Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


come from investigations of the antidiarrheal effect of
bifidobacteria. Indeed, the biotherapeutic potential of
foods containing bifidobacteria (and other LAB) has
been well established for antibiotic-associated diar-
rhea outbreaks and rotavirus infections. In a double-
blind, placebo-controlled trial in 55 hospitalized
infants, oral administration of Bifidobacterium bifi-
dum and Streptococcus thermophilus reduced the
incidence and severity of rotavirus diarrhea. There is
also some evidence that fermented milk products
containing bifidobacteria may protect against travel-
er’s diarrhea. However, work to date suggests that the
tbl0002 Table 2 Compilation of experimental results showing the effect of feeding bifidobacteria in humans
Organism Control Test Effect
Bifidobacterium bifidum,
Lactobacillus
acidophilus
15 humans receiving no
fermented milk
15 humans Increased total IgA levels
Fermented milk
B. bifidum, L. acidophilus Baseline
a
14 humans Increased phagocytic activity
B. bifidum, Streptococcus
thermophilus
Placebo 55 hospitalized infants Reduced incidence of diarrhea (70% of
observed diarrhea cases caused by
rotatvirus)
Bifidobacterium spp. Baseline Humans Lowered b-glucuronidase levels
Fermented milk
B. bifidum, L. acidophilus,
S.lactis,S.cremoris
Baseline Humans Lowered nitroreductase levels
Fermented milk
B. longum Baseline 10 humans Lower fecal pH
Fermented milk
B. longum, L. acidophilus Placebo 30 humans Lowered fecal volatile fatty acids levels and
reduced GI discomfortFermented milk
B. longum Baseline 6 humans Increased bifidobacterial dominance
Fermented milk
B. longum Placebo 12 humans Increased fecal bifidobacteria levels
Fermented milk
B. bifidum, L. acidophilus Placebo Humans Delayed reduction of bifidobacteria due to
antibiotic treatment and induced earlier
re-establishment of bifidobacteria,
postantibiotic therapy
Lyophilized bacteria
B. bifidum Baseline 12 elderly dyspepsia patients Increased fecal bifidobacteria levels and
reduced symptomsLyophilized bacteria
B. bifidum, L. acidophilus Placebo 20 humans No significant differences during amplicillin
administrationLyophilized bacteria
B. bifidum Placebo 15 humans Decreased chronic inflammation of sigmoid
colon and increased humoral immunityLyophilized bacteria
B. longum BB536 B. longum ATCC15707 48 Humans Reduced clostridia, bacteroides, coliforms
population levelsFermented milk
Bifidobacterium spp. Pasteurized bifidus-
fermented milk
60 Humans Reduced colonic transit time
Fermented milk
Bifidobacterium spp. Baseline Patients with hepatitis or liver
cirrhosis
Lowered blood ammonia and phenol levels,
reduced urinary indican levels, increased
fecal bifidobacteria levels and improved
appetite and weight gain
B. lactis 13 control subjects
given placebo milk
12 healthy elderly humans Increased interferon-a levels and
polymorphonuclear cell phagocytic capacitySupplemented milk
B. longum Baseline 5 humans Reduced lecithinase negative clostridia
numbers, lowered NH
3
and b-glucuronidase
levels in feces and reduced fecal pH
Lyophilized bacteria
Bifidobacterium spp. Baseline Bedridden elderly humans Increased stool frequency
Fermented milk
B. bifidum, L. acidophilus 10 elderly humans 15 elderly humans Reduced inflammation of sigmoid and
descending colon
Bifidobacterium spp. Baseline 15 humans Reduced stool frequency in intractable diarrhea
B. longum Yogurt without
bifidobacteria
10 Humans Reduced erythromycin induced gastrointestinal
effectsFermented milk
Bifidobacterium spp. Baseline Humans Lowered nitroreductase and glucuronidase
levelsFermented milk
a
Same subjects prior to feeding.
BIFIDOBACTERIA
IN FOODS 465

probiotic strain/mixture as well as the destination are
paramount.
Physiological Effects
0008 The production of organic acids (acetic and lactic
acid) by bifidobacteria inhibits the growth of patho-
genic organisms (directly and indirectly) and stimu-
lates intestinal peristalsis. Acetic acid is a stronger
antagonist against Gram-negative bacteria than lactic
acid. As such, the potential applications of bifido-
bacteria against microbial perturbation may surpass
those of lactobacilli. Additionally, the organic acids
produced by bifidobacteria have been shown to in-
hibit the growth of many nitrate-reducing bacteria.
Human studies using bifidobacteria as a dietary ad-
junct have shown the relief of symptoms in consti-
pated elderly persons (Table 2). Several investigations
have also reported a reduction of fecal pH in individ-
uals consuming fermented milk products containing
bifidobacteria. Lowering the pH of the intestinal tract
produces an environment less favorable to some
pathogenic bacteria and thus helps prevent their over-
growth.
Anticarcinogenic Activity
0009 Increasing evidence, from in vitro experiments and
animal studies, indicates the potential protective
influence of probiotic bacteria (including bifido-
bacteria) against cancer. To date, three plausible
mechanisms have been identified: (1) inhibition of
putrefactive organisms that produce carcinogens
(such as N-nitroso compounds, phenolic products of
tyrosine and tryptophan, and metabolites of biliary
steroids); (2) binding and/or inactivation of carcino-
gens; and (3) inhibition of tumor cell formation.
Bacterial enzymes (including b-glucuronidase, b-
glucosidase, nitroreductase, and azoreductase) are
responsible for converting some procarcinogens into
carcinogens. As such, the levels/activities of these
enzymes are considered a useful biomarker for cancer
risk in humans, enabling noninvasive estimation
of carcinogen levels. Several human studies have
employed this method to determine the effects of
different diets on the risk of colon cancer. A number
of investigations have shown a reduction in fecal
enzyme activity during ingestion of bifidobacteria-
containing milk products, compared with initial
levels before bifidobacterial consumption (i.e., base-
line levels). Although these results are encouraging,
they are inconclusive regarding the effects on cancer
risk. Much more research is required to establish clear
links between enzyme activity and cancer risk.
Animal studies have enabled more definitive investi-
gations of the anticancer effects of bifidobacterial
feeding. Several such reports from murine models
have identified an antitumor effect of bifidobacterial
feeding (with or without prebiotics). Cases affording
protection against both aberrant crypts (precancerous
lesions) and tumors have been published. However,
animal studies are speculative at best, regarding the
protective nature of these strains against the develop-
ment of cancer in humans.
Stimulation of Immune Functions
0010Several probiotics, including some bifidobacterial
strains, are claimed to enhance the immune system
in a nonspecific manner, thereby stimulating immun-
ity to a number of antigens. A number of studies have
also shown the ability of certain LAB strains to alter
cytokine production and/or increase secretory IgA
levels. However, most work in this area has either
involved inappropriate animal models or concen-
trated on intermediary endpoints (such as cytokine
production) rather than disease symptoms. Poor
study designs have also led to difficulty in confirming
the immunomodulatory agent. Preliminary data dem-
onstrate the potential of probiotics in modulating
certain immune responses and indicate their potential
role in allergy, autoimmunity, and gastrointestinal
disease.
Cholesterol-lowering Ability
0011Mann and Spoerry identified correlations between
daily consumption of fermented milk and low serum
cholesterol levels in East African Masai warriors.
Subsequent in vitro work has demonstrated the abil-
ity of bifidobacteria to both assimilate cholesterol
and coprecipitate it with deconjugated bile acids.
Such observations have led to great interest in the
cholesterol-lowering capacity of a diet containing fer-
mented milks. However, much contradictory data
exist regarding the effects of consuming foods con-
taining bifidobacteria on serum cholesterol levels.
Confounding the issue has been the use of different
strains, dosages, and/or food vehicles in the various
studies carried out so far. Additional criticisms of the
current data have included lack of stabilization of
baseline cholesterol levels, inadequate size and/or
duration of studies, and difficulty in controlling the
diet and physical activity of subjects.
Lactose Intolerance
0012Lactose malabsorption affects large portions of the
population (estimated by some to affect over half the
world’s population), with a higher prevalence in those
of Oriental or African ancestry. Symptoms normally
include abdominal discomfort, flatulence, and/or
diarrhea. However, lactose-intolerant individuals
466
BIFIDOBACTERIA
IN FOODS

can consume cultured milk products (containing
bifidobacteria and/or lactobacilli) without any dele-
terious effects. Two mechanisms have been proposed
for the improved digestibility of lactose in such
products: (1) the b-galactosidase activity of the pro-
biotic strains; and (2) stimulation of host mucosal
b-galactosidase activity by the ingested strains.
Nutritional Value
0013 Bifidobacteria are known to produce thiamine, ribo-
flavin, vitamin B
6
, and vitamin K. There have also
been reports of their ability to synthesize folic acid,
niacin, and pyridoxine. These vitamin B complexes
are slowly absorbed in the human body. However, the
impact on human nutrition of such vitamin synthesis
by bifidobacteria in the colon is unknown. Available
information on the nutritional properties of fer-
mented milks containing bifidobacteria indicates
that they have lower residual lactose and higher levels
of free amino acids and vitamins than nonfermented
milks. Additionally, they preferentially contain l(þ)-
lactic acid [produced by bifidobacteria in addition
to acetic acid, whereas lactobacilli produce d/l()-
lactic acid], which is more easily metabolized by
humans. This is particularly important for infants
less than 1 year old, in whom metabolic acidosis can
be a problem. Consuming bifidobacterial food prod-
ucts may also improve the bioavailability of certain
minerals, including calcium, zinc, and iron, by
lowering the gastric pH (facilitating ionization of
minerals, which is necessary for their uptake).
Product Development
0014 Technically, bifidobacteria may be incorporated into
foods either to produce certain product characteris-
tics, such as organoleptic and/or nutritional proper-
ties, or as a probiotic. In the latter case, the viability
of the strain is essential during both manufacture and
storage of the food product. Indeed, the generally
accepted recommendation is that probiotic strains
must be present at levels of > 10
6
viable cells per
milliliter at the time of consumption. This is based
on the minimum therapeutic daily dose of 10
8
–10
9
cells.
0015 Bifidobacterial cultures incorporated into foods for
therapeutic purposes may be added either during
fermentation (along with or as part of the starter
culture) or to the finished fermented or fresh product
prior to shipment. Storage of food products under the
appropriate refrigeration conditions after manufac-
turing restricts subsequent fermentation. As such,
products to which bifidobacteria are added immedi-
ately prior to distribution are organoleptically indis-
tinguishable from the unfortified version. Products
fermented with bifidobacteria, however, often have
a mild acidic flavor. The selection of culture strains
and ratios is as critical in determining the final prod-
uct characteristics as the fermentation parameters
(temperature, duration, chemical composition and
level of inoculum).
Selection Criterion
0016The selection of bacterial strains is vital since differ-
ent strains (even within the same species) have dis-
tinctive metabolic and probiotic characteristics, as
well as different growth rates. The main factors to
consider include rate of acid production, type of poly-
saccharide fermentation, ability to synthesize vita-
mins, proteolytic characteristics (which may result
in bitter compounds) and capacity to produce flavor
compounds. An additional consideration is the growth
characteristics of the bacterial strains. This is particu-
larly important regarding mixed inocula and deter-
mining both the optimal mixture and the ratio of
strains, as well as the appropriate time in processing
to add the organisms. Recent studies have shown that
bifidobacteria inoculated into sour cream and butter-
milk either at the time of setting or at the time of
breaking allowed appropriate numbers of bacteria to
survive during normal storage of the products. Add-
itional work suggests that the best procedure for in-
corporating bifidobacteria in cottage cheese is in the
creaming mixture, whereas addition immediately
after cooking (prior to hooping) is suitable for
Edam cheeses. In the production of yogurts contain-
ing bifdiobacteria, it is common practice to use
premixed cultures of Streptococcus thermophilus,
Lactobacillus delbrueckii ssp. bulgaricus, L. acidoph-
ilus, and Bifidobacterium species. However, bacterial
‘loading’ is also practiced, where the lactobacilli
and bifidobacteria are grown separately prior to
addition to the product. A number of studies have
also demonstrated successful manufature of probiotic
cheeses, especially hard cheeses (e.g. cheddar,
Canestrato Pug liese and Gouda-type), by either in-
corporating bifidobacterial strains in the starter cul-
ture or using bifidobacterial fermented cream.
0017As bifidobacteria are anaerobes, it is useful to select
species or strains that are less oxygen-sensitive (such
as B. infantis and B. longum), perform gas flushing
with nitrogen (a process often used for preserving
yogurts), or include an oxygen scavenger in the
fermentation inocula (such as S. thermophilus). The
growth and survival characteristics depend on the pH
(and its buffering capacity), the fermentation and
storage conditions (especially temperature and dur-
ation), and the presence of competing micro-
organisms or microbial inhibitors (such as H
2
O
2
and NaCl).
BIFIDOBACTERIA
IN FOODS 467

0018 Regarding biotherapeutic considerations, the
strain(s) must be selected, based on their ability to
elicit the desired beneficial effect at the target. This
necessitates their survivability within the product and
during transit through the upper GI tract. As such, it
is pertinent to use bifidobacteria of human intestinal
origin as they are better suited to the physiological
needs of the host than strains from other sources.
Finally, it is essential to demonstrate the safety of
probiotic strains. They must be confirmed as non-
pathogenic, have a well-established safety record, or
have ‘generally regarded as safe’ (GRAS) status.
Many publications have listed recommendations of
selection criteria for probiotic strains. These fall into
the four general categories of (1) appropriateness, (2)
technological suitability, (3) competitiveness, and (4)
performance and functionality.
Technical Considerations
0019 Besides strain selection, careful monitoring through-
out the manufacturing process is required to insure
efficient control of the final product (pH, metabolic
products, cell density, and flavor). Incorporation of
LAB above the necessary starter organism to produce
specific product characteristics demands examination
of the final product to confirm the desired property.
For example, in vitro demonstration of a strain’s abil-
ity to synthesize vitamins is inadequate on its own, as
the fermentation characteristics and final product
composition (including the levels of the desired vita-
mins) need to be determined.
0020 A number of methods have been reported to
increase the survival of LAB whilst maintaining
the desirable attributes of yogurt. These include
enrichment with whey protein concentrate; lowering
incubation temperature (to 37
C); terminating fer-
mentation at a higher pH (e.g., > 5); hydrostatic pres-
sure treatment or heat-shock of yogurt (to prevent
postmanufacture acidification); and storing below
3–4
C.
Market for Foods Containing
Bifidobacteria
0021 To date, food products containing bifidobacteria
have largely been of dairy origin and include yogurts,
fermented milks, fresh milks, cheeses, buttermilk,
sour cream, cream cheeses, cottage cheeses, frozen
dairy desserts (including icecream and frozen yogurts),
and dips. Research has further demonstrated the
feasibility to incorporate bifidobacteria in vegetable
spreads, fermented vegetable products (such as kim-
chi, a traditional Korean food), mayonnaise, cookies,
and some beverages. A list of some of the commer-
cially available fermented milk products containing
bifidobacteria and some of their claims is provided
in Table 3.
0022The increased awareness of consumers towards
general health and well-being, together with the
accepted impact of diet on health, has led to a greater
demand for healthy and/or functional food products.
As such, the upward trend currently seen in bifido-
bacterial foods is projected to continue. As much as
70% of milk products on the market in some Euro-
pean countries, such as Sweden, contain bifidobac-
teria. The Japanese and European food industry and
markets are well ahead of the USA and Canada,
regarding functional foods. However, the influx of
foods containing bifidobacteria on these markets has
begun. A recent article estimated double-digit annual
growth rates of the world-wide market for such
products.
Regulations and Legislation
0023The generally accepted minimum level of probiotics
in foods at the time of consumption is > 10
6
cells per
milliliter. However, the standard developed by the
Fermented Milks and Lactic Acid Bacteria Beverages
Association of Japan is > 10
7
cells per gram at the
time of manufacture. In the USA, 10
8
bacteria per
gram at the time of manufacture is the criterion of
the National Yogurt Association (NYA). As well as
the concern of viability of probiotics within the con-
sumed product, there is increasing interest (from the
scientific community, food industry, and national au-
thorities) in establishing codes of practice regarding
the legislation and regulations of health claims for
food products. To this end, definitive evidence
demonstrating the efficacy of products is essential.
As such, statically significant, well-designed (double-
blind, placebo-controlled) clinical trials are demanded
for health-promoting food products. The need to
prove safety is equally important to substantiating
efficacy. Key areas for demonstrating nutritional
safety include intake, extent of use, implications/
impact on gut microflora and metabolic pathways,
compositional analysis (including the presence of
antinutritional or toxic factors), and potential effects
in compromised individuals or specific target popula-
tions.
0024Currently, there is no world-wide regulatory or
legislative control on the labeling and health claims
of food products. However, most legislative bodies
prohibit the use of ‘medicinal’ claims for food prod-
ucts (including functional foods). That is, claims sug-
gesting the prevention of, treatment, and/or cure for
human disease are not permissible for food products.
However, health claims are deemed acceptable and
usually involve the promotion of health benefits,
468
BIFIDOBACTERIA
IN FOODS

reduction of disease risk, or general well-being of the
host. Health claims may not include specific claims
such as ‘improves resistance against pathogenic infec-
tion’ but could state ‘improves normal intestinal func-
tion.’ As one can see, the line between medical and
health claims is very gray, and the presentation or
wording of the claims is usually important in defining
under which heading they fall. Indeed, one published
explanation of medical foods (medicine) and func-
tional foods gave the following definitions. Medicines
include ‘a food product which has a direct preventa-
tive effect on a disease,’ whereas health claims may
tbl0003 Table 3 Commercially available fermented milk products containing bifidobacteria
Product Bacterial strains Claims Country
A-38 L. acidophilus, B. bifidum, Leuconostoc
mesenteroides ssp. cremoris, mesophilic
lactococci
Denmark
A-B Yogurt B. bifidum, L. acidophilus France
Aktifit S. thermophilus, L. casei, L. acidophilus,
B. bifidum
Defense system for bowel Switzerland
Akult B. bifidum, L. acidophilus, B. breve,
L. casei ssp. casei
Normalize the balance of human intestinal
flora
Japan
B-Active L. acidophilus, B. bifidum, S. thermophilus,
L. delbrueckii ssp. bulgaricus
France
Bifidus milk B. bifidum, B. longum Several
Bifiel B. breve strain Yakult, S. thermophilus,
Lactococcus lactis
Normalize the balance of human intestinal
flora
Japan
Bifighurt B. bifidum, S. thermophilus Germany
Bifilus B. longum, L. bulgaricus, S. thermophilus Sweden
Biokys B. bifidum, L. acidophilus, Pediococcus
acidilactici
Czech Republic
Biomilk Bifidobacterium spp., L. acidophilus Several
Bio Pot L. acidophilus, B. bifidum Improve intestinal flora Germany
Bio-Garde L. acidophilus, B. bifidum, S. thermophilus Australia
Biola B. lactis, L. acidophilus, L. GG Balance microflora Norway
Bulla AB Live L. acidophilus, Bifidus Singapore, Australia,
Indonesia
GEFILUS S. thermophilus, L. bulgaricus, L. acidophilus,
B. bifidum, L. casei GG
Finland
LA7 L. acidophilus LA7, Bifidus Improve intestinal flora Germany
Leisure Live L. acidophilus, B. bifidum UK
Little Swallow L. acidophilus, B. bifidum UK, Germany
Mil-Mil B. breve strain
Yakult, B. bifidum strain Yakult, L.
acidophilus
Normalize the balance of human intestinal
flora
Japan
Mil-Mil E B. breve strain Yakult, L. acidophilus,
S. thermophilus
Normalize the balance of human intestinal
flora
Japan
Morinaga Bifidus B. longum, L. acidophilus Normalize the balance of human intestinal
flora
Japan
Morinaga Caldus B. longum, L. acidophilus Normalize the balance of human intestinal
flora
Japan
Nu-trish A/B B. bifidum, L. acidophilus USA
Procult 3 B. lo ngum Germany
Progurt B. bifidum, L. acidophilus, mesophilic
lactococci
Several
Ski L. acidophilus, Bifidus Promote health Australia, Singapore
Sym-Balance L. reuteri, L. acidophilus, L. casei,
bifidobacteria
Defense system for bowel Switzerland
VAALIA L. acidophilus, B. lactis, L. GG Promote health, balance intestinal
microflora
Australia
Vifit S. thermophilus, L. bulgaricus, L. acidophilus,
B. bifidum, L. casei GG
Keep the intestinal flora in good shape,
enhance immune response
Netherlands, Belgium,
UK
Vifit S. thermophilus, L. bulgaricus, L. acidophilus,
B. bifidum, L. casei GG
Keep the intestinal flora in good shape,
enhance the immune response
Germany
Yoplait Yoplus L. acidophilus, Bifidus Singapore, Australia
Yukijirushi Nachure L. acidophilus, B. longum Promote the maintenance of a good
intestinal environment
Japan
BIFIDOBACTERIA
IN FOODS 469

include maintenance of ‘good health as part of a life
style’ and/or potential reduction of ‘the risk of disease
occurring.’
0025 In Japan, a voluntary system is in place where
industry may apply for ‘Foods for Specified Health
Use’ (FOSHU) approval. Information on the safety
and efficacy in humans (including definitive scientific
evidence) and nutritional analyses are required for
such approval. FOSHU labeling, therefore, effectively
demonstrates that the product is approved as a func-
tional food, according to the advertised claim.
0026 In the USA, Federal Drug Agency (FDA) approval
is necessary regarding any labeling of health claims on
food products. Furthermore, the claims must be
founded on an ‘authoritative statement’ from an
appropriate Government scientific agency respon-
sible for ‘public health protection or research.’ How-
ever, ‘structure/function’ statements are permitted on
dietary supplements relating the food ingredient to
‘normal, healthy functioning of the human body.’
Such claims are not normally used on conventional
foods, but rather for foods intended to supplement
the diet. That said, foods containing bifidobacteria
are generally marketed as dietary supplements in the
USA, and therefore fall within a loophole. Currently,
however, most manufacturers of such products
simply name the added bacteria (at least to genus
level) for labeling purposes and do not state any
functions or claims.
0027 In Europe, there is no collective regulation or legis-
lation regarding functional foods. In general, the
primary focus is on the safety of the products. Food
products or food ingredients that were not available
on the European market for human consumption
prior to April 1997, and can be classified as novel
(owing to raw materials, composition, or manufac-
turing process), must undergo regulatory approval.
The second regulatory focus in Europe is the labeling
and health claims of the food product. The general
rule of thumb is that health claims may not attribute
the property of preventing, treating, or curing a
human disease, or any such implications. Currently,
seven European Union member states are developing
their own Codes of Practice regarding legislation and
regulation of health claims and labeling of functional
foods. The level of scientific substantiation needed for
such health claims differs between some codes. How-
ever, the Codes of Practice for the UK, Belgium, and
The Netherlands all include principles for human
(clinical) trials.
Summary
0028 Results to date clearly indicate the potential benefits
of consuming a diet incorporating foods containing
bifidobacteria. Several food vehicles have been
identified within the dairy industry, and appropriate
manufacturing techniques are available to insure that
the desired product characteristics and viability of
probiotic strains are attained. Essential to the future
of functional foods are adequate studies confirming
the safety, efficacy, and viability of such products
(especially well-designed human clinical trials). Cur-
rent developments within the scientific community,
food industry, and regulatory bodies are all pursuing
this end.
See also: Dairy Products – Nutritional Contribution;
Fermented Milks: Types of Fermented Milks; Products
from Northern Europe; Other Relevant Products; Dietary
Importance; Lactic Acid Bacteria; Microbiology:
Detection of Foodborne Pathogens and their Toxins;
Microflora of the Intestine: Role and Effects; Probiotics;
Prebiotics; Probiotics; Yogurt: The Product and its
Manufacture; Yogurt-based Products; Dietary Importance
Further Reading
American Society for Nutritional Sciences (various authors)
(2000) Symposium: Probiotic bacteria: Implications
for human health. Journal of Nutrition 130: 382S–
416S.
Arunachalam KD (1999) Role of bifidobacteria in nutri-
tion, medicine and technology. Nutrition Research 19:
1559–1597.
de Roos NM and Katan MB (2000) Effects of probiotic
bacteria on diarrhea, lipid metabolism, and carcino-
genesis: a review of papers published between 1988
and 1998. American Journal of Clinical Nutrition 71:
405–411.
Gibson GR and Williams CA (2000) Functional Foods:
Concept to Product. Cambridge: Woodhead Publishing.
Heller KJ (2001) Probiotic bacteria in fermented foods:
product characteristics and starter organisms. Amer-
ican Journal of Clinical Nutrition 73(Suppl): 374S–
379S.
Kailasapathy K and Rybka S (1997) L. acidophilus and
Bifidobacterium spp. – their therapeutic potential and
survival in yogurt. The Australian Journal of Dairy
Technology 52: 28–34.
Lee Yuan-Kun Nomoto K, Salminen S and Gorbach SL
(1999) Handbook of Probiotics. Wiley-interscience
Publication. New York: Wiley.
Rasic JL and Kurmann JA (1983) Bifidobacteria and Their
Role. Experientia Supplementum, vol. 39. Basel: Birk-
hauser.
Sanders ME and in’t Veld JH (1999) Bringing a probiotic-
containing functional food to the market: microbio-
logical, product, regulatory and labeling issues. Antonie
van Leeuwehoek 76: 293–315.
Tannock GW (1999) Probiotics: A Critical Review.
Wymondham, Norfolk, UK: Horizon Scientific Press.
470
BIFIDOBACTERIA
IN FOODS

BILE
A Lanzini, University of Brescia, Brescia, Italy
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Bile is a complex aqueous solution of organic and
inorganic compounds secreted by the liver. It contains
bile acids, a class of detergent-like molecules that
exert their biological functions as lipid solubilizers
within the biliary tree and the gut lumen. The bile
acids are restricted to the enterohepatic circulation,
so as to reutilize many times these biologically
valuable molecules. This chapter reviews the com-
position and physicochemical properties of bile, the
mechanism involved in bile formation, and the func-
tion of bile acids as solubilizers of biliary and dietary
lipids.
Composition of Bile
0002 Inorganic compounds in human hepatic bile comprise
electrolytes in concentrations similar to those in
plasma, with the noticeable exception of HCO
3
con-
centration which is higher in bile. The osmolarity of
human hepatic bile is also similar to plasma osmolar-
ity, at about 300 mOsm l
1
.
0003 Organic compounds in bile comprise protein
and bile pigments in addition to the three biliary
lipids, bile acid, cholesterol, and phospholipid. Pro-
teins account for 4.5% of organic compounds in bile,
and their concentration ranges between 0.3 and 3.0 g
ml
1
in typical human bile. The most abundant bil-
iary protein is albumin derived from the plasma pool.
In general, the biliary concentration of other plasma
proteins is inversely related to their molecular weight.
Bile also contains immunoglobulin A, lysosomal
enzymes, and plasma membrane enzymes. The secre-
tion of these latter proteins is influenced by the bile
acid secretion rate.
0004 Bile pigments constitute less than 0.3% of organic
compounds in bile, and their concentrations range
between 0.8 and 3.2 mmol l
1
in human hepatic and
gallbladder bile, respectively. Conjugation with glu-
curonic acid is essential for biliary bilirubin excretion,
and bilirubin diglucuronide is the major pigment in
human bile. The maximal bilirubin excretion capacity
depends on bile flow and is increased during enhanced
bile acid secretion rates. About 30% of bilirubin ex-
cretion is thought to be bile acid-independent, sug-
gesting that incorporation of bilirubin into mixed
micelles is not essential for its biliary excretion.
0005Many other endogenous substances are present in
human bile in addition to those listed above. These
include vitamins (mainly D
2
,B
12
, folic acid), steroid
(estrogens), and thyroid hormones. Exogenous com-
pounds such as contrast media, some antibiotics
(ampicillin, metronidazole), cardiac glycosides, and
opiates may also be excreted in bile and undergo
some degree of enterohepatic circulation.
Biliary Lipid Chemistry and
Physicochemical Properties
Bile Acids
0006Bile acids comprise a class of molecules derived from
the hepatic catabolism of cholesterol. More than 200
bile acids have been isolated in human bile, and 92–
99% of total bile acids are constituted by mono-, di-,
and tri-hydroxy derivatives of a 24-carbon atom ster-
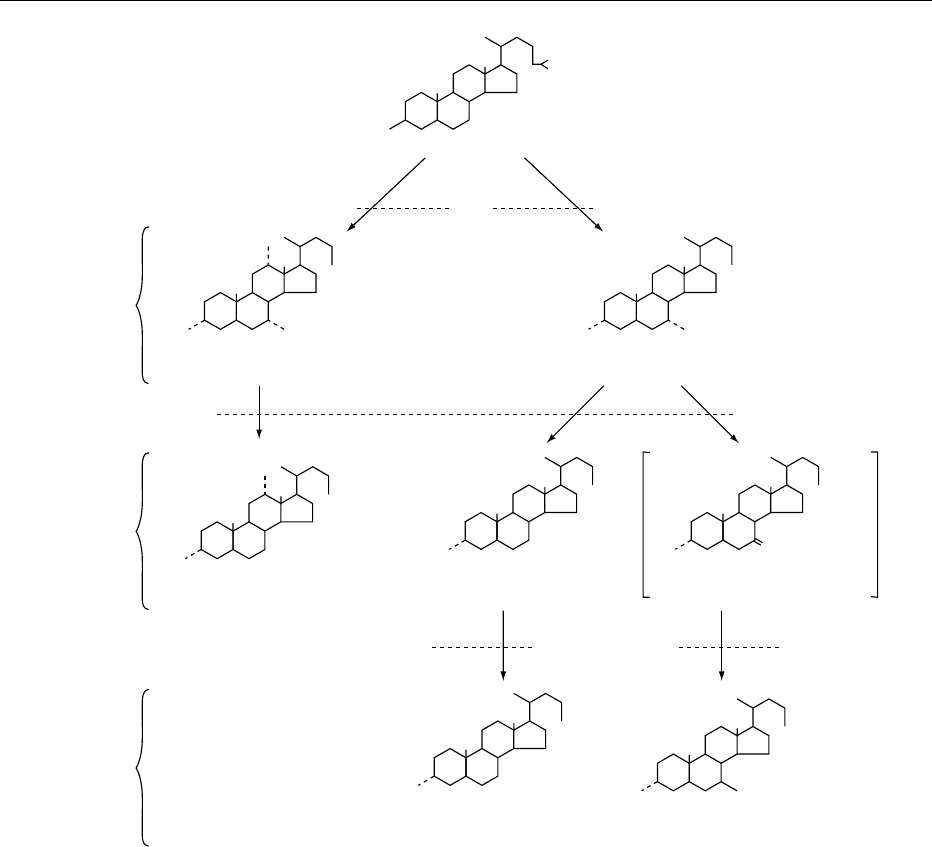
oid, cholanoic acid (Figure 1). The brief and system-
atic names and the relative proportions of individual
bile acids in human bile are shown in Table 1.
0007Virtually all biliary bile acid are amidated in man
almost exclusively with glycine or taurine at a ratio
of about 2:1. Sulfation on the steroid nucleus of
bile acids occurs to a significant extent in man only
for lithocholic acid, and glucuronidation is a trace
metabolic pathway in man.
0008From a physicochemical point of view, bile acids
are planar amphiphiles in that they exhibit both
hydrophilicity with one part of the molecule and
hydrophobicity with the remainder. In commonly oc-
curring bile acid molecules, the hydrophobic face
is constituted by the b side of the steroid nucleus,
and the hydrophilic face by the a side of the nucleus
and by the side chain. The relative potency of the
hydrophilic and hydrophobic functional groups in
affecting the physicochemical properties of bile
acids is referred to as the hydrophilic–hydrophobic
balance. The order of increasing hydrophilicity,
as assessed by high-pressure liquid chromatogra-
phy, follows the order taurine conjugates > glycine
conjugates > unconjugated bile acid; and trihydroxy-
lated > dihydroxylated>monohydroxylated bile acids.
0009Bile acid solubility is strongly dependent on amida-
tion. Unconjugated bile acids (pK
a
5.0) are insoluble
at a pH below 6–7, and glycine-(pK
a
3.8) and taurine-
conjugates (pK
a
< 1.0) are soluble at pH 4–5and
below 2, respectively. Fully ionized common bile
acids are present in physiological solutions as their
sodium salts. They are extremely water-soluble, with
a monomeric solubility of 1–3 mmol l
1
(Figure 2).
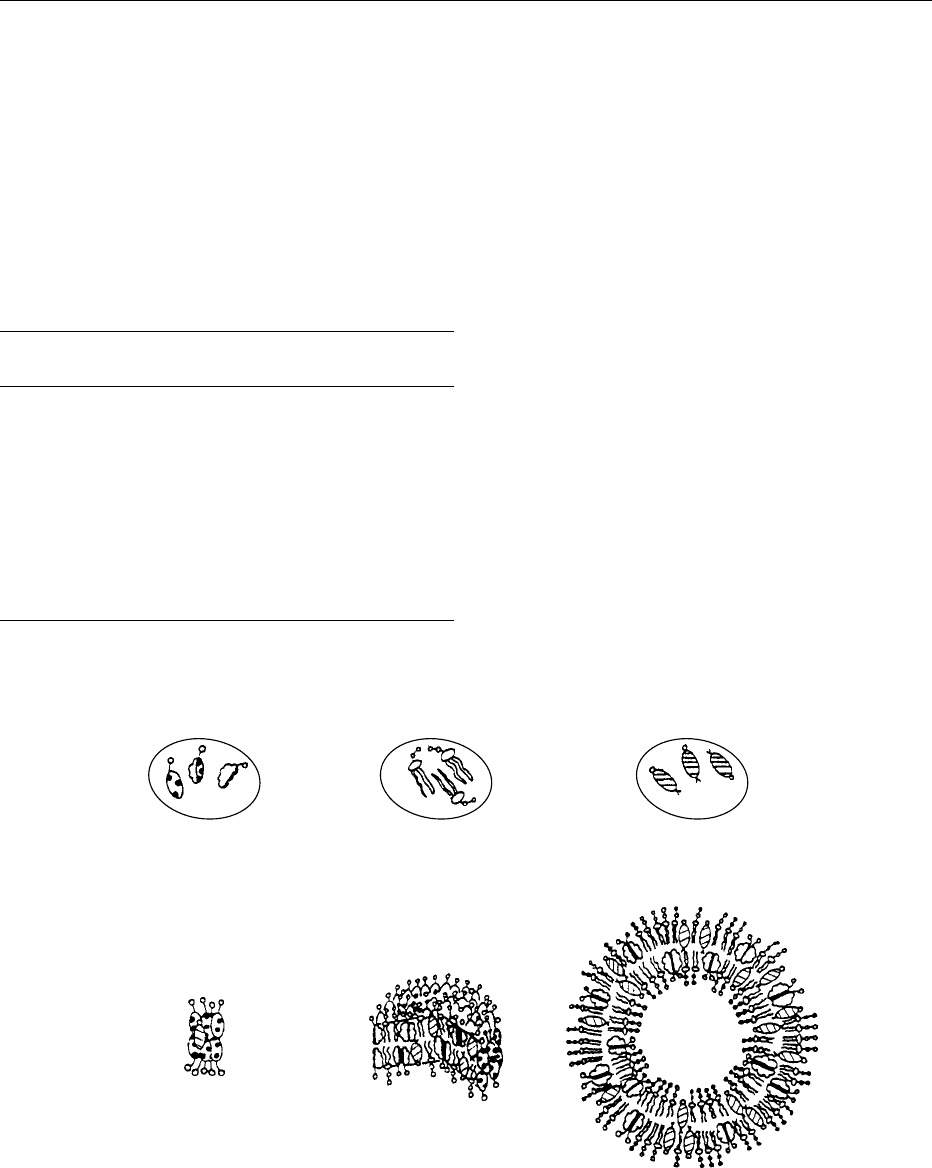
BILE 471
They are present in diluted water solutions as mono-
mers in the bulk water phase, and as an unstable film
on the water surface. Upon concentration, a critical
monomeric concentration is reached, called the crit-
ical micellar concentration (CMC), at which bile acid
monomers self associate to form multimolecular
aggregates called micelles. Micelle formation involves
back-to-back agglomeration of the hydrophobic side
of the bile acid molecules, with the hydrophilic
side facing water. At concentrations above the
CMC, bile-salt micelles have the capacity of incorpor-
ating and solubilizing otherwise insoluble com-
pounds, thus acting as detergents. In general terms,
hydrophobic bile acids form micelles at lower concen-
tration than hydrophilic bile acids, and the CMC
ranges between 0.5 and 11 mmol l
1
for common
bile acid (Table 1).
Phospholipids
0010The most abundant biliary phospholipid species are
phosphatidylcholines (lecithins), which account for
80–95% of phospholipids in human bile. Phosphati-
dylcholines are insoluble swelling amphiphiles, with a
monomeric solubility in water of about 1 pmol l
1
(Figure 2). Upon hydration (> 45% water), phospho-
lipids swell to form liquid crystalline phases consist-
ing of choline bilayers with interposed water layers.
These liquid crystalline phases may fold and aggre-
gate to form vesicular structures.
COOH
HO
Cholesterol
Liver
HO
Primary
Secondary
Intestinal
bacteria
Intestinal
bacteria
OH
COOH
OH
Cholic acid
HO
Tertiary
OH
Deoxycholic acid Lithocholic acid
Sulpholithocholic acid Ursodeoxycholic acid
HO
OH
COOH
Chenodeoxycholic acid
HO
OH
COOH
HO
COOH
−
O
3
SO
COOH
7-Oxolithocholic acid
HO
O
COOH
Liver
Liver
fig0001 Figure 1 Structural formulae and sites of synthesis and metabolism of common bile acids in man. From Carey MC and Duane WC
(1994) Enterohepatic circulation. In: Arias IM, Boyer JL, Fausto N et al. (eds.) The Liver: Biology and Pathobiology, 3rd edn, pp. 719–767.
New York: Raven Press, with permission.
472 BILE
Cholesterol
0011 From a physicochemical point of view, cholesterol
is an insoluble nonswelling amphiphile, with a mono-
meric solubility in water of about 1 nmol l
1
at 37
C
(Figure 2). Upon hydration, crystals of cholesterol
monohydrate are in equilibrium with water-contain-
ing monomers of cholesterol monohydrate.
Bile Formation
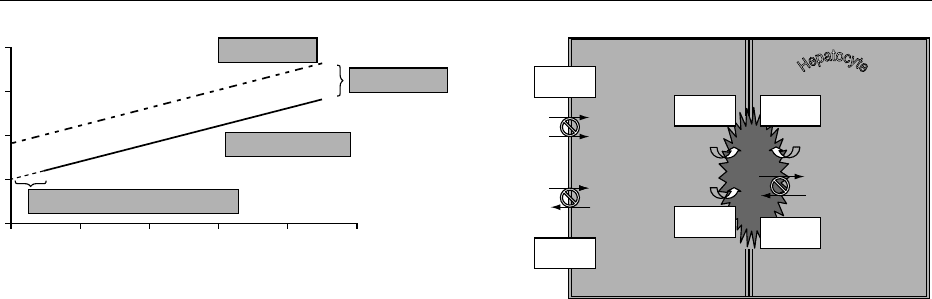
0012Bile formation involves a number of secretory
processes in the hepatocyte and in the ductular cells
generating an osmotic gradient between the cells and
the biliary canaliculus and ductulus, with passive
water movement along this gradient until osmotic
equilibration is achieved. Canalicular and ductular
bile flow contribute 470 and 150 ml, respectively to
the typical total daily bile flow of 620 ml in man. Bile
flow varies from 1.5 to 15.4 ml min
1
kg
1
,andis
linearly related to bile acid secretion rate (Figure 3).
The contribution of canalicular to total bile flow is
defined by the linear relationship between bile acid
secretion and biliary clearance of sugars (erythritol or
mannitol) acting as bile-flow markers. This relation-
ship identifies the so-called bile acid-dependent cana-
licular bile flow (about 60% of total canalicular bile
flow) generated by active bile acid transport into the
canaliculus. Extrapolation of the linear regression
function relating bile acid secretion rate and erythri-
tol clearance to a value of zero bile acid secretion rate
yields a positive zero intercept. This linear extrapo-
lation identifies the so called bile acid-independent
bile flow (about 40% of total canalicular bile flow).
0013Bile acid-independent bile flow is generated by
active transport of glutathione and organic anions
tbl0001 Table 1 Name and composition of biliary bile acids of typical
human bile
Brief name
of bile acid
Systematicname CMC
(mmoll
1
)
Percentage
in bile
a
Cholic 3a,7a,12a-
Trihydroxy-
5-cholanoic acid
11 35
Chenodeoxycholic 3a,7a-Dihydroxy-
5-cholanoic acid
435
Deoxycolic 3a,12a-Dihydroxy-
5-cholanoic acid
324
Ursodeoxycholic 3a,7b-Dihydroxy-
5-cholanoic acid
7 tr.–4
Lithocolic 3a-Monohydroxy-
5-cholanoic acid
0.5 tr.–3
a
Individual bile acids as a percentage of total bile acids. CMC, critical
micellar concentration; tr, trace.
(A)
(B)
BS−Ch micelle
(Rh, 1 nm)
'Mixed disc'
BS−L−Ch micelle
(Rh, 2−3 nm)
Unilamellar
BS−L−Ch vesicles
(Rh, 30−40 nm)
BS
1−3 mmoll
−1
L
~1 pmol l
−1
Ch
~1 nmol l
−1
fig0002 Figure 2 Molecular models of (A) monomeric and (B) aggregated biliary lipids. Monomeric solubility in water and mean hydro-
dynamic radius (Rh) of aggregated lipids are also shown. BS, bile acids; L, lecithin; Ch, cholesterol). From Carey MC and Duane WC
(1994) Enterohepatic circulation. In: Arias IM, Boyer JL, Fausto N et al. (eds.) The Liver: Biology and Pathobiology, 3rd edn, pp. 719–767.
New York: Raven Press, with permission.
BILE 473
(bilirubin glucuronide, thyroid and steroid hormones,
drugs) into the canaliculus. Glutathione is synthesized
in the hepatocyte, secreted into bile, where it is hydro-
lyzed into the constituent amino acids. These amino
acids exert the osmotic gradient responsible for bile
acid-independent paracellular entry of water into
the biliary canaliculus. Na
þ
and HCO
3
also contrib-
ute to generating the osmotic gradient responsible for
the bile acid-independent bile flow.
0014 Bile acid-dependent bile flow is a complex phe-
nomenon involving captation, intracellular transport
and biliary secretion of bile acids (Figure 4). Bile acid
captation occurs on the basolateral membrane of the
hepatocyte and involves two transport systems.
The first is a Na
þ
-taurocholate cotransporter poly-
peptide dependent on a sodium gradient energized by
the Na
þ
–K
þ
–ATPase system. The second transport
mechanism is independent of sodium gradient and
consists of an organic anion transporting polypep-
tide. This system is not specific for bile acids, and
transports other organic anions such as bilirubin,
bromosulfophthalein, and estrogens.
0015 The process of intracellular bile acid transport is
poorly understood, and it is thought to occur by
diffusion of bile acid bound to cytosolic protein
from the sinusoidal to the canalicular pole of the
hepatocyte. Vesicular transport may also play a role
in regulating intracellular bile acid traffic, as sug-
gested by the finding that vesicle poisoning results in
cholestasis.
0016 Bile acid secretion into the biliary canaliculus in-
volves two ATP-dependent transport systems and
an electrogenic system driven by membrane poten-
tial. Monovalent bile acids are transported by an
ATP-dependent canalicular bile salt export pump,
and sulfated or glucuronated divalent bile acids are
transported by a canalicular multispecific organic
anion transporter. This latter transport system is
also involved in biliary excretion of bilirubin diglu-
curonide, glutathione, bromosulfophthalein, and
other anionic dyes.
0017Phospholipid secretion into bile is also an active
ATP-dependent process mediated by p-glycoprotein
encoded by two genes in humans (multidrug-
resistant; MDR1 and MDR3) that make cells resist-
ant to a variety of amphipatic drugs (colchicine,
vincristine, doxorubicin, and others) by stimulating
their extrusion from the cell. MDR3 protein is ex-
pressed exclusively on the bile canalicular membrane
and is specialized in biliary export of phospholipid,
as indicated by the finding that homozygous
disruption of the murine mdr2 gene (the equivalent
of human MDR3) leads to complete absence of phos-
pholipid in bile.
0018Cholesterol secretion into bile is tightly coupled
with that of phospholipid, but it is not clear whether
cholesterol secretion is carrier-mediated, as demon-
strated for phospholipids. It is likely that cholesterol
translocation to the outer leaflet of the bile canalicu-
lar membrane is mediated by a spontaneous flip-flop
mechanism.
0019The current hypothesis to explain the phenomenon
of lipid secretion into bile is summarized in Figure 5.
Bile acids are continuously pumped out of the bile
canalicular membrane into bile. When the outer leaf-
let of the bile canalicular membrane is sufficiently
enriched with phosphatidylcholine, it ‘buds out,’
forming a phospholipid–cholesterol bilayer that ad-
sorbs to bile acids and dissolves into mixed bile acid–
cholesterol–phospholipid micelles (see below).
0020Bile flow and composition changes along the bil-
iary tree, due to water and inorganic electrolyte
movements across the duct system. The net result of
these movements is an increase in bile flow due to
secretion of bicarbonate and water. This process is
under control of the enteric hormone secretin that
stimulates secretion of Cl
by a ‘cystic fibrosis
GSH
HCO
−
3
HCO
−
BA
−
,OA
−
BA
−
,OA
−
OA
−
Na
+
NTCP
OATP
MOAT
AE2
MDR3BSEP
BA
−
BA
−
Cl
−
PL
3
fig0004Figure 4 Major membrane transporters involved in bile forma-
tion. NTCP, Na
þ
taurocholate cotransporter polypetide; OATP,
organic anion transporting polypetide; BSEP, bile salt export
pump; MOAT, multispecific organic anion transporter; MDR3,
multi drug resistance 3; AE2, anion exchanger 2.
Bile acid secretion
Bile acid independent flow
Bile flow
Ductal flow
Canalicular flow
fig0003 Figure 3 Relationship between bile acid secretion into bile and
bile flow.
474 BILE
