stability when
compared with enzyme-based sensors. Whole-cell
biosensors have been utilized commercially for envir-
onmental monitoring and capitalize on the broad
sensitivity of microorganisms to a wide range of
toxins. Whole-cell biosensors may contain living bac-
teria, yeast, fungi, plants, or animal cells. Biosensors
able to monitor indicators of pollution, such
as biochemical oxygen demand (BOD), have been
developed. Sensors such as the ARAS BOD Biosensor
System (Dr Bruno Lange GMbH, Du
¨
sseldorf,
fig0005 Figure 5 YSI 2700 SELECT
TM
Biochemical Analyzer. Photo
courtesy of YSI incorporated, Yellow Springs, USA.
BIOSENSORS 495

Germany), the BOD module biosensor produced by
(Prufgerate-Werk Medigen, Germany), and Nissin’s
(Japan) BOD Sensor are commercially available. A
range of biosensors based on this principle have been
developed for polluting compounds such as pesti-
cides, in particular for water monitoring.
0034 Electrochemical sensor approaches to hygiene
monitoring based on the metabolism of living cells
have also been commercialized. The Bactometer (Bac-
tomatic Inc., Princeton, NJ) and the Malthus 2000
(Malthus, Stoke-on-Trent, UK) use impedance and
conductance, respectively, to plot bacterial growth.
A more rapid approach is the use of amperometry.
The method comprises the use of mediators to detect
the metabolic activity of the cells, and the resulting
current can be related to the numbers of organism
present in the sample.
0035 Catalytic optical biosensors are usually based on
optical fibers or planar wave-guide films. Various
fiber-optic biosensors based on pH opt(r)odes have
been constructed for glucose, urea, penicillin, and
creatinine analysis. Commercial examples of fiber
optic sensors for monitoring blood pH, dissolved
oxygen and carbon dioxide concentrations. The
transfer of these technologies to the agrofood and
environmental sector can be anticipated in the near
future.
Affinity Sensors
0036 Antibodies have been used as recognition elements in
tests and assays since the 1950s and in biosensor
devices since 1970s. In the past decade, there has
been phenomenal growth in affinity-based sensor re-
search and development. Affinity-based sensors can
be define as analytical devices that use an antibody,
receptor protein, or other molecules with affinity-
recognition powers interfaced to a signal transducer
to measure a binding event. Various research groups
have pioneered different types of affinity-based sensor
configurations. By utilizing the immunorecognition
properties of antibodies in immunosensors and affin-
ity sensors, the range of analytes that can be measured
has been broadened considerably.
0037 Immunosensors offer a wide range of potential
applications to the food industry, water companies,
and regulatory authorities. In immunosensors or
immunoprobes, the antibody (Ab) or the antigen
(Ag) constitutes the biospecific component in the
sensor structure. A range of immunosensors are being
developed, and with minor adaptations, these can
replace immunoassays. At present, immunodiagnos-
tic tests are used in pollution detection in food
and water samples and also on-farm monitoring of
livestock reproduction (milk progesterone), and
quality control of foodstuffs originating from live-
stock production (authenticity and adulteration
testing). These tests can be developed to a new gener-
ation of rapid immunosensors for real-time and on-
site applications. A range of research projects have
been undertaken to develop immunosensors for resi-
dues in food (antibiotics, toxins, and pesticides), the
presence of additives, and hormones. The availability
of the required antibody can limit the potential for
diverse analyte detection by immunosensors. In-
creased research in the development of specifically
tailored antibodies such as plantibodies produced in
plants, recombinant antibodies, catalytic antibodies
or abzymes, artificial receptors, and molecularly
imprinted polymers should overcome some of these
problems.
0038Immunosensors can be divided into classes
depending on the transducer technology employed.
Electrochemical immunosensors generally rely on the
use of electroactive labels, usually based on enzyme
labeling and amplification techniques. The sensors
can be inexpensive and may achieve low detection
limits (< 1 p.p.b.). Different types of electrochemical
immunosensors and affinity sensors have been de-
veloped for environmental analysis using various
transducers. The scientific literature increasingly con-
tains reports of the development of one-shot dispos-
able or on-line immunosensors for a diverse range of
analytes. However, the technology required to manu-
facture these types of sensors has not yet reached the
level of sophistication achieved for enzyme elec-
trodes, and hence, these devices are at an earlier
stage of commercialization.
0039Optical devices have a clear advantage over elec-
trochemical methods due to their ability to monitor
binding reactions directly. The major drawback to the
application of optics remains the high cost of many
optical components, but these costs are constantly
falling. Optical phenomena such as SPR and evanes-
cent wave (EW) technology have shown promise in
providing direct measurement of Ag–Ab interactions
occurring at the surface–solution interface, the sur-
face usually consisting of a glass prism on a gold or
silver metal layer. SPR is a phenomenon that occurs
when a beam of light is directed on to a glass–metal
interface, which results in changes in the resonance
angle. The BIAcore
TM
biosensor system based on SPR
technology was developed by a Pharmacia (Uppsala,
Sweden) spin-off company and is now commercially
available from Biacore AB (Uppsala, Sweden). This
instrument represents a significant breakthrough in
optical immunosensor technology. BIA technology
enables detection of biomolecules in real time without
the use of labels, but the instruments are expensive
and mainly laboratory-based, and require trained
496 BIOSENSORS
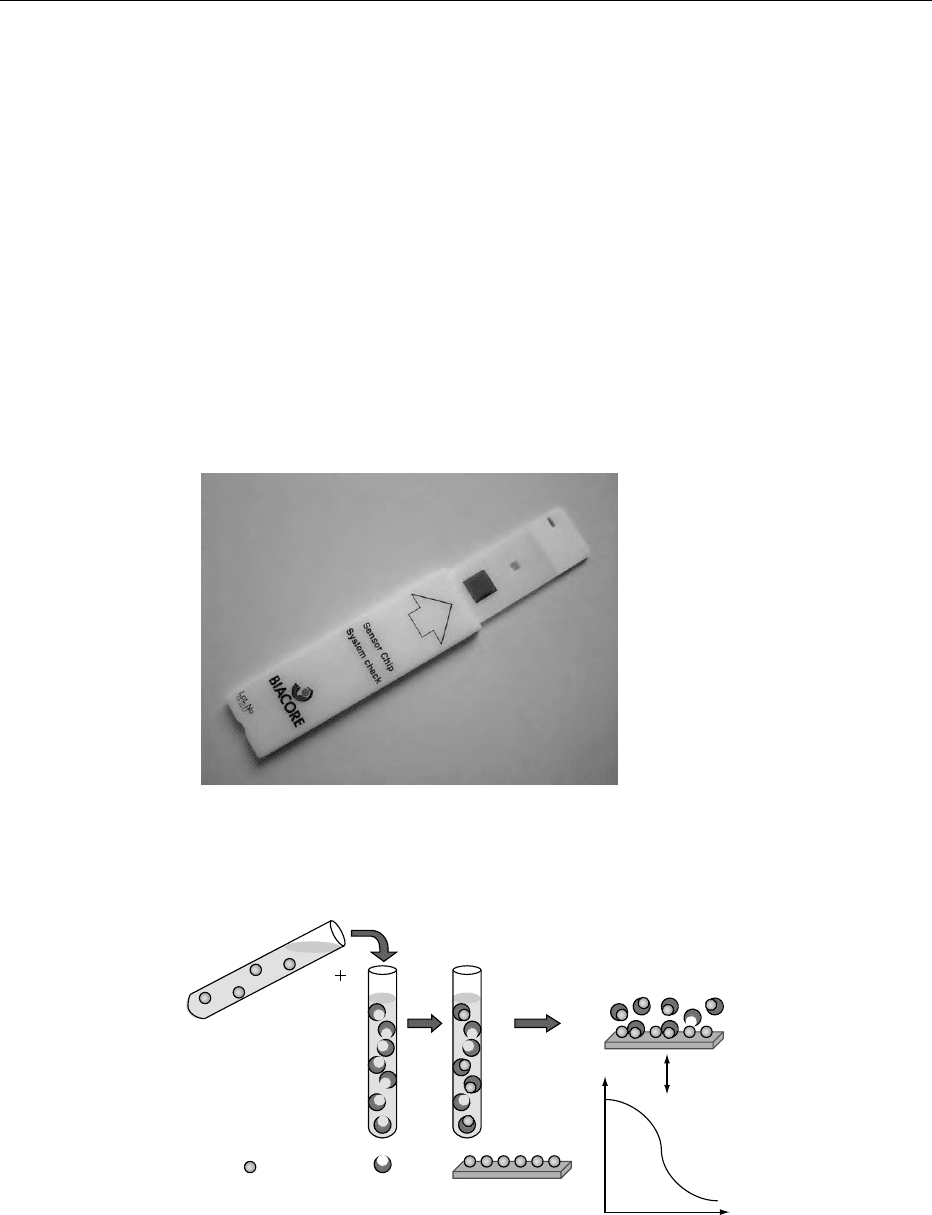
personnel to interpret the results. The Biacore system
has been reported to be able to detect atrazine at
0.05 mgl
1
and E. coli O157 in food samples. Re-
cently, Biacore AB have launched the BIAcore-
Quant
TM
, for the automated analysis of vitamins in
foods (Figure 6). The assay is designed as an inhib-
ition assay, and similar principles are applied for the
determination of drug residues in meat and milk
products using BIACORE 2000. Amersham Inter-
national plc has been active in research into SPR-
based immunosensors for infectious diseases and has
developed immunotechnology that employs anti-
bodies labeled with latex particles (beads). The
beads amplify the change in refractive index at the
sensor surface–solution interface, which occurs when
the antibodies bind to the immobilized antigen layer
and thus cause a large change in the resonance angle.
An alternative direct optical immunosensor to com-
pete with SPR technology has been developed by
Affinity Sensors (Cambridge, UK). The resonant
mirror technology is marketed as the IAsys (IAsys
and IAsys Autoþ) and also enables analysis of bio-
molecular interactions in real-time. Reusable cuvettes
offer a choice of derivatized sensor surfaces and
chemistries. The ligand is simply and efficiently im-
mobilized on to the sensor surface, and binding of the
analyte can be detected immediately.
0040The alternative approach of using fluorecent labels
detected via evanescent wave interactions with an
optical fiber has also been applied successfully. One
example is the Raptor developed by the US Navy
Research Laboratory for the detection of biological
Vitamin-specific molecules are added to sample extract
(a)
(
b
)
Vitamin-specific
molecule
Vitamin Vitamin sensor
chip
Measurement of unbound
vitamin-specific molecules
Vitamin
concentration
Response (RU)
Sensor chip
fig0006 Figure 6 The BiacoreQuant
TM
surface plasmon resonance system for the automated analysis of foods, (a) the sensor chip, (b) the
procedure. Photo courtesy of Biacore AB.
BIOSENSORS 497

warfare agents. Unilever (Bedfordshire, UK) has de-
veloped a fluorescence-based EW immunosensor that
incorporates a novel capillary-fill design. The system
consists of two glass plates separated by a narrow
capillary gap of * 100 mm. The lower plate acts as
an optical waveguide and contains an immobilized
layer of antibodies on its surface. These sensors bene-
fit from the capillary fill system, which draws a fixed
volume of sample into the space between the plates,
regardless of bulk sample volume.
0041 Piezoelectric transduction approaches and, in par-
ticular, SAW devices have also been widely used to
detect antibody binding to an immobilized antigen.
The most widely used acoustic transducer is the
quartz crystal microbalance, which measures small
changes in surface properties, such as bound surface
mass and viscosity, resulting from the binding of mol-
ecules to the sensor surface. Again, the attraction
of piezoelectric sensors is their ability to monitor
directly the binding of Ab–Ag reaction encountered
in affinity sensing, but they suffer from the disadvan-
tage of having a low sensitivity for small molecules.
0042 Potential areas of application for affinity-based
sensors are similar to those for biocatalytic sensors.
However, affinity sensors can offer enhanced sensitiv-
ity and specificity when compared to biocatalytic
sensors. Commercial immunosensors may not have
been developed specifically for use in the agrofood
and environmental sectors yet, but their long-term
potential is evident. As the commercial success of
immunoassays becomes more evident in health care,
food, and environmental monitoring, the demand for
faster techniques will be sufficient for continued af-
finity sensors development. The development of dis-
posable electrochemical affinity sensors for the rapid
detection of residues of anabolically active illegal
androgens, antibiotics, and other residual compounds
in food samples would allow regulatory bodies to
improve their screening program. A potential market
for immunosensors is genetically modified (GM)
foods testing for protein analysis. Immunoassay tests
as a plate test or dipstick devices for the detection of
GM foods in plant tissues, raw agricultural commod-
ities, and also food ingredients are marketed today by
SDI Europe Ltd. (Hampshire, UK). These types of
tests may well be developed into immunosensors
devices in the near future.
DNA Biosensors
0043 DNA is a double-stranded helical molecule that can
be split into its two complementary strands by treat-
ment with alkali. By using the correct environmental
conditions, the two strands can come together again
(reassociate) in a process that forms the basis of all
DNA hybridization techniques. This technique in-
volves a small quantity of a pure DNA probe that is
labelled and made single-stranded so that it can seek
out and hybridize to its complementary sequence in a
large amount of DNA. The probes can be labelled
with an enzyme marker. Nucleic acids, the building
blocks of DNA and RNA, are universally present in
all living cells and are utilized as a general method of
microbial detection and identification. PCR is a
widely used method for amplifying trace amounts of
DNA for analysis. To date, it has been used mainly for
qualitative analysis, but the need for quantitative in-
formation has resulted in further development of PCR
protocols. Quantitative PCR methods are shown to
be extremely sensitive, and fully automated detection
systems that should be rapid, simple to use, and inex-
pensive are being developed. DNA biosensors are
currently used in the detection of infectious diseases
and genetic abnormalities, where the main approach
has been by the use of immunobased sensors. This
technology is well established, and the performance
of these tests is good. PCR is widely used to amplify
the signal in DNA probes, but it is time-consuming.
There is intense current interest in microsystems for
DNA analysis. An electrochemical DNA sensor based
on a gold electrode modified with DNA probes and
an electroactive hybridization indicator has been
reported in the literature. A range of detection
systems have also been applied in DNA probes such
as the use of enzyme label, an SPR-based biosensor,
an EW biosensor and an acoustic-based sensor.
Affymetrix (Santa Clara, CA) has developed a plat-
form for acquiring, analyzing, and managing com-
plex genetic information. The system is based on
disposable DNA probe arrays (GeneChip) containing
selected gene sequences on a chip, and an instrument
to process each chip, and analyze the information.
This ‘lab-on-a-chip,’ offers traditional, laboratory-
based biological assays in convenient on-site or
on-line instruments. DNA chips are used for micro-
organism identification or detection of GM foods.
The company is developing new chips for microbial
contamination diagnosis in environmental samples.
GeneScan Europe (Hanse Analytik GmbH, Freiburg,
Germany) markets test kits for the detection of gen-
etically modified components in human and animal
foods by DNA analysis using PCR techniques. These
tests can also be developed into DNA biosensor
devices.
Electronic Noses
0044The application of the electronic nose concept or
artificial olfaction has been of tremendous emerging
importance in recent years. Electronic noses are based
498 BIOSENSORS

on the use of artificial receptors in the sensor array,
and their application in food analysis justifies their
inclusion in this article. In recent years, a great deal of
research in the development of the electronic nose has
taken place with a diverse range of applications. This
type of sensor instrument mimics the olfactory system
in the nose. The instrument consists of an array of gas
sensors with different selectivity patterns, a signal
collecting unit, and data analysis software, which
analyses the signal by pattern-recognition methods,
such as principal component analysis, discriminant
function analysis, cluster analysis, and artificial
neural networks. The results are comparative, rather
than quantitative, and are presented as a ‘fingerprint.’
A variety of sensors are used, ranging from metal
oxides to conducting polymers, which can be highly
sensitive but not specific and can respond to volatile
compounds with molecular weight ranging from 30
to 300. Molecules such as alcohols, ketones, fatty
acids, and esters give a strong response, whereas
fully oxidized species, such as CO
2
,NO
2
, and H
2
O
have a lower response. The sensor array can also
recognize molecules containing sulfur and amine
groups. Furthermore, they are not in direct contact
with the measuring media, since they are used for gas-
phase measurements.
0045 There has always been considerable waste during
the storage and handling of food. Moreover, food
often constitutes an ideal growth medium for micro-
organisms. The composition of volatile compounds
evolved from the food can reflect the activity and type
of microorganisms present and also can be related to
food quality. Thus, electronic noses appear to be a
useful means of detecting these kinds of changes.
Electronic noses have been used in medical, environ-
mental, and food diagnoses. The majority of the
current research concentrates on quality control in
the foods and drinks industry, such as the detection
of microbial contamination (bacteria, fungi, and
yeast), freshness of meat and fish, and authenticity
(beverages, coffee, and meat).
Conclusions
0046 The food and drink industry deals with a wide range
of materials and products with different characteris-
tics and diverse processing procedures. The shared
theme, however, is that the raw materials are organic,
subject to environmental pollution, degradations
and microbial contamination. The food diagnostics
market is expanding rapidly and covers a wide range
of disciplines. The establishment of appropriate tech-
nologies to apply biosensors to practical agriculture
and horticulture is expected to produce a significant
effect on quality improvement and cost reduction.
Biosensor applications in the medical diagnostics
market have been highly successful, but their poten-
tial success in the food, agriculture, veterinary diag-
nosis, and environmental market still remains to be
established. Biosensor systems, which are relatively
small portable instruments, have an on-site applica-
tion, and their relatively low cost is clearly desirable
in agrofood analysis. Research is being undertaken in
diagnostic companies and research institutions to
develop biosensor technologies for the food and en-
vironmental sector. Nevertheless, moving the technol-
ogy to the market place involves many challenges.
Biosensors will have to offer perceived advantages
over existing and competing technologies.
See also: Enzymes: Functions and Characteristics;
Nucleic Acids: Properties and Determination;
Genetically Modified Foods.
Further Reading
Cunningham AJ (ed.) (1998) Introduction to Bioanalytical
Sensors. New York: Wiley-Interscience.
Fellows PJ (ed.) (1996) Food Processing Technology,
Principles and Practice, Series in Food Science and
Technology. Cambridge: Woodhead.
Kress-Rogers E (ed.) Handbook of Biosensors & Electronic
Noses. Boca Raton, FL: CRC Press.
Mathewson PR and Finley JW (eds) (1992) Biosensors
Design and Application, ACS Symposium Series 511.
Washington, DC: American Chemical Society.
Mulchandani A and Rogers KM (eds) (1998) Methods in
Biotechnology; Enzyme and Microbial Biosensors.
Techniques and Protocols. Totowa, NJ: Humana Press.
Rogers KR and Mulchandani A (eds) (1998) Methods in
Biotechnology 7, Affinity Biosensors, Techniques and
Protocols. Totowa, NJ: Humana Press.
Scott AO (ed.) (1998) Biosensors for Food Analysis. Cam-
bridge: The Royal Society of Chemistry.
Turner APF (ed.) Advances in Biosensors. volumes 1; 2;
Suppl 1; 3; 4 (1991; 1992; 1993; 1995; 1998). London/
Amsterdam: JAI Press/Elsevier.
Turner APF (2000) Biosensors McGraw Hill Yearbook of
Science and Technology 2000. New York: McGraw Hill.
Turner APF, Karube I and Wilson GS (eds) (1989) Biosen-
sors: Fundamentals and Applications. Oxford: Oxford
University Press.
BIOSENSORS 499

BIOTECHNOLOGY IN FOOD PRODUCTION
SPe
´
rez-Magarin
˜
o and M L Gonza
´
lez-Sanjose
´
,
University of Burgos, Burgos, Spain
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Biotechnology’s origins lie in the ancient crafts of
brewing, baking, and the production of fermented
foods such as yogurt and cheese, although it was
not until 1859 that microorganisms were identi-
fied as the cause of both desirable and undesirable
changes in food. During the 1940s, microorgan-
isms, as submerged cultures in large vessels, were
developed. Nowadays, similar fermentation tech-
nology is applied to obtain a wide range of bio-
products.
0002 In the 1950s, the primary structure of proteins and
DNA was first determined. These discoveries led to
the development of molecular biology in the 1960s
and genetic engineering techniques in the 1970s.
Techniques developed using microorganisms have
been applied to plants and animals, and these trans-
genic plants and animals have been produced to make
many useful products.
0003 For centuries, humans have been selecting, sowing,
and harvesting seeds to produce better food more
efficiently. In recent decades, global food demand
has increased enormously, and biotechnology has
offered the technology required to produce more
nutritious and better-tasting foods, higher crop
yields, and plants protected against disease and
insects.
Definition of Biotechnology
0004 The term ‘biotechnology’ has come to mean different
things to different people. In general, biotechnology is
the integration of natural sciences and engineering in
order to provide new products and services by the
application of organisms, cells, parts thereof, and
molecular analogs.
0005 In a broad sense, biotechnology covers many of the
tools and techniques that are commonplace in agri-
culture and food production. However, in a narrow
sense, biotechnology considers only the new DNA
techniques, molecular biology, and reproductive
technological applications. Thus, ‘biotechnology’ for
many people refers specifically to the genetic engin-
eering techniques that have been developed in the last
30 years.
Food Biotechnology
0006Biotechnology provides powerful tools for the sus-
tainable development of agriculture as well as the
food industry, improving the production of foods
and making them more available and nutritious.
Food biotechnology is a rapidly expanding field of
science with many different applications, which is
clear from the large number of scientific papers pub-
lished in the last 10 years. The Food Science and
Technology Abstracts (FSTA) database reveals at
least 4150 articles on Food Biotechnology published
between 1990 and 2000, which represents almost
4% of the number of total published papers on food.
0007According to FSTA information and the current
situation of the food industry, some of the main
areas of application of food biotechnology are: the
production of new kinds of foods and drinks, both by
modern developments of conventional techniques
and by genetically modifying the products them-
selves, or producing them using genetically modified
(GM) organisms or their products.
0008The majority of articles deal with fermentation
processes, the use of enzymes and microorganisms
(Figure 1). These fields have been the most important
and traditional applications of biotechnology in food
production. However, in the last few years, investi-
gations into genetic engineering, molecular biology,
and cell cultures have been increasing significantly.
Traditional Food Biotechnology
0009Traditional biotechnology refers to the conventional
techniques that have been used for many centuries to
produce beer, wine, cheese, bread, and other foods,
all of them foods obtained by fermentation processes.
0010The fermentation of food is the oldest biotechno-
logical process, and is the oldest form of food preser-
vation. In brief, fermentation is anaerobic conversion
of certain carbohydrates (such as reducing sugars,
mainly glucose) to other products such as:
.
0011alcohol, glycerol and carbon dioxide, the principal
products of the fermentation of yeast sugars (alco-
holic fermentation);
.
0012butyl alcohol, acetone, lactic acid, and acetic acid,
the principal products of bacterial action (such as
acetic, lactic, and malolactic fermentation).
Of the many possible types of fermentation processes,
lactic acid fermentation and alcohol fermentation are
the two most common. These processes are carried
out by specific microorganisms (several yeasts and
500 BIOTECHNOLOGY IN FOOD PRODUCTION
bacteria), and they are regulated by the action of
specific enzymes.
0013 Traditional biotechnology is most closely related to
food production both in the selective breeding of food
plants and animals and in food processing using
microbial enzymes. Traditional selection techniques
have been employed to develop a great variety of
plants, animals, and microorganisms for the produc-
tion of a wide range of food products and ingredients
for processed foods.
0014 In addition, enzymes have been applied to practical
uses for thousands of years, in the form of crude
animal or plant preparations or as a consequence of
permitted microbial development. Improvements in
modern techniques, which allow purification and
purification of specific fungal and microbial enzymes
and their production at an industrial level, has made
it possible to use enzymes in order to facilitate and
improve some food manufacturing processes.
0015 Current technology for dealing with complex or-
ganisms advances modern biology and permits us to
understand many traditional processes in which bio-
logical agents are involved, allowing the application
of new fermentation and enzyme technological pro-
cesses to food production. This new situation has
opened the doors to an exciting dimension of food
biotechnology, which has been called ‘modern food
biotechnology.’
Modern Food Biotechnology
0016 Modern biotechnology embraces all methods of
genetic modification by recombinant DNA and
cell-fusion techniques together with the modern
developments of ‘traditional’ food biotechnological
processes. Genetic modification techniques (GMT)
are now being used in the production of new foods
and drinks. Genetic modification involves the inser-
tion of one or a small number of scientifically well-
characterized genes into the food plant, animal,
or microorganism. These techniques will play an
increasingly useful and economic role, and they are
strongly related to fields such as genetic engineering,
molecular biology, protein engineering, biochemical
engineering, and processes involving monoclonal
antibodies, which are beginning to have a consider-
able impact on food processing.
0017Modern biotechnology will pursue not only the
production of new foods but also the preservation of
raw materials, increased shelf-life of final products,
and the planned alteration of their nutritional and
functional properties. It also affects the development
of processing aids and direct additives that can
improve the overall utilization of materials. GMT
are being used to achieve many of the same aims as
traditional breeding and selection methods but have
at least two main advantages. First, they provide the
means of controlling the introduction of genes with
much greater prediction and precision than can be
achieved by traditional methods. Second, they make
it possible to introduce copies of genetic material into
unrelated species hitherto impossible to achieve by
traditional techniques.
0018Some applications of genetic engineering tech-
niques are:
.
0019increasing the resistance of plants to diseases and
pests, herbicides or pesticides;
.
0020developing plants to withstand more extreme
environmental conditions (drought, frost, and soil
salinity, amongst others);
.
0021developing foods with specific properties and im-
proved quality (sensorial and nutritional character-
istics);
.
0022adapting microorganisms for more efficient food
production and to produce natural food ingredients
(amino acids, organic acids, volatile fatty acids,
vitamins, etc.);
.
0023obtaining enzymes, antibodies and microorgan-
isms to monitor food production and processing
Fermentation
Enzymes
Microorganisms
Genetic engineering
Molecular biology
Cell cultures
Other
17%
23%
26%
19%
3%
4%
8%
fig0001 Figure 1 Percentage of published papers on different fields of biotechnology in food production.
BIOTECHNOLOGY IN FOOD PRODUCTION 501

systems for quality control. Microbial probes and
biosensors are being used experimentally as indica-
tors of bacterial and other types of contamination;
and
.
0024 improving animal farming by genetic modification
of crop plants for animal feed, of microorganisms
or enzymes to improve the nutritional value of
animal food stuffs and in the animal health sector
for pharmaceuticals.
Benefits and Risks of Food Biotechnology
0025 For centuries, farmers have relied on the newest tech-
nology to produce and enhance foods that possess
specific beneficial traits. The use of biotechnology
benefits not only the grower or producer but also
the consumer.
0026 The current benefits of biotechnology include dis-
ease resistance, reduced pesticide use, herbicide toler-
ance, more rapid growth of crops, producing higher
yields. Hence, biotechnology benefits the environ-
ment, including water and soil conservation, and is
safer for workers and the ecosystem. Also, it can
enhance the nutritional characteristics of foods and
improve food taste and quality.
0027 Biotechnology has been used in a number of crops
for several years. The volume of biotechnological
crops in developmental stages continues to grow. In
the early 1990s, many people were skeptical that
transgenic crops could provide improved products.
During the last five years, the planting of transgenic
crops has increased, thus demonstrating that the early
promises of transgenic crops are meeting the expect-
ations of farmers of both large and small farms
planting these crops in both industrial and developing
countries. However, two-thirds of total transgenic
crops are in the developing countries, where yields
are lower, and the need for improved production of
food is the greatest.
0028 More genetically enhanced products are expected
to be on the market in the years to come, many of
which are similar in nature to products already on the
market. Benefits that can be expected in the near
future include:
.
0029 reduced levels of natural toxins in plants;
.
0030 simpler and faster methods to locate pathogens,
toxins, and contaminants; and
.
0031 extending the time before spoilage.
The world population is expected to double by the
year 2050. Few other new technologies will be able to
approach biotechnology’s potential to help avoid
starvation in the next century. Another economic
and environmental benefit is in the area of fertilizer
use. Much of the fertilizer used is wasted and can end
up in water sources, damaging the environment.
Some plants, such as corn, might also be modified to
draw nitrogen from the soil, thereby reducing the
need for fertilizer. Benefits that can be expected fur-
ther down the road include:
.
0032producing safer foods through reduction of aller-
genic proteins;
.
0033drought and flood tolerance;
.
0034salt and metal tolerance; and
.
0035heat and cold tolerance.
In general, according to most scientific papers pub-
lished, the use of biotechnology poses no specific risk,
and products should not be discriminated against on
the grounds of their method of production. The sci-
entific consensus is that the risks associated with food
biotechnology are fundamentally the same as for
other foods. Current science shows that foods made
using biotechnology are safe to consume and safe for
the environment. Although there is no such thing as
‘zero risk’ for any food, consumers can be confident
that foods produced using biotechnology meet the
government’s most stringent food-safety standards.
Years of research and no evidence of promoting
health harm indicate that the benefits of agricultural
biotechnology far outweigh any risks.
0036However, there are people, amongst them ecolo-
gists and activist groups, who do not agree with this.
Some of their arguments against biotechnology are
that the use of GM products could reduce genetic
diversity indirectly if, for example, farmers adopt
genetically uniform varieties of plants and other
organisms. The inclusion of novel genes for herbicide
resistance in plants may increase the occurrence
of weeds with resistance to certain agrochemicals.
Others are concerned about the possible appearance
of allergens or toxins and possible causes of different
human diseases.
0037Thus, despite the benefits to be gained from modern
biotechnology, the products obtained need to have
appropriate legislation and safeguards to protect
human health and the environment.
Current Concerns, Regulations, and
Labeling
0038According to the concerns expressed about the pos-
sible existence of health hazards associated with the
consumption of food containing modified genetic
material, it is clear that rational policies are needed,
regarding the regulatory status of bioengineering
products.
0039Studies about public perceptions, consumer atti-
tudes, and ethical implications of biotechnology in
food and drink production have shown that certain
502 BIOTECHNOLOGY IN FOOD PRODUCTION

applications are more acceptable than others. For
example, according to the European Federation of
Biotechnology, the use of biotechnology to change
plants was considered more acceptable than using it
to change animals. In addition, around 85% of inter-
viewees said that food obtained with genetic tech-
niques should be clearly labeled. It will be necessary,
therefore, to develop programs to educate the public
about the benefits and problems of biotechnology in
the production and processing of food. Governments
and scientific institutions must be encouraged to
share knowledge on transgenic foods with society,
such that people are well informed about the impact
of biotechnology on the environment, food safety,
sustainability, and global food safety. In addition, as
with any technology, there are limits that will and
must be subjected to law. Countries must be helped
to develop appropriate legislation and to set up
proper regulatory bodies for all aspects of biosafety.
Appropriate regulation is a prerequisite from the
point of view of both the general public and the
food industry.
0040 In the USA, there are three organizations respon-
sible for controlling GM organisms and products.
The Environmental Protection Agency (EPA) and
the United States Department of Agriculture (USDA)
oversee the effects of GM organisms on the environ-
ment and on plant production. The Food and Drug
Administration (FDA) is responsible for overseeing
the safety of food, which is determined by the charac-
teristics of the consumed or processed product rather
than the method by which it was produced. The FDA
policy statement also addresses the labeling of foods
obtained from new plant varieties. It states that label-
ing is only required when a food obtained from a GM
variety differs from that obtained from a conven-
tional counterpart.
0041 Nowadays, in Europe, there is no equivalent over-
all agency for food regulation, although a European
Institute will be in operation in the near future. Until
this time, the European Commission has therefore
proposed harmonizing regulation between member
states. The Council of the European Community
(EC) adopted the Directive 90/220/EC on the deliber-
ate release into the environment of GM organisms.
This directive had two main objectives: to protect
human health and the environment and to provide a
harmonized regulatory framework of GM organisms
with the EC.
0042 In the EC, the safety of GM foods is controlled by
Regulation (EC) No. 258/97 concerning novel foods
and novel food ingredients, and the labeling of GM
foods is regulated by two regulations, with important
differences with American Regulations. First, Com-
mission Regulation (EC) No. 49/2000 establishes that
the foodstuffs shall not be subject to the additional
specific labeling requirements when the material de-
rived from the GM organisms is present in a propor-
tion no higher than 1% of the food ingredients
individually considered or food comprising a single
ingredient, or this presence is adventitious. Second,
Commission Regulation (EC) No. 50/2000 estab-
lishes the labeling of foodstuffs and food ingredients
containing additives and flavorings that have been
genetically modified or have been produced from
GM organisms. In addition, novel foods and GM
foods also have to fulfill the general labeling require-
ments set out in the Directive 2000/13/EC, which
deals with an approximation of the laws of the
Member States relating to the labeling, presentation,
and advertising of foodstuffs.
0043With so many foods traded internationally, there is
a clear need to reach a harmonized approach to label-
ing GM foods if potential trade barriers are to be
eliminated. Therefore, it will be necessary to develop
international safety guidelines.
0044In 1990, Food and Agriculture Organization (FAO)
and the World Health Organization (WHO) took the
first steps towards international harmonization of the
food safety assessment of GM foods. It was recom-
mended that the safety assessment of foods produced
by biotechnology should take into account the mo-
lecular, biological, and chemical characteristics of
these foods. The 1996 FAO/WHO report defined
substantial equivalence as ‘established by a demon-
stration that if the characteristics assessed for the GM
organisms, or the specific food product derived there-
from, are equivalent to the same characteristics of the
conventional food, then it can be assumed that the
new food is as safe as the conventional equivalent.’ In
1999, the FAO/WHO of Codex Alimentarius estab-
lished the Codex Ad Hoc Intergovernmental Task
Force on Foods derived from Biotechnology to elab-
orate norms and guidelines for the foods derived from
biotechnology. The Task Force held its first session in
March 2000, and approved the elaboration of major
texts with a set of broad general principles for risk
analysis and specific guidance on the risk assessment
of foods derived from biotechnology. It will also have
to prepare a list of available analytical methods in-
cluding those for the detection or identification of
these kinds of foods.
0045The Task Force agreed that the labeling was
covered by the Codex Committee on Food Labeling,
and the environmental risk was addressed by other
bodies such as the Cartagena Biosafety Protocol
under the Convention on Biological Diversity, the
International Plant Protection Convention, and the
Commission on Genetic Resources for Food and
Agriculture.
BIOTECHNOLOGY IN FOOD PRODUCTION 503
Biotechnology in the Food Industry
0046 As we said earlier, biotechnology applied to food
production has a long history, beginning in cottage
industries such as bread-making, cheese-making, and
brewing. Many biotechnology advances have been
made in the food industry over the last century. A
major advance has been in food preservation, which
is basically the prevention of undesirable microbial
and enzyme activity in foodstuffs. This allows the
storage of food over much longer periods and permits
transport over great distances. The development of
genetic techniques also represents a great advance,
providing opportunities for much innovation.
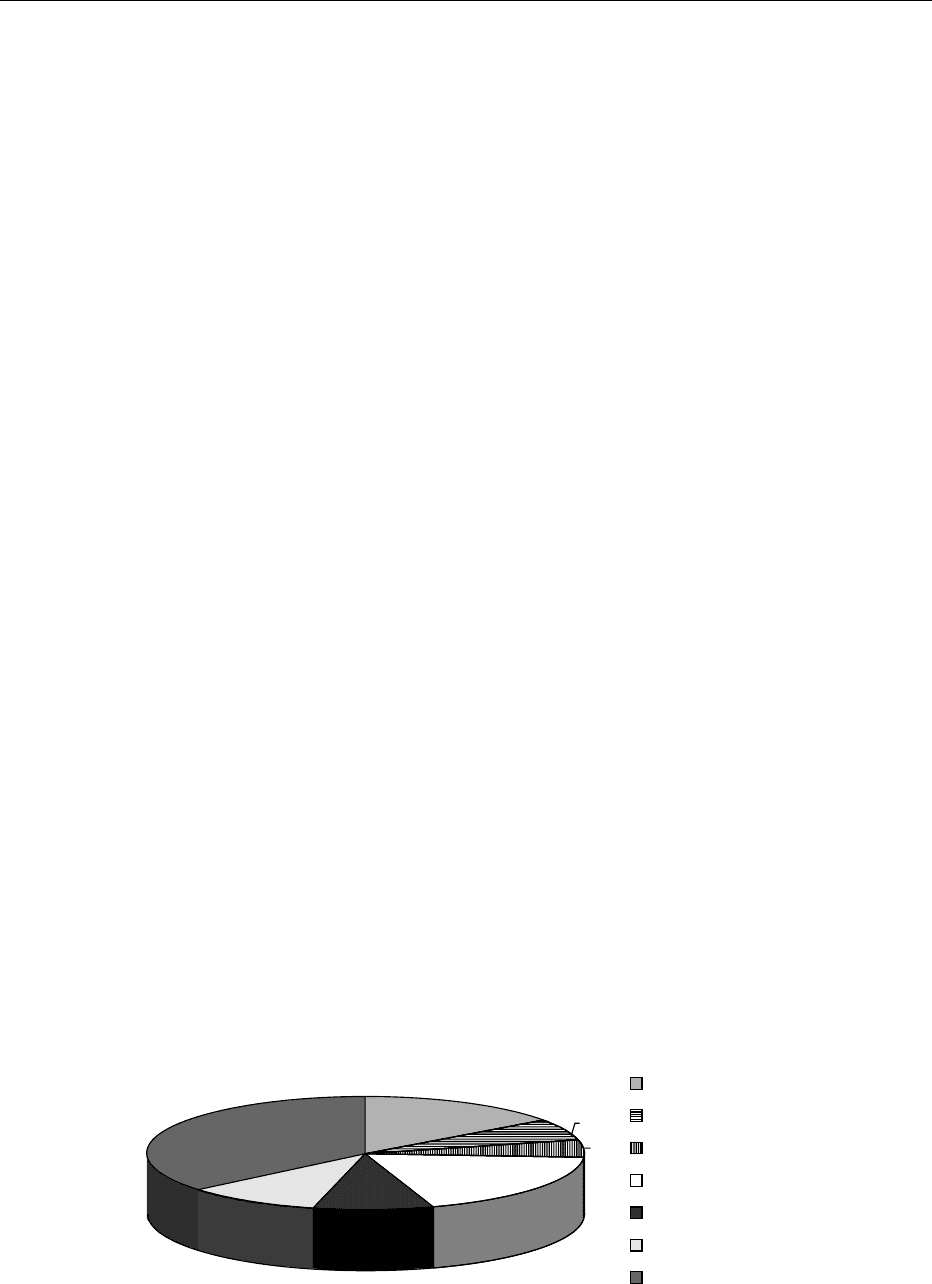
0047 According to the data obtained from the FSTA data
base (Figure 2), food biotechnology processes are
applied more to manufacturing foods of vegetable
origin (38%) than to foods of animal origin (26%).
In addition, the fields of study covered by the two
largest groups of papers were vegetables and fruits
first (19%) with dairy products in second place
(15%). Papers on beverages, cereal products, meat
products and fish products have been published
more or less in the same quantity. During the last
few years, the most frequent applications of biotech-
nology in food industries have been as follows.
Biotechnology in the Vegetable and Fruit Industries
0048 Enzyme applications are very common in this indus-
try. The presence of acid phenols and tannins in fruits
has been studied, owing to their effect on bacterial
growth during lactic fermentation.
0049 Pectinolytic enzymes and cellulolytic enzymes are
the most commonly used. These enzymes have appli-
cations in plant tissue maceration and in fruit juice
processing, improving the clarification of juices and
manufacturing processes of fruit such as peeling.
Owing to the importance of these enzymes, many
works have studied their activities during the ripening
of fruits. These enzymes have even been extracted
from fruits and purified later to be characterized
and applied in different food industries.
0050Vegetables and fruits are used as substrates in some
reactions, owing to their enzymatic systems, to
produce other chemical compounds, such as volatile
flavor compounds, lipids, vitamins, enzymes, pig-
ments, etc.
0051The genetic variation of some vegetable and fruit
crops, and the effect of genotype and environment
on yield and quality, have been investigated, using
random amplified polymorphic DNA. This DNA-
based technology and others have resulted in a sub-
stantial increase in the number of genes identified and
characterized, which will make it possible to produce
transgenic crops of several vegetables and fruits.
Biotechnology in the Beverage Industries
0052In general, fermented beverages are obtained by the
presence of determinate microorganisms, so the
majority of published papers deal with this topic.
0053Many applications focus on the selection of yeasts
capable of performing alcoholic fermentation under
extreme conditions (high sugar concentration, high
amounts of SO
2
or ethanol). GM amylolytic yeasts,
which can hydrolyze starch, have been used to
improve fermentation processes.
0054Some studies on the evaluation of the use of non-
Saccharomyces yeasts, separating those that could
positively affect the taste and flavor of alcoholic
beverages from others that produce large amounts
of negative byproducts, have also been developed.
Yeasts selected have been applied to obtain fruit
drinks with a slightly acid and less sugary taste. The
use of immobilized cells in fermented beverages for
continuous production has also been studied.
0055Another field is the use of different methods such as
the combination of pressure, heat, and bacteriocins,
and a flow-through photoreactor, to render inactive
foodborne pathogens in some juices and some micro-
organisms in water, respectively.
0056Other studies have investigated the molecular and
catalytic properties of different types of enzymes
(proteinases, glucosidases), which have particular
Dairy
Meat
Fish
Vegetables & fruits
Cereals
Beverages
Others
10%
9%
19%
5%
6%
15%
36%
fig0002 Figure 2 Percentage of food biotechnology published papers in different sorts of food industries.
504 BIOTECHNOLOGY IN FOOD PRODUCTION
