Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0020 The problem of calibrating and handling such com-
plex fluorescence information will be discussed
below. Likewise, autofluorescence of animal tissue
components and parasites, detected in the fluores-
cence microscope and defined in the spectrofluorom-
eter, can be exploited in digital video techniques
coupled to image analyzers and robots, e.g., to facili-
tate automatic butchering operations and cutting out
bones from cod fillets (Figure 7:3A–D). In the labora-
tory, naturally fluorescent compounds such as vita-
min E components can be separated and detected in
an HPLC system coupled to a fluorometer. In com-
mercial-scale fermentation processes, the fluorescing
reduced forms (NADH and NADPH) of the respira-
tory coenzymes nicotinamide adenine dinucleotide
(NAD) and nicotinamide adenine dinucleotide phos-
phate (NADP) can be used online to follow the rate of
the fermentation process. (See Tocopherols: Proper-
ties and Determination.)
0021Similarly, a food chemist in need of a certain assay,
e.g., to monitor the breakdown of mixed-linkage
b-glucan in the malting and brewing industry or to
Ellipsoid
M (E) 5
Source
S
Mirror
M (S) 3
Mirror
M (T) 4
Mirror
M (F) 2
1
Mirror
M (F) 2
Mirror
M (T) 1
1
Grazing
G (1200)
G2
Mirror
M (S) 3
1
Sample
photomultiplier
Mirror
M (T) 1
Grating
G (1440)
Reference
photomultiplier
Sample
A
B
R
G1
Beam
splitter
Slit
excitation
entry
Slit
excitation
exit
Slit
emission
entry
Slit
emission
exit
Zero-order
mirror
accessory
Excitation
polarizer
Emission
polarizer
Emission
filter
C
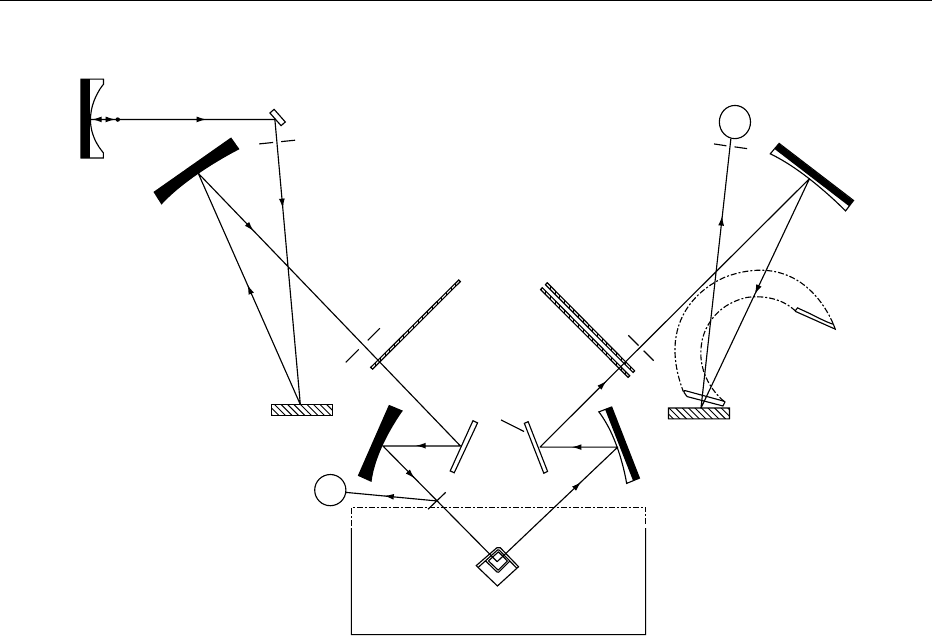
fig0006 Figure 6 Fluorescence spectrophotometer (Perkin Elmer LS-50). The source (S) is a pulse xenon flashtube (less than 002 s for a
complete pulse). Light from S is reflected by a mirror through a slit to the diffraction grating (G1) which has 1440 lines per millimeter.
The resulting narrow-wavelength band is transferred via another system of mirrors and slit to the sample (A). The effective wavelength
of the excitation beam is determined by the setting of the grating, the angle of which is controlled by means of a stepping motor. A very
small proportion of the excitation light is reflected by a beam splitter (B) to a reference photomultiplier. To correct for the response of
the reference multiplier, a rhodamine correction curve is stored within the software in the instrument computer. Light emitted by the
sample is transferred via slits and mirrors to a second diffraction grating (G2), which has 1200 lines per millimeter and is controlled by
another stepping motor, and on to the sample photo multiplier (C). A microprocessor controls the xenon tube, grating stepping motors,
slit apertures, and photo multipliers. The digitized signal is accumulated for about 008 s, i.e., four flashes of the source. The sample
accumulation is divided by the corrected reference accumulation to obtain a ratio that is unaffected by variation in source intensity.
Output is given by the microprocessor on a data screen and a recorder. The computer is programmed so as to allow calibration
spectra to be subtracted from sample spectra to generate difference spectra. The wavelengths of the instrument can be calibrated by
the characteristic fluorescence spectrum of benzene vapors, for example, or from fluorescent standards cast into plastic blocks
furnished by the instrument manufacturers. It is important in some applications to be able to record accurately several of the internal
settings of the instrument, including spectral correction and different scanning modes, and this can be done with a high-specification
spectrofluorometer. The practicable wavelength ranges for the standard version of the instrument are about 230–600 nm for excitation
and about 250–650 nm for emission. Reproduced from Spectroscopy: Fluorescence, Encyclopaedia of Food Science, Food Technology
and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
SPECTROSCOPY/Fluorescence 5437
ABCD
0
1
3
5
A
Days
B
1
12
275 µm 1600 µm 100 µm
3
Video Cutting robot
Conveyor
Separator
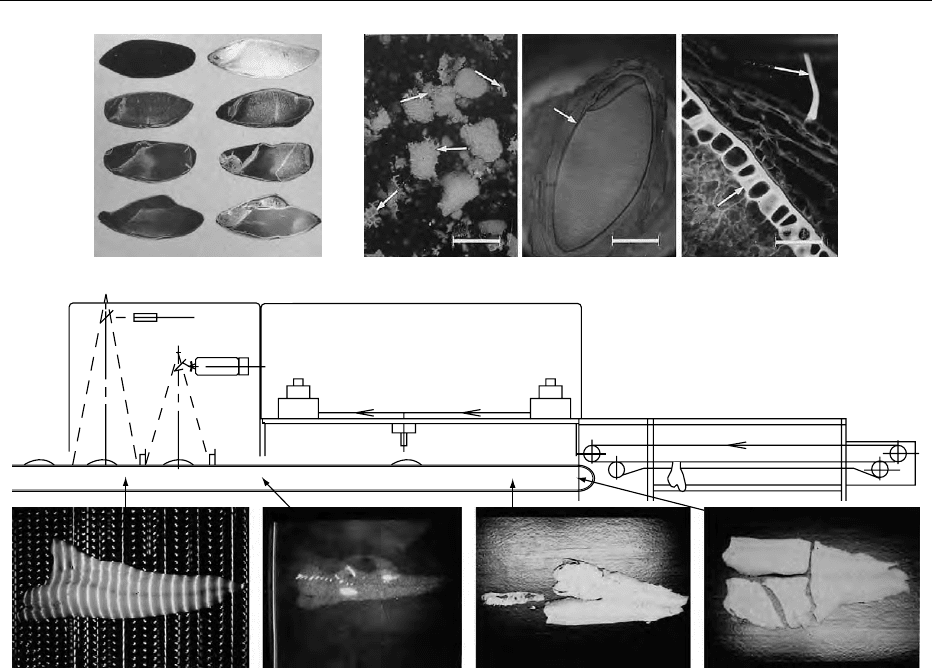
fig0007 Figure 7 (see color plate 129) Examples of fluorescence microscopy and imaging reproduced from articles referred to below in
Munck L. (ed.) (1989) Fluorescence Analysis in Foods, Harlow: Longman. pp. 289. 7:1. Cryostat sections of 0, 1-, 3- and 5-day malted
barley viewed through a 4 microscope objective. The sections were stained by (A) Calcofluor/Fast green photographed at 400 nm
excitation/418 nm emission in order to show the patterns of cell wall (b-D-glucan) breakdown and by (B) FITC-labelled antibodies
against a-amylase viewed with BP 455-490 nm exciter/LWP515 nm emission filter. Sections are identical pairwise – first stained with
method B followed by A. It is seen that a-amylase is spread from the germ (the structure on the left of each section) and from the
aleurone layer enclosing the starchy endosperm. The enzyme invades only the endosperm areas where cell walls are absent.
(Compare B with A on each day of malting.) From Munck L. Practical experiences in the development of fluorescence analyses in an
applied food research laboratory, ibid. p. 1-32. 7:2:1-3. represent a wheat and barley material with natural and added fluorochromes.
7:2:1. A view of ground whole wheat flattened slightly with cover glass, excited at 365 nm with a barrier filter from 420 nm. Bran
fragmentsarereadilydetectedbythenaturalbrightbluefluorescencecharacteristicofthealeuronelayer(arrows).Seealso Figure
2b.7:2:2.Alow-powerviewofalongitudinallyhalvedbarleykerneltreatedwithamultiplefluorescentstain(Safranin,BasicFuchsin,
Alcian Blue) to show several grain components simultaneously. The starchy endosperm (central region of the kernel) fluoresces bright
yellow due to starch-safranin interaction under 450-490 nm excitation, while the outer bran layers are deep red as a result of Basic
Fuchsin interaction with lignified and other phenolic-enriched structures such as cell walls (arrows). In this case, safranin is used to
detect starch, while the other stains are added as counterstains to surpress non-starch specific fluorescence. 7:2:3. As in 7:2:2, but
showing the bran tissues at higher magnification and 365 nm excitation. In this case, the procedure clearly differentiates between I.
ferulic acid-enriched aleurone cell walls (lower left) detected due to their natural fluorescence (as in 7:2:1), II. surface trichomes
(arrows) which do not bind significant amounts of the red fuchsin stain and III. the pericarp cell walls (right diagonal) which are
intensely stained deep red. Starch does not fluoresce significantly at these wavelengths. From Fulcher RG, Irwing DW and de
Francisco A. Fluorescence microscopy: Applications in food analysis, ibid. 59-109. 7:3:A-D. Operation of an automatic Fish Fillet
Deboning Line based on fluorescent technology. Skinned cod fillets are fed onto a steel conveyor while moving at one m/s. The video
detector (see drawing above the photographs) detects a fillet (A). The detector consists of two cameras and two light sources for
visible and UV light for fluorescence, respectively. The visual light is projected in stripes (A) in order to detect (I) the boundaries of the
filet and (II) the topography of the fillet for later partition (D) into desired weight portions. The video UV fluorescence system detects the
naturally fluorescent bones (ex. max 340/em. max 390) seen on a TV monitor (B). The information from the two cameras is directed to a
computer which directs a water jet nozzle (diameter 0.1 mm, pressure 300 bar) robot with an operating space of 1 m. The cutting of the
fillet bones (C) and further partition of the fillet (D) is performed while the steel conveyor is moving and completed within the time span
of one second. Bones and fillet pieces are separated and graded in the separator unit drawn above. This principle may also be used to
remove bones from chicken breast fillets. From Jensen SAa, Reenberg S and Munck L. Fluorescence analysis in fish and meat
technology, ibid. 171–180.
5438 SPECTROSCOPY/Fluorescence
(a)
Raw data: 268 samples
from a sugar campaign
Raw data
(b)
PARAFAC
1. Initialize B and C
2. A =
X
k
BD
k
Σ
K
k=1
−1
(B'B)*(C'C)
3. B =
X'
k
AD
k
Σ
K
k=1
(A' A)*(C' C)
−1
4. D
k
=
5. Go to 2 until convergence
−1
(B' B)*(A' A) diag (A' X
k
B)
30
(c)
1
0
1
0
1
0
1
1
0
0
1
0
1
0
1
0
40 50
24 26 28 30 32 34
24 26 28 30 32 34
24 26 28 30 32 34
24 26 28 30 32 34
30 40 50
30 40 50
30 40
Emission (nm 10) Excitation (nm 10)
Comp 2 Tryp Try Comp
1
50
Deconvoluted spectra
(d)
0
0
036 9
12345
2
4
6
8
10
12
14
16
18
Time/day
Score
Concentrations
Tyrosine
Comp. 2
Tryptopha
Comp. 1
6789
1
2
2
3
3
(e)
Predicting color
Day
Predicting CaO
(f)
0123456789
Predicted
Reference
Day
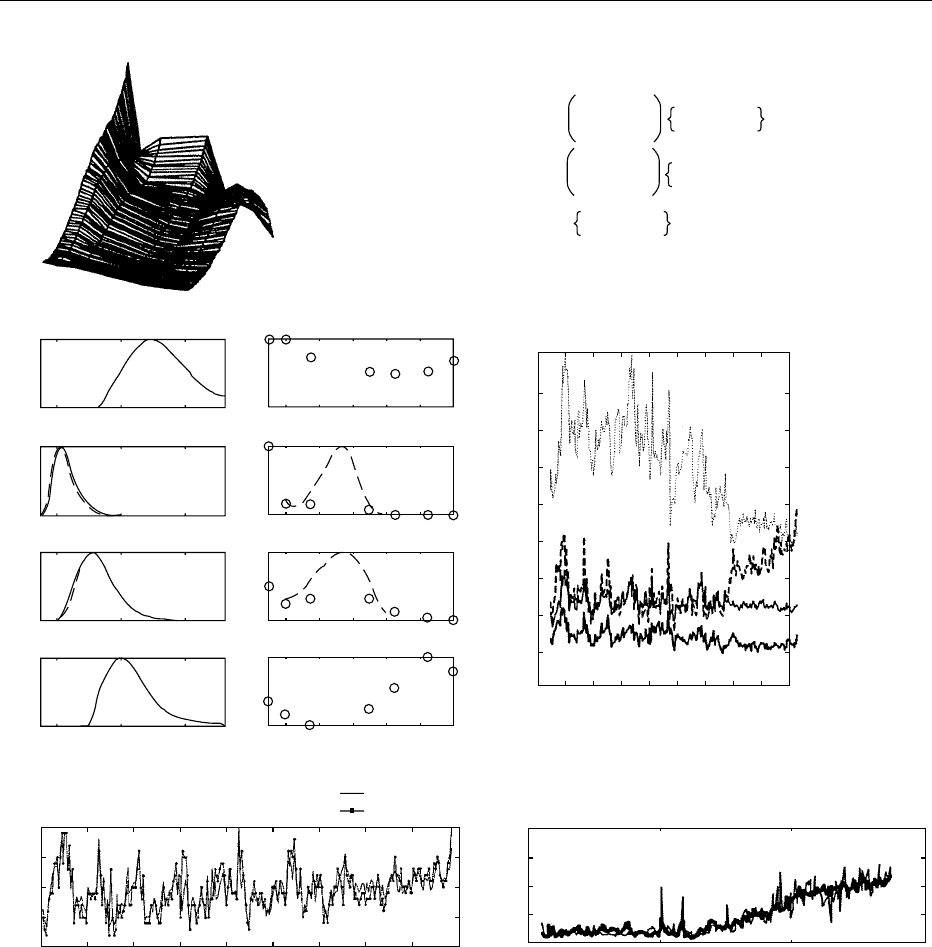
fig0008 Figure 8 An example of mathematical chromatography is multiway modeling of fluorescence landscapes of sugar into the pure
excitation and emission spectra of the underlying fluorophores. A total of 268 samples of sugar have been dissolved in water and
measured spectrofluorometrically, spanning the sugar campaign with three samples representing three 8-h periods per 24 h. Each
such measurement gives a characteristic landscape of a sample (a). By using the so-called parallel factor analysis (PARAFAC) model
(b), it is possible to describe these landscapes by the variation in four estimated fluorophores (c). Thus, at any time the sugar
landscape of any sugar sample can be described by having different amounts expressed in scores of these four fluorophores, defined
by their estimated excitation and emission spectra. The former is described less accurately than the latter due to sampling (20 nm).
The amounts of the four fluorophores in the 268 samples thoughout the season are shown in (d). Furthermore, it is possible to qualify
which chemical analytes these four fluorophores represent, just as in ordinary chemical chromatography. In this case, two of the
analytes have been identified as the uncolored amino acids tyrosine and tryptophan which have the potential for color formation
through reaction with reducing sugars. (Estimated and true emission spectra are shown.) The other two fluorophores represent
colored fluorescent high-molecular Maillard products. These interpretations have been checked with high-performance liquid
chromatography. Thus, a chemical understanding of the fluctuations during the campaign is provided. In (e) and (f) it is demonstrated
that this spectrofluorometric chemically based variation can be used for creating cheap online screening analyses for prediction of,
e.g., important process variables such as CaO in sugar juice or quality parameters such as color in sugar by using a multiple linear
regression model based on scores of the four fluorophores.
SPECTROSCOPY/Fluorescence 5439

screen for lipases in oat products, may select from
potentially useful fluorochomes (Table 1) to solve the
analytical problems by employing secondary fluores-
cence techniques: for example, calcofluor white Mr2
new and fluorescein dibutyrate can be used to deter-
mine mixed-linkage b-glucan and esterase/lipase con-
tents, respectively, of cereal grains. In the case of the
calcofluor/b-glucan assay, two official European
brewing convention methods, one visual – malt
modification analysis (Figure 7:1A) – and one chem-
ical – FIA for mixed-linkage b-glucan in wort and
beer – have been developed. These methods have
helped to strengthen the insight of brewing chemists
into the strong relationship between the physical cell
wall structure of barley and malt and the chemistry of
wort and beer related to viscosity and filterability due
to the influence of b-glucans. Mg-ANS (1-aniline-8-
naphthalene sulfonic acid) can be used to detect dead
cells in pitching brewer’s yeast, and fluorescein iso-
thiocyanate (FITC)-immunofluorescent visual and
chemical methods can also be applied successfully,
for example, to monitor the distribution of a-amylase
activity in malting barley seeds (Figure 7:1B). A range
of components such as aleurone, starch, b-glucans,
protein bodies, fat globules, protein, and crystalline
inclusions can be convincingly demonstrated by
employing a range of fluorochromes and treatments
one at a time or in combination as demonstrated in
an example with wheat in Figure 7:2:2 and 3. Several
of these methods can also be used quantitatively to
determine components in homogenized samples by
spectrofluorometry.
Fluorescence Spectrofluorometry for Prediction of
Food Quality Calibrated with Chemometrics
0022 Due to its specificity and sensitivity, spectrofluoro-
metric analysis allows a unique characterization of a
fluorescent food sample, thus complementing near
infrared spectrophotometry. Fluorescence measure-
ment with fluorochromes in vitro, e.g., to detect
lipase (esterase) activity with fluorescein dibutyrate
at 495 nm excitation and 525 nm emission (Figure 1)
is a rather straightforward analysis using the equa-
tions given above. However, spectra from complex
fluorescence systems, such as that from wheat flour
(Figure 2), need advanced data analysis techniques
(chemometrics) similar to those developed for protein
analysis of seeds by near infrared spectroscopy. These
use complete spectra, and involve principal compon-
ent analysis for discrimination between normal and
deviating samples and partial least-squares analy-
sis (PLS) for calibration to chemical analyses for
prediction. Precise calibration of the spectrofluorom-
eter that is stable from day to day is obligatory
here, if data from whole spectra are to be utilized.
Spectrofluorometric data in the form of excitation/
emission landscapes are ideally resolved by multiway
chemometric algorithms such as parallel factor analy-
sis (PARAFAC: Figure 8) which in the fluorescence
example from process control in a beet sugar factory
covers three dimensions: excitation, emission, and
sample. In a PLS evaluation of a complex fluores-
cence landscape the latent factors, or loadings, are
difficult to interpret due to orthogonality. In contrast,
PARAFAC loadings are interpretable and expressed
in the form of estimates of the underlying excitation
and emission spectra of the corresponding fluoro-
phores. If stable computerized instruments with en-
larged measurement area for fluorescence monitoring
(mathematical chromatography) of inhomogeneous
samples in the food industry can be developed, the
chemometric software already developed will be able
to create a measurement system with unique potential
with regard to sensitivity and specificity to measure
those quality characteristics which depend on fluor-
ophores.
See also: Carotenoids: Occurrence, Properties, and
Determination; Chromatography: Thin-layer
Chromatography; High-performance Liquid
Chromatography; Polycyclic Aromatic Hydrocarbons;
Retinol: Properties and Determination; Spectroscopy:
Near-infrared; Tocopherols: Properties and
Determination
Further Reading
Bro R (1999) Exploratory study of sugar production using
fluorescence spectroscopy and multi-way analysis. Jour-
nal of the Chemometrics and Intelligent Laboratory
Systems 46: 133–147.
Engelsen SB (1997) Explorative spectrometric evaluations
of frying oil deterioration. Journal of the American Oil
Chemist Society 74: 1495–1508.
Engelsen SB, Mikkelsen E and Munck L (1998) New ap-
proaches to rapid spectroscopic evaluation of properties
in pectic polymers. Progress in Colloid Polymer Science
108: 166–174.
Guilbault GG (ed.) (1990) Practical Fluorescence – Theory,
Methods and Techniques. New York: Marcel Dekker.
Hurtubuise RJ (1984) Solid Surface Luminescence Analysis.
New York: Marcel Dekker.
Lakowicz JR (1999) Principles of Fluorescence Spectros-
copy. New York: Kluwer Academic/Plenum Publishers.
Morgan LC, Gormally J, Hubbard AR, d’Lacey C and
Ockleford CD (1999) Confocal microscopy: theory and
applications. Methods in Molecular Biology 114: 51–74.
Munck L (ed.) (1989) Fluorescence Analysis in Foods.
Harlow: Longman.
Munck L, Nørgaard L, Engelsen SB, Bro R and Andersson
CA (1998) Chemometrics in food science – a demonstra-
tion of the feasibility of a highly exploratory, inductive
5440 SPECTROSCOPY/Fluorescence
evaluation strategy of fundamental scientific signifi-
cance. Journal of the Chemometrics and Intelligent
Laboratory Systems 44: 31–60.
Rost FWD (1992) Fluorescence Microscopy, vols 1 and 2.
Cambridge: Cambridge University Press.
Von Sengbusch G and Thaer AA (1973) In: Thaer AA and
Sernetz M (eds) Fluorescence Techniques in Cell Biology,
p. 31. New York: Springer.
Wang XF and Herman B (eds) (1996) Fluorescence Imaging
Spectroscopy and Microscopy. New York: Wiley.
Wolfbeis OS (ed.) (1993) Fluorescence Spectroscopy: New
Methods and Applications. Berlin: Springer.
Atomic Emission and
Absorption
N Ulrich, University of Hannover, Hannover, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Summary
0001 Atomic spectrometry is suitable for the determination
of trace concentrations of most elements. The
principle is the absorption or emission of light of
certain wavelengths after the atomization and excita-
tion of the sample. The wavelength gives qualitative
information about the element, whereas the intensity
of the emission or the quotient of the intensity before
and after the absorption is proportional to the con-
centration of the analyte. Several spectral and non-
spectral interferences must be considered.
Background
0002 Atomic emission and absorption spectrometry are
used for the qualitative and quantitative determin-
ation of chemical elements, mainly metals and semi-
metals in a huge variety of different matrices. Both
techniques are based on the interaction of atoms
and electromagnetic energy. Kirchhoff and Bunsen
grounded the atomic spectrometry in the middle of
the nineteenth century. They observed that free, gas-
eous atoms of an element can absorb or emit radi-
ation of discrete and specific wavelengths. In atomic
spectrometry, wavelengths between 170 and 800 nm
are investigated.
The Atomizing Process – Important for
Both Techniques
0003 Prior to the excitation or absorption step, free atoms
of the elements must be formed. In classic techniques,
the samples are introduced into the system as aerosols,
e.g., solutions of sodium chloride. These aerosols are
desolvated and evaporated rapidly by adding thermal
energy to form microparticles. By interactions with
the environment in the flame or the plasma, free
atoms are formed that are capable of absorbing light
of specific wavelengths (atomic absorption) or, if
excited by thermal energy, can emit light of specific
wavelengths (atomic emission). The wavelength is
equal for both processes. Figure 1 shows the atomiza-
tion process for aerosols using sodium chloride
(NaCl) as an example.
Excitation and Relaxation of Valence
Electrons
0004After the atomization, the excitation of the electrons
(absorption) and relaxation (emission) take place.
Only the valence electrons are capable of the
radiation used in atomic spectrometry. In their basic
form, these electrons are in certain energy levels,
representing the ground state for an atom of a given
element. The electrons can absorb energy and form
energy-enriched, excited states. Two forms of excita-
tion are of importance for atomic spectrometry:
1.
0005Thermal excitation: The electrons can be excited
by thermal energy. At temperatures of 2000–
3000
C, most of the atoms are in the ground
state, following the Boltzmann distribution.
More states can be populated at higher tempera-
tures, e.g., in the Ar-plasma of an inductively
coupled plasma (ICP). The relaxation takes place
as electromagnetic radiation, which is measured in
atomic emission spectrometry (AES).
2.
0006Electromagnetic excitation: The electrons can
absorb light of a certain wavelength according to
Einstein’s law of absorption and emission, which
is measured in atomic absorption spectrometry
(AAS). The wavelength depends on the energy dif-
ference between the ground and excited electronic
state of the atom. The relaxation may take place as
thermal or electromagnetic radiation.
There are many emission lines in different series
starting from the ground state or an excited state, but
Drying:
Na
+
aq
+ CI
−
aq
+∆E
NaCI
s
Evaporation:
NaCI
s
NaCI
g
+∆E
Ionization:
Na
∗
+∆E
Na
+
Excitation:
Na
•
+CI
•
+∆E
AES:
Na
∗
Na
∗
Na
•
+hn
Dissociation:
NaCI
g
Na
•
+CI
•
+∆E
AAS:
Na
•
+hn
Na
∗
fig0001Figure 1 Processes in the atomizer of an atomic spectrometer.
SPECTROSCOPY/Atomic Emission and Absorption 5441
not every emission line is also a detectable absorption
line in AAS. The intensity of the absorbance line
depends on the population of that state (,) which
absorbs the light. In most cases, only the ground
state is populated to such an extent that intensive
radiation is emitted.
Atomic Emission Spectrometry
0007 In AES, the atoms are excited by thermal energy and
emit light of specific wavelengths. The frequency of
the light is proportional to the energy difference of
both states. For optical emission spectrometry, the
wavelengths are in the ultraviolet/visible region. This
light is measured and can be correlated by the wave-
length to the element (qualitative information) and
by the intensity to the concentration (quantitative
information).
0008 The intensity of the emitted light depends on the
number of atoms in the excited state. This number
will be larger for higher temperatures given by the
Boltzmann distribution. At temperatures below
2500
C, only transitions between the ground state
and one of the first excited states are possible for most
of the elements. The alkali metals can be excited in
sufficient rates at temperatures of c. 2000
C. The
concentration of the analyte in the sample is directly
proportional to the intensity of the emitted light.
Principal Components of an Atomic
Emission Spectrometer
0009 The main components of an atomic emission spec-
trometer are the sample introduction device, which is
often a pneumatic nebulizer system, the atomization
and excitation source, one or more monochromators,
and a detector, mostly a photomultiplier. The princi-
pal set-up of an AES is shown in Figure 2. AES can be
used as single element method or as a simultaneous
measurement device, as detailed below.
Atomizing and Excitation Techniques
0010 There are many techniques for atomizing and
exciting samples, like bow and spark excitation or
glow discharge excitation. The two most important
methods are flame photometry, which is suitable pri-
marily for alkaline and some earth alkaline elements,
and ICP, which is used widely as an energy source in
atomic emission spectrometry.
Flame Photometry (Atomic Emission Spectrometry)
0011 Flame photometry was established by Bunsen. It is
called photometry, although it is an emission tech-
nique. The correlation between the intensity and
concentration is linear, and the Lambert–Beer law
cannot be applied. The atoms are excited in a flame,
and the intensity of the emitted light is measured.
0012The apparatus of FAES contains a nebulizer system,
a burner, suitable for different gases like nitrous oxide
or methane, a monochromator, which is important
for the quality of the measurement, and a detector.
Often, flame atomization absorption spectrometry
systems with the lamps turned off are used as the
FAES device.
0013Applications and analytical performance In the
early years, FAES was used for all metals. However,
the exciting efficiency of the flames was poor, and
the detection limits were quite high. Today, FAES is
mainly used for the determination of alkali metals in
solutions. The sensitivity for these elements, espe-
cially Na and K, can be better than for the FAAS.
Typical detection limits are 0.01 mg l
1
(Na) and
0.1 mg l
1
(K) in liquid biological samples. For other
elements, AES with plasma excitation, atomic
absorption spectrometry, or mass spectrometry is
used nowadays.
0014Problems and interferences Spectrometric determin-
ations are relative methods, so calibration is neces-
sary. Differences between the samples and the
standard solutions can lead to systematic errors,
called interferences. In spectrometry, there are two
types of interferences. The spectral interferences are
problems caused by unspecific radiation or absorp-
tion, whereas nonspectral interferences affect the con-
centration of the analyte in the atomizer and exciting
zone.
0015Spectral interferences in flames include the spectral
lines of matrix compounds and the high continuous
background of the flame, emitting light of all wave-
lengths. In relatively cold flames (2000
C), emission
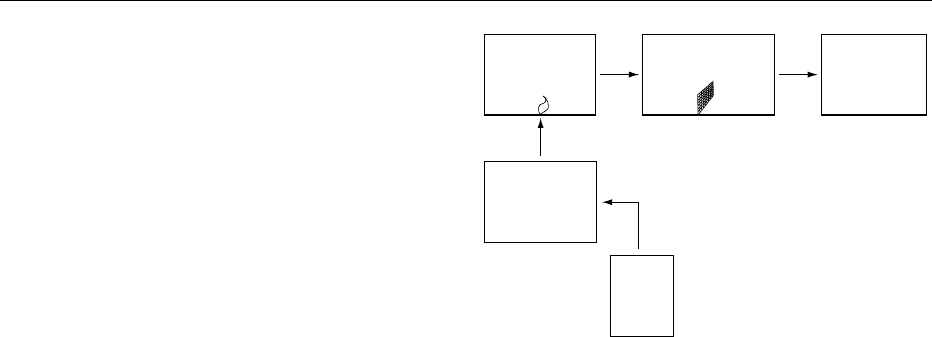
Atomizer
(excitation)
Monochromator
Detector
Nebulizer
Sample
fig0002Figure 2 Principal set-up of an AES apparatus.
5442 SPECTROSCOPY/Atomic Emission and Absorption

spectra with few lines are observed. However, excesses
of one element, e.g., 1000-fold excess of Na for Li
determination can lead to an overlapping of the sig-
nals. In addition, Ca and Sr can lead to interferences.
0016 Nonspectral interferences can be classified as
chemical interferences, transport interferences, ion-
ization interferences, and self-absorption. If stable
compounds of the analyte and matrix components
are formed, these will hinder or even completely
suppress the atomization and excitation. Organic
substances can alter the flame temperature, which is
important for the FAES, and can emit continuous
background radiation. In addition, the analytes can
react with the flame gases, e.g., Ca can form CaO
with oxygen.
0017 Transport interferences occur when the amount of
sample introduced into the atomizer changes over
time. The main reason is a difference in viscosity
between water solution and organic solvents. In
addition, larger concentrations of salts and matrix
compounds can disturb the nebulizing process.
0018 Ionization interferences occur, when part of the
analyte is ionized within the flame. It only takes
place to a small extent, but causes a reduction of the
population in the excited state. One problem with a
high concentration of the analyte is self-absorption.
Because emission and absorption take place at the
same wavelength, analyte atoms can absorb light,
which is emitted by other analytes. This leads to a
significant curving of the calibration graph.
Inductively Coupled Plasma–Atomic Emission
Spectrometry (ICP-AES)
0019 The inductively coupled plasma was introduced into
atomic emission spectrometry by Greenfield in 1964.
ICP-AES is today one of the most widely used tech-
niques for trace metal and semimetal determination
in a huge variety of different samples.
0020 An argon plasma with a gas consumption of c.17l
min
1
is used as atomizer. The gas is delivered
through three concentric quartz tubes (the torch),
which are surrounded by an induction coil. This coil
is operated at a frequency of 27.1 MHz and a power
of 1000–2000 W. Gas temperatures of 6000–8000
C
are observed in the plasma, which, in combination
with the relative long residence time of the analytes in
the plasma, lead to an effective energy transfer on to
the analytes. Even refractive metals and oxides can be
atomized to a great extent. The spectra obtained are
often complicated and contain many lines, especially
for the transition metals. The plasma can be observed
either axially or radially.
0021 One of the main advantages with respect to AAS is
the ability of multielement determinations, because
sequential and simultaneous set-ups can be used.
Analytical wavelengths range from 170 to 800 nm.
For sequential investigations, the monochromator,
often of the Czerny–Turner type, is tuned for
each element. Several monochromators of un-
changeable wavelengths are fixed in the detector,
mostly forming a Rowland circle, for simultaneous
measurements.
0022Applications and analytical performance The ICP-
AES is suitable for a wide range of elements, because
the high temperatures provide very good atomizing
conditions. All metals and semimetals and even some
of the nonmetals (e.g., sulfur, phosphorus, and iodine)
can be detected. The simultaneous spectrometers
are limited to six to 30 elements, depending on the
number of monochromators in the detector. Quanti-
tative results for these elements can be obtained
in c. 1 min. Typical detection limits range from
0.01 mgl
1
for Ca, Mn, Mg, and Mo to 20 mgl
1
for
As, Bi, and Sn. The dynamic range reaches usually
over five to six decades, so that matrix compounds
and trace elements often can be detected in one
analysis.
0023Gaseous, liquid, and solid samples can be detected
using ICP-AES. Liquids can be nebulized with any of
the standard nebulizers. Flow injection and on-line
coupling to chromatographic devices are other
options. Gases, e.g., metal hydrides, can be brought
directly into the plasma. The dry plasma and the
nearly complete sample introduction lead to a signifi-
cant enhancement of the detection limits. For solid
samples, mostly slurry techniques with particles less
than 2 mm in diameter are used.
0024Problems and interferences The drawbacks of ICP-
AES are the high costs of the apparatus and mainten-
ance, and the high spectroscopic background of the
plasma.
0025In addition, there are numerous interferences ob-
served in the spectra. Spectral interferences arise from
the plasma itself, which is an intensive source of
unspecific radiation and argon emission lines. Both
depend on the temperature and the composition of
the plasma gas and vary, e.g., with the introduction of
water from the sample. Further, there are emission
lines of hydrogen, nitrogen, and oxygen. Different
molecular species are formed in the plasma, e.g.,
N
2
þ
, OH, NH, or NO, which show a large number
of rotation and vibration bands.
0026Nearly all elements in the sample emit characteris-
tic spectral lines not only from the ground state but
from several excited states, because the high tempera-
tures in the plasma lead to a remarkable population
of numerous states. These interferences can be re-
solved to some extent by the use of high-efficiency
SPECTROSCOPY/Atomic Emission and Absorption 5443
monochromators, but many lines are too close
together for separation.
0027 Chemical interference is not important in most
cases. Transport interference can occur, as described
for FAES.
Other Plasma Sources for Atomization
0028 Microwave-induced plasmas (MIP) are formed by
microwaves (frequency 2.45 GHz, power 50–200 W).
This plasma is mainly used as a detector for gas
chromatography. Mostly, He is used as the plasma
gas, providing very high electron temperatures and
excitation energies, e.g., for the nonmetals.
0029 Several other plasma sources have been applied for
AES. Glow discharges, sparks, and bows are mostly
used for metallic samples. In addition, capacity
coupled plasmas can be utilized to excite metal atoms.
Atomic Absorption Spectrometry
0030 This technique is based on the selective absorption of
light by the gaseous free atoms. The absorbance is the
logarithm of the quotient of the radiation intensity
before the absorption, I
0
, and after the absorption, I.
According to the Lambert–Beer law, it is directly
proportional to the concentration of the absorbing
atoms in the atomizer, c, the length of the atomizer,
d, and the absorption coefficient, a.
A ¼ 1
g
I
0
I
¼ adc: ð1Þ
The coefficient, a, depends on the element and the
wavelength, g, and can be related to the Einstein
transition probability of an absorption process, B
and the Planck constant, h.
a ¼ B
h
l
: ð2Þ
Principal Components of an Atomic
Absorption Spectrometer
0031 The main component of an analytical absorption
spectrometer is the light source, providing monochro-
matic light for the absorption process. Two types of
light sources are mostly used. Hollow-cathode lamps
contain a cathode of the analyte element and an
anode, and are filled with a noble gas. There is a
glow discharge between the cathode and the anode,
in which positive gas ions are formed, which sputter
element atoms of the cathode at relatively low
temperatures. These lamps emit mostly lines excited
from the ground state and show only slight line
broadening. The other type of lamps are electrodeless
discharge lamps that contain the element in a small
quartz tube filled with a noble gas. A high-frequency
field (c. 27 MHz) leads to a plasma within the tube, in
which the element is excited and emits specific light.
The advantages are the better stability of the lamp,
especially for elements like antimony, mercury and
tin, and the higher intensity. A problem is the forma-
tion of ions, which reduce the effective concentration
of the excited atoms. Both lamp types generate
narrow emission lines and are available for all elem-
ents that can be determined by AAS. AAS is mostly a
single element or sequential analytical technique, be-
cause a specific light source is used for each analyte.
0032The light beam is sent through the atomized sample
and then via a monochromator to the detection
system similar to that of AES. Pneumatic nebulizers
are used as well for AAS. In addition, slurry and solid
samples have been investigated. The atomizer in the
light path can be of different types. The principal set-
up of an AAS is shown in Figure 3.
Atomizing Techniques for Atomic
Absorption Spectrometry
0033The purpose of the atomizer is the reproducible for-
mation of gaseous atoms of the analyte in the ground
state. There are three main atomizing techniques in
atomic absorption spectrometry. The classical method
is flame atomization. Electrothermal atomization pro-
vides better detection limits for most elements, and
chemical vapor atomization is well suited for gaseous
samples like the metal and semimetal hydrides and
gaseous mercury.
Flame Atomic Absorption Spectrometry
0034In FAAS, the liquid sample is introduced as an aerosol
in a flame with temperatures between 2000 and
3000
C. The flame should have a low self-absorption
at the analyte wavelength and be a low source of
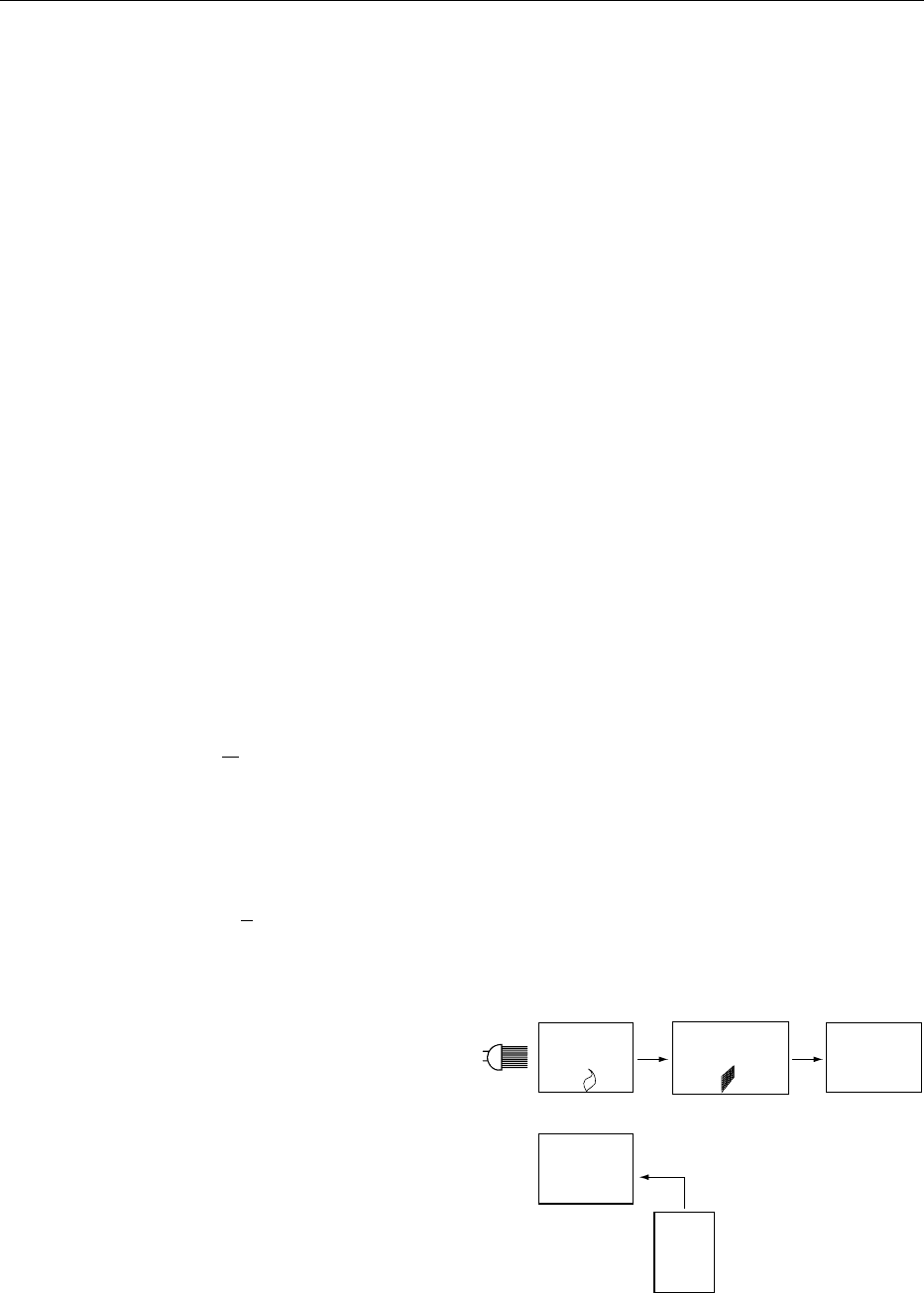
Atomizer
Monochromator
Detector
Nebulizer
Sample
Lamp
fig0003Figure 3 Principal set-up of an AAS apparatus.
5444 SPECTROSCOPY/Atomic Emission and Absorption

white light to provide adequate signal-to-noise ratios.
In addition, the residence time of the analyte atoms in
the flame should be sufficient for the absorbing
process, and therefore, lower flow rates for the
flame gases should be used.
0035 The most important is the acetylene/air flame,
which is rather transparent over a broad region of
wavelengths and, at temperatures of c. 2500
C, is
suitable for most analytes. Higher temperatures are
needed for some elements like Be, Ca, Ti, V, Mo, and
the rare earths, so acetylene/nitrous oxide flames with
temperatures of c. 3000
C are used. Other flame
types include hydrogen/air and methane/air.
Applications and Analytical Performance
0036 FAAS is suitable for samples with analyte concentra-
tions of mg l
1
to the higher mgl
1
. The sensitivity
is often given as a characteristic concentration, c
0
,
which depends on the parameter of the instrument
(e.g., the geometry of the atomizer) and the element.
Linear calibration functions are obtained up to 100
c
0
, and the precision is frequently better than 0.5%.
0037 FAAS can be used to determine the macronutrient
elements in human body fluids and other biological
matrices. Whereas sodium and potassium are mainly
investigated by flame photometry, calcium and mag-
nesium are analyzed by FAAS by direct measurement
after dilution.
Problems and Interferences
0038 The matrix in the samples can influence the drying,
crystal formation, atomization, and distribution of
elements in the flame. Mostly, the kinetic of the atom-
ization is changed by analyte compounds with high
boiling points. The energy of the flame can lead to an
ionization of certain elements like the alkali or earth
alkali metals and to curved calibration functions. The
addition of electron donators like Cs shifts the equi-
librium to the atoms and provides a better linearity
of the calibration. Chemical reactions and spectral
effects are less important for the FAAS, because of
the dilution of the analytes and the matrix by the
flame gases. Problems can arise from organic solvents
and high salt concentrations, which alter the viscosity
of the solution.
Electrothermal Atomic Absorption
Spectrometry (ETAAS)
0039 One major problem of the FAAS is the residence time
in the atomizer. In order to improve this time, the
ETAAS has been invented by L’vov in the middle of
the twentieth century and is now one of the most
widely applied techniques for trace and ultratrace
metal determinations.
0040In the ETAAS, the atomizer is a small quartz tube
mounted between two electrodes. Samples containing
a few microliters of liquid or micrograms of solid are
injected through a small hole on top of the tube. The
oven is heated electrically following a temperature
program:
1.
0041The drying step, in which the water or the solvent
is evaporated.
2.
0042The sample preparation step, in which matrix
components are evaporated or altered.
3.
0043The atomization step, in which the analytes
are atomized at constant temperatures, 1500–
2500
C, depending on the analyte.
4.
0044The cleaning step, in which the temperature is
brought to a maximum, and the graphite furnace
is cleaned.
In order to improve the sensitivity of the ETAAS and
to inhibit the formation of carbides by elements like
Mo, V, and Ti, the furnace can be coated by different
materials including Os and Pd. In contrast to the
continuous signal of the FAAS, in ETAAS, a transient
signal, mostly a heavy tailing peak, is obtained.
Analytical Performance
0045The ETAAS has several advantages in respect to the
FAAS. Slurry and solid samples can be used without
dissolution, and the analytes can be separated from
the matrix using a program of different temperatures.
The method provides a high sensitivity and very
low sample consumption, because nearly the whole
sample is introduced into the atomizer. Drawbacks
are the complicated apparatus and handling, the
low sample throughput, and the large number of
interferences. The ETAAS is now widely used for
the determination of trace elements in biological
samples.
0046The detection limits of the ETAAS are from
0.5 mgl
1
for elements like V and Li to 0.005 mgl
1
for elements like K, Cd, and Zn (10–1000-fold better
than for the FAAS). The absolute sensitivity depends
on the dimensions and geometry of the quartz tube
and further experimental parameters. It is given as
characteristic mass, m
0
. The precision is c. 1% stand-
ard deviation at 10 m
0
, and the linear range ends by
c. 100 m
0
, similar to the FAAS.
Problems and Interferences
0047In the ETAAS, numerous spectral interferences have
been determined. These interferences are based on
the insufficient separation of the analytes and matrix.
The radiation from the light source can be absorbed
unspecifically by atoms and molecules, leading to
overlapping atomic and molecular bands or can be
scattered on particles like undissociated metal salts
SPECTROSCOPY/Atomic Emission and Absorption 5445

in the light beam. At high atomizing temperatures,
sublimating graphite particles can lead to problems.
0048 The background depends strongly on the matrix,
so that background correction is much more import-
ant than for FAAS. Modern apparatus use either a
deuterium lamp or the Zeeman effect for correction
of the data. The standard addition method should be
used for complex matrices.
Chemical Vapor Atomic Absorption
Spectrometry (CVAAS)
0049 CVAAS is used for the determination of gaseous metal
and semimetal compounds. It is an important detector
for the speciation of hydride-forming elements. There
are two sectors of the CVAAS: the cold vapor tech-
nique, which is only suitable for mercury, and the
hydride generation process, which can be applied to
Se, Te, As, Sb, Bi, Sn, and Pb. In the latter case, the
elements are reduced to the corresponding hydrides
mostly with sodium tetrahydridoborate in acidic solu-
tion. A quartz furnace is used as the atomization unit,
which is typically heated electrically to 900–1000
C.
The samples are introduced as gases to nearly 100% by
an inert carrier gas stream. The residence time in the
atomizer depends on this gas stream.
0050 Mercury compounds can be reduced by sodium
tetrahydridoborate to elemental mercury, which can
be introduced by inert gas into the atomizer nearly
without heating. The quartz furnace is used at 120
C
in order to inhibit the condensation of mercury and to
reduce the scattering on water particles in the oven.
Analytical Performance
0051 The absolute performance of the hydride generation
is c. 100-fold less than the ETAAS, but the larger
sample volumes (up to 100 ml in one batch reactor)
lead to an increase in the detection ability usually at a
factor of 10. The determination of 0.01 mg per liter of
arsenic or selenium is possible. In addition, the oxi-
dation states and organic species of the hydride
formers can be detected separately, so that more infor-
mation about the species distribution of these elem-
ents in different matrices can be gathered. In addition,
the matrix is nearly completely separated from the
analytes. The drawbacks are the complicated experi-
mental set-up, relatively long analysis times, the in-
stability of the formed hydrides, and the high risk of
contaminations by the addition of high excesses of
solid and difficult-to-clean reagents (e.g., sodium
tetrahydridoborate, sodium hydroxide).
0052 The CVAAS for the mercury determination pro-
vides very good detection limits (0.001 mgl
1
). Even
enrichment is possible by using gold foils for
amalgam formation.
Problems and Interferences
0053In the CVAAS, the separation of the analytes of the
matrix is nearly complete for nonhydride formers.
However, the hydride-forming elements can strongly
interfere with the reduction of one another (e.g., As
interfering with the reduction of Sb). In addition, the
reduction process can be inhibited by oxidants or com-
plexing agents in the matrix or even by some transition
metals. The platinum group elements adsorb large
amounts of hydrogen, so that the effective reduction
ability of the solution is lowered. Standard addition
for calibration is therefore strongly recommended.
0054The volume of the sample must be constant. Foam
formation can influence the degassing of the analytes,
especially in biological matrices. This should be
suppressed.
Conclusion
0055Atomic spectrometry is suitable for trace concentra-
tions of most elements. The principle is the absorp-
tion and emission of light of a certain wavelength
after atomization of the sample. The wavelength
provides qualitative information about the element,
whereas the intensity of the emission (AES) or the
quotient of the intensity before and after the absorp-
tion (AAS) is proportional to the concentration of the
analyte. Several spectral and nonspectral interfer-
ences must be considered.
See also: Aluminum (Aluminium): Properties and
Determination; Copper: Properties and Determination;
Electrolytes: Analysis; Iron: Properties and
Determination; Lead: Properties and Determination;
Lithium; Mass Spectrometry: Principles and
Instrumentation; Mercury: Properties and Determination;
Mineral Water: Types of Mineral Water; Potassium:
Properties and Determination; Selenium: Properties and
Determination; Sodium: Properties and Determination;
Spectroscopy: Overview; Tin; Trace Elements; Water
Supplies: Chemical Analysis
Further Reading
Dean JR (1997) Atomic Absorption and Plasma Spectros-
copy. Chichester, UK: John Wiley.
Dedina J and Tsalev D (1995) Hydride Generation Atomic
Absorption Spectrometry. Chichester, UK: John Wiley.
Ebdon L and Hywel E (1998) An Introduction to Analytical
Atomic Spectrometry. Chichester, UK: John Wiley.
Hill SJ (1999) Inductively Coupled Plasma Spectrometry
and its Applications. Sheffield, UK: Sheffield Academic
Press.
Jackson KW (1999) Electrothermal Atomization for
Analytical Atomic Spectrometry. Chichester, UK: John
Wiley.
5446 SPECTROSCOPY/Atomic Emission and Absorption
