Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0004 Since the energy required to stimulate an electronic
transition is greater than that for a vibrational transi-
tion, which in turn is greater than for rotational
transition, it is found that more than one transition
is usually stimulated so that, for example, pure vibra-
tional spectra are not seen and are nearly always
complicated by rotational transitions.
0005 The normal way for interaction of the radiation to
occur is through one of four processes: (1) absorption,
(2) emission, (3) elastic scatttering, although there is
not net energy absorption, and (4) inelastic scattering.
These mechanisms are described in more detail under
the appropriate headings as the spectroscopies are
discussed in more detail. The first to be discussed
are ultraviolet and visible (UV/VIS) spectroscopies,
both of which involve electronic transitions.
Electronic Transitions
0006 In UV/VIS spectroscopies, absorption of radiation is
the result of the excitation of bonding electrons. The
types of bonds that give rise to absorption are known
as chromophores and in the UV the electrons of the
chromophore are either directly used in bond forma-
tion or are nonbonding or unshared outer electrons of
an electronegative atom, such as oxygen, nitrogen, or
sulfur. The general mechanism in a chromophore such
as C
—
—
C, in which orbitals are used for bonding, in-
volves the promotion of an electron in a bonding p
orbital into a nonbonding p* orbital (a so-called pp*
transition), which typically requires about 7 eV, cor-
responding to a wavelength of 180 nm. It is also pos-
sible for a nonbonding electron to be promoted to a p*
(an np* transition). These two are the most common
transitions, although similar ones exist for single (s)
bonds (ns*orss*). However, because the latter
required much higher energies, they are seen in the
vacuum-UV and are harder to observe. The frequen-
cies of the absorption can be influenced by solvents
and by delocalization in conjugated systems.
0007Transition-metal ions absorb in the UV/VIS region
and the transitions responsible involve 4f and 5d
electrons. Alternatively, in some inorganic complexes
the process of charge-transfer absorption occurs.
0008Most UV/VIS spectroscopy involves absorption
processes and normally a spectrophotometer is used
to measure a spectrum. The major components are a
source, a dispersing system, and a detector. Normally,
light from a suitable source is passed to a prism or
grating where it is dispersed into its component fre-
quencies. The dispersing element may be rotated so
that each frequency is passed in turn through a
narrow slit. This light may be divided so that half
passes through a channel containing the sample and
half through a reference channel. The emerging
beams can be directed in ratios at a detector and the
absorbance of the sample as a function of frequency
(or wavelength), i.e., the spectrum, can be plotted.
0009It should be noted that UV/VIS spectra do not
consist of discrete lines. The reason is that the high
energy of the UV/VIS region can be transferred into
the vibrational and rotational substates so that both
types of transition are simultaneously stimulated. In
Figure 2 the energy level diagram for a chromophore
is shown. E
0
and E
1
represent the ground and excited
electronic energy levels of a molecule. Each electronic
level has associated with it various vibrational sub-
levels, v
0,1
v
0,2
, etc., which in turn have rotational
sublevels. An electron may be promoted from the E
0
to E
1
electronic state but may go from the v
0,0
to v
1,0
or v
1,1
state; i.e., there is a simultaneous vibrational
transition. The range of vibration subtransitions pos-
sible, combined with rotational transitions, means
that there is no discrete frequency at which transition
occurs.
0010Furthermore, if an electron is promoted from the
v
0,0
state of E
0
to the v
1,1
state of E
1
it may lose
energy by collision, for example, and may become
lowered into the v
1,0
state. During relaxation to
the ground electronic state a photon is emitted of
Radio frequency
NMR ESR UV/VISMicrowave Infrared X-ray
γ-ray
10
−8
eV 10
6
eV
Increasing energy
10 m 100 cm 1 cm
100 µm1 µm 10 nm 10 pm
Increasing wavelength
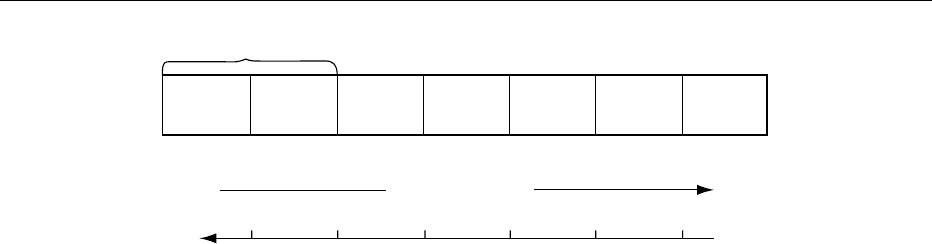
fig0001 Figure 1 The electromagnetic spectrum. NMR, nuclear magnetic resonance; ESR, electron spin resonance; UV/VIS, ultraviolet and
visible. Reproduced from Spectroscopy: Overview, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
SPECTROSCOPY/Overview 5407
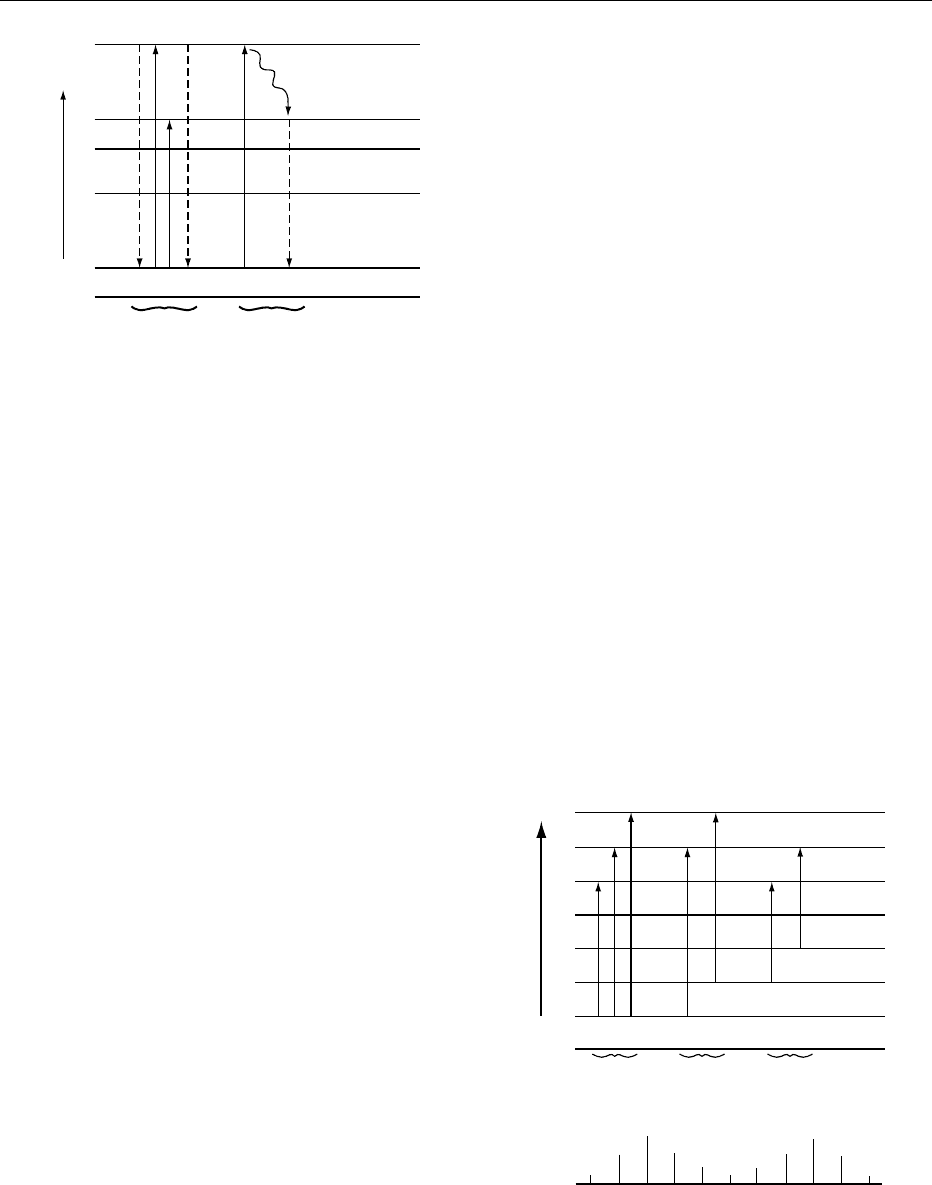
different energy from that absorbed, and this process
is called fluorescence.
Vibrational Spectroscopy
0011 The transitions between the vibrational energy levels
are the basis of infrared and Raman spectroscopies.
The infrared region is divided into the near, middle (or
mid) and far infrared. This division is on the basis of
instrumental factors as well as the types of vibration
that occur in each region. It is easiest to consider first
the middle infrared, which is usually considered to lie
between 2.5 and 25 mm in wavelength. It is common
practice, however, for vibrational spectroscopists to
usetheunitwavenumber(reciprocalofthewavelength
in centimeters) rather than wavelength; this has units
of cm
1
, which is a frequency term. The middle infra-
red then stretches from 4000 to 400 cm
1
.
0012 The bond between two atoms can be considered to
be rather like a spring that has a certain strength of
force constant (k). The bond, or spring, can be
stretched and caused to oscillate. It will do so at
some natural frequency, f, that depends upon k and
the masses of the atoms according to Hooke’s law:
f ¼ð1=2 pÞ
ffiffiffiffiffiffiffiffi
k=m
p
ð2Þ
where m is the reduced mass given, defined as:
m ¼ðm
1
m
2
Þ=ðm
1
þ m
2
Þð3Þ
m
1
and m
2
being the masses of the individual atoms
constituting the bond. Equation (2) shows that a
particular bond will give rise to a characteristic
frequency that depends upon the masses of the atoms
and the strength of the bond. Therefore, a C
—
—
O bond,
which has a greater force constant than a C—O bond,
will have a vibrational frequency which is larger. In
practice, different functional groups give rise to char-
acteristic vibrational frequencies. This is the major use
for vibrational spectroscopy; it is a highly useful probe
for the identification of functional groups and for
structural determination. It has greater selectivity
than UV/VIS in this respect.
0013Equation (2) is derived from classical physics, but
of course the actual process is quantized and (2)
should be written as:
F ¼ðh=2p Þ
ffiffiffiffiffiffiffiffi
k =m
p
ð4Þ
where h is Planck’s constant.
0014In the main, an infrared spectrum is generated by
absorption using a similar arrangement to that used
for UV/VIS but with different source, detector, and
dispersing optics. The process is illustrated in
Figure 3. The energy level diagram shows the ground
(v
0,0
) and excited (v
1,0
) vibrational states. Also shown
are the various rotational substates (J
0
and J
00
). Exci-
tation can occur from v
0,0
(J
00
¼ 0) to v
1,0
(J
0
¼ 0),
corresponding to the band center of the absorption
band. However, excitation from the J
00
¼ 1toJ
0
¼ 1
rotational substates (i.e., DJ ¼ 0) will produce a
slightly different frequency as the rotational sublevels
are not equally spaced. It is also possible for DJ to be
+1, giving rise now to a complicated absorption
v
1,1
v
1,0
v
0,1
v
0,0
E
1
E
0
Energy
Electronic absorption
with simultaneous
vibrational transition
Emitted radiation
equal to absorbed
Origin of
fluorescence
Emitted energy
of different
from absorbed
fig0002 Figure 2 Energy levels for a chromophore, showing electronic
and vibrational levels. Reproduced from Spectroscopy: Over-
view, Encyclopaedia of Food Science, Food Technology and Nutrition,
Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
J' = 2
J' = 1
J' = 0
J'' = 2
J'' = 1
J'' = 0
v
1,0
v
0,0
Energy
∆J = 0 ∆J = +1
∆J = +1
∆J = −1
∆J = −1
Q branch R branch P branch
nb: For all absorptions ∆v = 1
PQR
fig0003Figure 3 Energy levels for infrared transitions, showing vibra-
tional and rotational levels. Reproduced from Spectroscopy:
Overview, Encyclopaedia of Food Science, Food Technology and Nu-
trition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Aca-
demic Press.
5408 SPECTROSCOPY/Overview

band comprising of a central absorption (Q branch)
with equally spaced lines either side called the P branch
(DJ ¼1), and the R branch (DJ ¼þ1). This structure
is only seen as such in the gas phase. In solid or solution
state the result is that a broad absorption rather than a
sharp line is seen. However, no absorption will be seen
at all unless the selection rule is applied. This states
that for absorption to occur there must be a change in
dipole moment during the vibration. Consequently,
homonuclear bonds do not absorb.
0015 At normal room temperature most molecules will
be in the ground vibrational state. However, as the
temperature is increased, a more significant popula-
tion will develop in the excited state. As a result
transitions from the v
1
to v
0
state can occur with the
emission of a photon. This is the process of infrared
emission which is, albeit rare, an alternative to
absorption spectroscopy. In this case the (heated)
sample acts as the infrared source.
0016 Infrared spectroscopy has, until recently, been of
little use for industrial, biological, and food use owing
to the difficulties of sample handling and the time of
data acquisition. However, the recent development of
Fourier transform methods involving the replacement
of the dispersing element with an interferometer has
benefits of increased speed, throughput, and fre-
quency reproducibility. Coupled with new methods
of sample presentation, this has led to a reawakening
of interest in the middle infrared.
0017 The absorptions in the middle infrared are known
as the fundamentals. However, various overtones and
combinations of the fundamentals can arise. For
example, a molecule with two fundamentals at fre-
quencies v
1
and v
2
may give overtones at 2v
1
,3v
1
,
4v
1
,or2v
2
, etc., or combinations at, say, v
1
þv
2
or
2v
1
þv
2
. In practice, not all fundamentals give rise to
overtones, usually only bonds in which a heavy atom
such as N or O is coupled to hydrogen. The overtone
and combinations constitute the near infrared
(2.50.7 mm) which, despite the apparent complexity
of the spectra, has found considerable application to
food problems.
Raman Spectroscopy
0018 If a sample is illuminated with monochromatic visible
light, it is found that much of the light is scattered and
that the scattered light is of the same frequency as the
illuminating light. This is elastic or Raleigh scattering.
However, analysis shows that a small amount
(< 10
6
) of the incident radiation is scattered with a
different frequency. A series of lines is found with
frequencies less than the incident light. A weaker
series is found with higher frequencies. When the
former set of lines are presented as a spectrum of
intensity versus frequency shift, the result is some-
thing similar to an infrared spectrum with the shift
scale from about 4000 to 20 cm
1
. This effect is the
Raman effect and the spectrum is called the Raman
spectrum. The lines comprising the spectrum are
called Stokes lines. Those of higher frequency than
the exciting line are called anti-Stokes lines and con-
sist of the same peaks with the same shift, but there
may be different intensity ratios.
0019The electrical field of the incident radiation inter-
acts with the electrons in the sample and causes peri-
odic polarization and depolarization so that energy is
momentarily absorbed in a distorted, polarized state
or virtual state. Most molecules relax by the emission
of energy of the same frequency to that absorbed. In
a few cases some of the energy will be dissipated
amongst the vibrational energy levels, causing vibra-
tional excitation and giving rise to the Raman spec-
trum. Even fewer molecules will not be in the ground
vibrational state before excitation but in the virtual
state may relax and the emitted photon will be of
higher energy than that incident, leading to the anti-
Stokes lines. In contrast to infrared spectroscopy, the
selection rule for absorption is that, during vibration
of the bond, there must be a change in the electronic
polarizability. There is thus a distinct difference in the
two spectra and vibrations that may be weak or absent
from infrared spectra, e.g., C—C are present and per-
haps strong in the Raman. The two spectroscopies are
thus complementary and together provide a complete
picture of the vibrational states of a molecule.
Far Infrared/Microwave
0020To complete the picture, at lower energy there is the
far infrared (40010 cm
1
), which has major appli-
cations in inorganic chemistry as bonds between
metals and organic ligands appear here as well as
skeletal vibrations of molecular backbones. This
region is of limited application in food and nutritional
studies.
0021In the microwave region, at even lower energy,
pure rotational spectra can be produced. However,
they will not be addressed here as this is also of
limited application.
0022At the radiofrequency end of the spectrum is NMR
spectroscopy which involves transitions between
magnetic quantum levels of atomic nuclei. Nuclei
have properties of spin and magnetic moment. Split-
ting of the energy levels can be induced by placement
in a magnetic field and transitions can be induced by
the application of radiofrequency radiation. Today,
this is usually achieved by irradiating the sample ex-
posed to a high magnetic field with a pulse of broad-
band radiation. After excitation the nuclei reemit
SPECTROSCOPY/Overview 5409

energy at their resonance frequencies and the observed
signal is a combination of these frequencies, and this
decays with time. A spectrum can be produced by
Fourier transformation of this decaying signal. The
usefulness of the technique lies in the fact that the
resonance frequency of a given nucleus depends upon
its chemical environment. However, the range of
NMR experiments possible is very large indeed and
it is a very powerful method for structural analysis.
0023 In the food industry the use of NMR spectra as
such is increasing but relaxation time measurements
are still more important, particularly in the determin-
ation of solid/liquid ratios. The relaxation rate from
the excited state depends on environmental factors
and molecular mobility.
Absorption Laws
0024 In UV, near infrared, and mid-infrared adsorption
spectroscopy, the fundamental law governing adsorp-
tion is the Beer–Lambert relationship. For a sample
illuminated by radiation of intensity I
0
the amount
transmitted, I, is given by:
I ¼ I
0
e
ecl
ð5Þ
where c is the concentration of absorbing species, l is
the pathlength through which the light passes, and e
is the molar absorptivity.
0025 For quantitative analysis, spectra are usually pre-
sented in absorbance units, where absorbance, A,is
defined as
A ¼logðI=I
0
Þ¼ecl ð6Þ
so that A is directly proportional to the concentration
at constant pathlength.
0026 Practically, optical spectroscopy requires that e
be determined for any absorbing species. This is
achieved by calibration and their absorbance is meas-
ured. Solutions of the sample to be determined are
prepared at known concentration and their absor-
bances are measured. When the latter are plotted
against the concentration, a linear plot results of
slope e. Unknown concentrations can be calculated
by measuring absorbance and interpolating from the
calibration curve.
0027 Deviations from the Beer–Lambert relationship can
occur if too wide a range of concentration is chosen so
that solute–solute interactions occur, or where there is
chemical interaction between components.
0028 A particular problem that exists in the near and
mid-infrared is where significant overlap of absorb-
ance peaks occurs. Clearly, the absorbance at a given
wavelength may then depend upon more than one
concentration, so that:
A ¼ e
1
c
1
¼ e
2
c
2
þ e
3
c
3
... ð 7Þ
Hence, more complicated solutions to the Beer–
Lambert relationship may be required for multi-
component analysis. Such methods include p and k
matrix, partial least-squares, or principal components
regression.
0029In NMR single-pulse experiments the signal ob-
served is directly proportional to the number of
nuclei, provided sufficient time is allowed between
pulses for the reestablishment of equilibrium. Under
such circumstances the NMR experiment is quantita-
tive and requires no calibration. Double-resonance
experiments can, however, lead to enhanced signals
for certain nuclei (nuclear Overhauser effect) so that
some form of calibration is necessary. In relaxation
measurements the magnetization decay can be broken
down into components from fast (solid) and slow
(liquid) components, the relative magnitude of each
reflecting the relative concentrations.
0030Practical details and applications of the most rele-
vant spectroscopies in food analysis can be found in
following chapters.
See also: Spectroscopy: Infrared and Raman; Near-
infrared; Fluorescence; Atomic Emission and Absorption;
Nuclear Magnetic Resonance; Visible Spectroscopy and
Colorimetry
Further Reading
Andrews DL (ed.) (1990) Perspectives in Modern Chemical
Spectroscopy. Berlin: Springer-Verlag.
Banwell CN and McCash EE (1994) Fundamentals of
Molecular Spectroscopy, 4th edn. London: McGraw
Hill.
Colquhoun IJ and Goodfellow BJ (1994) Nuclear magnetic
resonance spectroscopy. In: Wilson RH (ed.) Spectro-
scopic Techniques for Food Analysis. New York: VCH.
Wilson RH (ed.) (1994) Spectroscopic Techniques for Food
Analysis. New York: VCH.
Infrared and Raman
B Schrader, Essen, Germany
S Keller, Hamburg, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Principles
0001A molecule can be identified uniquely by its vibra-
tional spectrum, since this depends on every struc-
tural feature. The vibrational spectrum consists of
the frequencies and intensities of the vibrations
5410 SPECTROSCOPY/Infrared and Raman
observed by infrared and Raman spectroscopy. Both
are nondestructive methods. This means that the
samples can be investigated afterwards by other
methods. The intensity of the bands in the infrared
and Raman spectra gives complementary information
about the nature of the vibrating bonds. Therefore, it
is advisable to evaluate both the infrared and Raman
spectra in order to obtain the maximum amount of
analytical information.
0002 Now, the advantages of both methods, IR and
Raman spectroscopy, can be employed by power-
ful spectrometers, thus making vibrational spectros-
copy a very useful tool in food analysis. State-of
the-art instruments for vibrational spectroscopy
are equipped with interferometers coupled to com-
puters for the transformation of interferograms
into spectra, so-called Fourier transform infrared
(FTIR), Fourier transform near-infrared (FTNIR),
or FT Raman spectrometers. Compared with
grating instruments, interferometers have several
advantages, in particular the fact that the radiant
flux analyzed is larger by about two orders of
magnitude. Interferometers therefore permit reliable
routine analyses of small samples and of low concen-
trations.
2000 0/cm
−1
4000
(a)
(b)
m
1
m
1
f
2500 1800 1500
XH
XY
OH NH CH SH
XYX Y X Y
ZYZ
Z
=
1
f
2πc
1
m
1
1
m
2
+
~
n
~
n
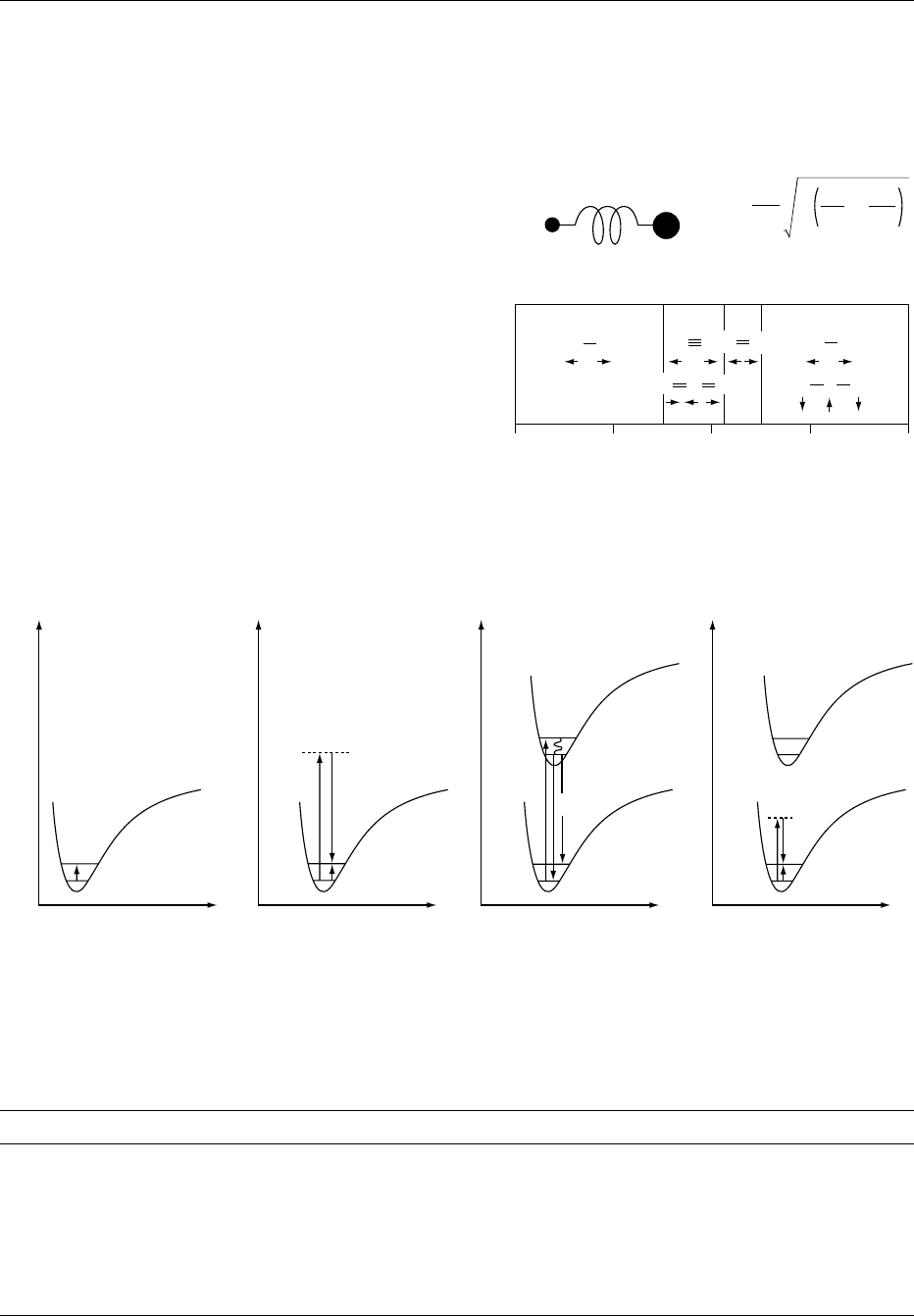
fig0001Figure 1 Principles of infrared and Raman spectroscopy: (a)
model and equation describing frequency of diatomic molecule;
(b) frequency ranges of different small molecules.
VIS-Raman
488 nm
100%
(b)
QY: 10
−10
E
hn
o
hn
o
hn
r
hn
s
hn
r
hn
s
hn
F0
hn
F1
S
1
S
0
S
1
S
0
q
(c)
Fluorescence
QY:
~
1
E
q
(d)
NIR-Raman
1064 nm
46%
QY: 10
−12
E
q
Infrared
(a)
Quantum
yield (QY)
QY: 10
−2
E
1
1
0
0
1
0
1
0
1
0
1
0
hn
s
q
fig0002 Figure 2 Term scheme of spectra: (a) infrared; (b) Raman (excitation in the VIS); (c) fluorescence; (d) Raman (excitation in the NIR).
tbl0001 Table 1 Comparison of infrared, near-infrared and Raman spectrometry
Infraredspectrometry Raman spectrometry Near-infrared spectrometry
Sample preparation Partly expensive, moisture-sensitive Simple Simple
Sample container Cells of KBr, NaCl, ZnSe, Si, Csl, TlBr/TlJ Glass, quartz Glass, quartz
Bands of water Strongly disturbing Low intensity Strong intensity
Observed vibrations Antisymmetric polar groups, substituents Symmetric unpolar
groups, skeletons
Overtones and combinations
of C—H, N—H and O—H
Intensity, I, , concentration, c log(l
0
/l) * cl* c log (l
0
/l) * c
Spectral range 400–4000 cm
1
standard, 10–400 cm
1
with FIR-optics
10–4000 cm
1
4000–12 500 cm
1
SPECTROSCOPY/Infrared and Raman 5411

Basic Theory
0003In Figure 1, the theory necessary to understand infra-
red and Raman spectroscopy is outlined. Every mol-
ecule can be considered as being built up from atoms
with a definite mass, connected by chemical bonds
behaving like elastic springs (Figure 1a). The fre-
quency,
~
nn, is usually measured in wavelengths per
centimeter, called the wavenumber (cm
1
). For a di-
atomic molecule of two bound atoms, the frequency
of the vibration is proportional to the square root of
the force constant (f ), – which is approximately pro-
portional to the bond order – and the sum of the
reciprocal masses.
0004For larger molecules, the vibrations of the frag-
ments couple and produce complicated ‘fingerprint’
patterns. However, most of the structural units
keep their typical frequency range. Thus, X—H
bonds, with X any element, show their typical vibra-
tions between 4000 and 2500 cm
1
(Figure 1b),
followed by groups with triple bonds or cumulated
double bonds in the range 2500–1800 cm
1
. Groups
with ‘double’ bonds show their characteristic vibra-
tions between 1800 and 1500 cm
1
, and groups
tbl0002 Table 2 Characteristic frequencies and Raman and infrared
intensities of food components
Assignment Infrared
(cm
1
)
Raman
(cm
1
)
d(C—O), sugar 479
n(S—S) 520–540
Phenylalanine (skeletal) 622
Tyrosine (skeletal) 642
Adenine 725
Thymine 750
Breathing vib. (a-anomers,
Type III, sugar)
756–776
Tryptophan 759
Breathing vib. (b-anomers,
Type III, sugar)
765–783
Cytosine, uracil (ring, str) 785
n(—O—P—O—)
s
, A-helix 810–814
Tyrosine 829
n(—O—P—O—)
s
, B-helix 830
C
1
—H bending (a-anomers,
Type II, sugar)
836–852 840–850
Tyrosine 852
C
1
—H bending (b-anomers,
Type II, sugar)
884–898 890–910
Ring vibration (a-anomers,
Type I, sugar)
904–930
Ring vibration (b-anomers,
Type I, sugar)
915–925
n(PO
4
)
s,as
960
n
as
(C—N
þ
—C), choline 970
Phenylalanine 1004
n(C—N) and n(C—C) 1061
Deoxyribose – Z-DNA 1065
n(O—P
—
—O)
s
1070–1100 (m) 1080–1100 (s)
n(C—N) and n(C—C) 1093
n(C—N), n(C—C) 1129
n(C—C), carotenoids 1157
n(PO
4
3
)
as
– Z-DNA 1215
n(O—P
—
—O)
as
1225 (s) 1225 (vw)
Amide III, (b-structure) 1225–1245 (s)
n(PO
4
3
)
as
– B-DNA 1225
n(PO
4
3
)
as
– A-DNA 1240
Amide III (random coil) 1241–1251
n
as
(PO
2
) 1250
n(
—
—C—H)
i.p.b.
unsaturated
fatty acids
1267
Amide III (a-helix) 1270–1300
(vw, br)
d(CH
2
), twisting 1295
g
w
(CH
2
)
n
1342–1180
Tryptophan 1358
n(CH
3
) 1374
Aspartic acid, n(COO
) 1408
Deoxyribose – A-DNA 1418
Deoxyribose – B-DNA 1425
Glutamic acid, n(COO
) 1437
d(C—H) 1445
d(CH
2
) 1470
Amide II, d(N—H)
(antiparallel b-sheet)
1510–1540
n(C
—
—C), aromate (tyrosine),
carotinoids
1515 1519
d(N—H), amide II (b-sheet
structure)
1530–1545
Amide II, d(N—H) (a-helix) 1540–1560
Amide II, d(N—H) (random coil) 1535
Amide II, d(N—H)
(parallel b-sheet)
1550
Guanine, adenine (ring, str) 1575
Tryptophan 1578
Phenylalanine 1606
n(COO
), carboxylate 1610
Tyrosine 1614
Tryptophane 1621
Amide I n(C
—
—O) (parallel
b-sheet structure)
1632–1648
Amide I, n(C
—
—O), (a-helical
structure)
1655 1658
Amide I, n(C
—
—O) (random coil) 1655–1660 1665
n(C
—
—C) unsaturated fatty lipids 1657
Amide I, n(C
—
—O) (antiparallel
b-sheet and b-turn structure)
1685–1700 1665–1675
– Z-DNA 1695
– A-DNA 1705
– B-DNA 1715
n(C
—
—O), ester 1720–1750 1746
n(C
—
—O), carboxylic acid 1730
n(CH
2
)
s
lipid 2849 2847
Methyl 2872
n(CH
2
)
s
, protein, n(CH
2
)
as
, lipid 2890
n
as
(CH
2
) 2918
n(CH
3
)
s
, lipid, n(CH
2
)
a
, protein 2935
n
as
(CH
3
) 2956
n(CH
3
)
as
, protein, n(CH
3
)
as
, lipid 2975
n
as
(CH
3
)
3
N
þ
3028
n(C
—
—C—H)
s
, lipid 3010
n(NH), n(OH), base sugar 3300–3600 (m)
5412 SPECTROSCOPY/Infrared and Raman
2400 2200 2000 1800 1600 1400 1200 1000 800 600 400
A
B
C
0.2
200 026002800300032003400
3600
HO
.
CO
.
CH
.
CH
2
.
S
.
S
.
CH
2
.
CH
.
CO
.
OH
NH
2
NH
2
I
II
III
L-Cystine
ν
Raman Infrared
499
3678
3784
92917
62969
10101 W
4200 W
393 S
1.5616 M
847 S
1127 S
0.51298 S
2.51340 S
21410 VS
1485 VS
1585 VS
~3030 S
(a)
Grubb Parsons IS 3 Solid B 10 mg/150 mg PE; C 25 mg/150 mg PE 5 cm
−1
Bspl : 6.25 / 25 MW 240.30 m.p. 261d(lit):FIR
: Perkin Elmer 521 Solid A 0.8 mg/400 mg K J 2 cm
−1
C
6
H
12
N
2
O
4
S
2
IR
B 7 − 01
Coderg PHO Solid ~ 2 mg I 1; II 0.2III Pellet 140 mg 0.3 6/1 cm
−1
EGA5145 A; 700 mWDC; s20; 612; AAA:Ra
40
~
2400
MW 294.48
bp.: 215
20
(lit.)
C2 − 08
I
A
B
II
P
P
P
2 cm
−1
C
19
H
34
O
2
GC > 98%IR: Perkin−Elmer 521
Liq A 30 µ Cs Br; B 100 µ Cs Br;
2200 2000
1800
1600 1400 1200 1000 800 600 400 200 026002800300032003400
3600
4 cm
−1
DC; S20; 708v; BBB
FLUKA
Ra: Coderg PHO
Liq 0.3; 90; I 1 ⊥;II 0.2 ⊥
5145 A; 1000 mW
Infrared
M
S
S
VS
S
VS
S
0.2
0.9
0.8
0.9
0.1
0.5
0.5
0.2
0.2
0.2
0.9
Raman
7
6
15
20
33
48
2
4
45
25
7.5
838
1072
1265
1440
1658
2905
725
1170
1435
1743
2855
2925
3010
972
ν
~
H
3
C
CH
2
CH
2
H
CH
2
H
CH
2
H
H
C
3
C
O
O
Linoleic acid methyl ester
H
3
C
C
C
C
CH
2
6
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
(b)
o
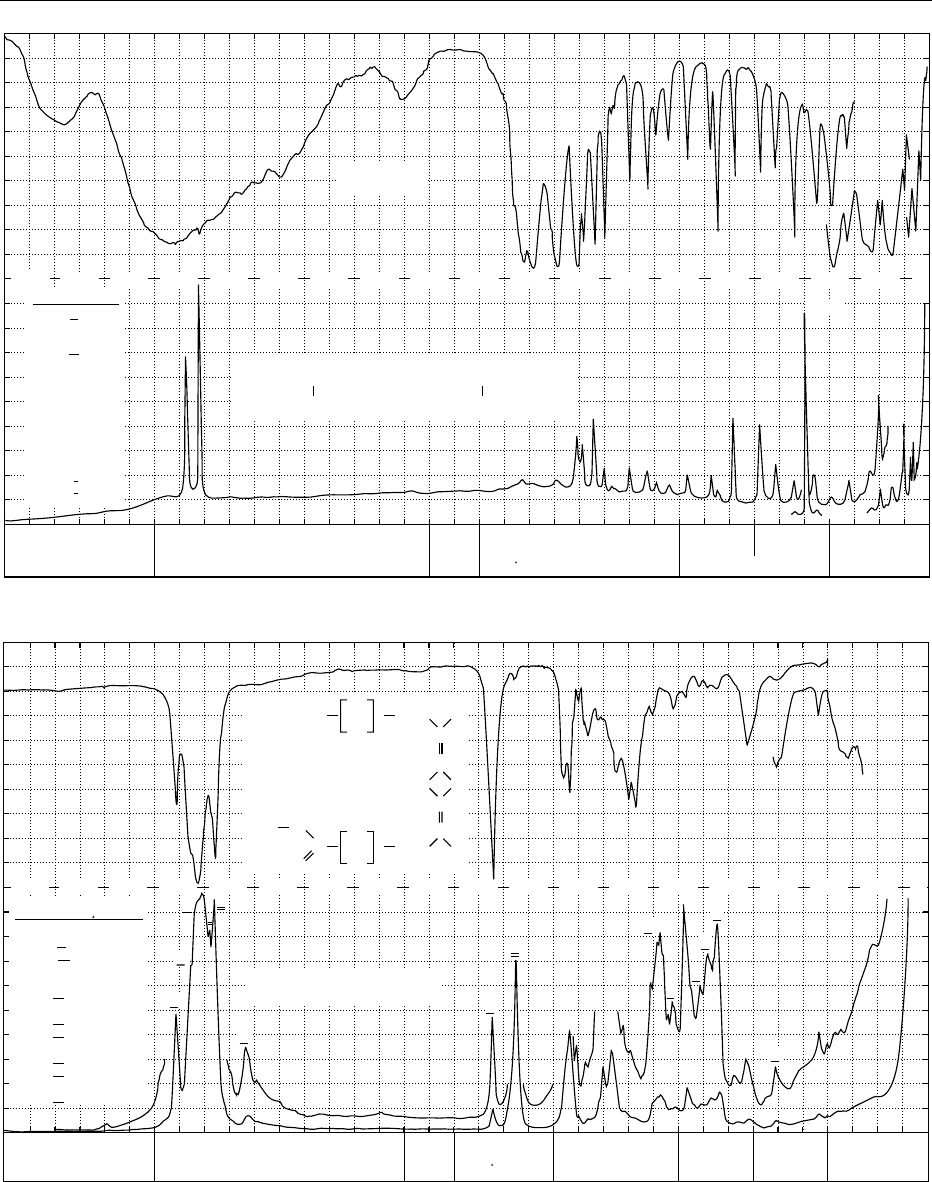
fig0003 Figure 3 Infrared transmission (above) and Raman spectrum (below) of: (a) cystine; (b) linolenic acid methyl ester; (c) retinol,
vitamin A; (d) caffeine; (e) thiamine hydrochloride, vitamin B
1
; (f) L-ascorbic acid, vitamin C; (g) a-D-glucose; (h) sucrose. From
Schrader B (1989) Raman/Infrared Atlas of Organic Compounds, 2nd edn. Weinheim: VCH Verlagsgesellschaft with permission.
SPECTROSCOPY/Infrared and Raman 5413
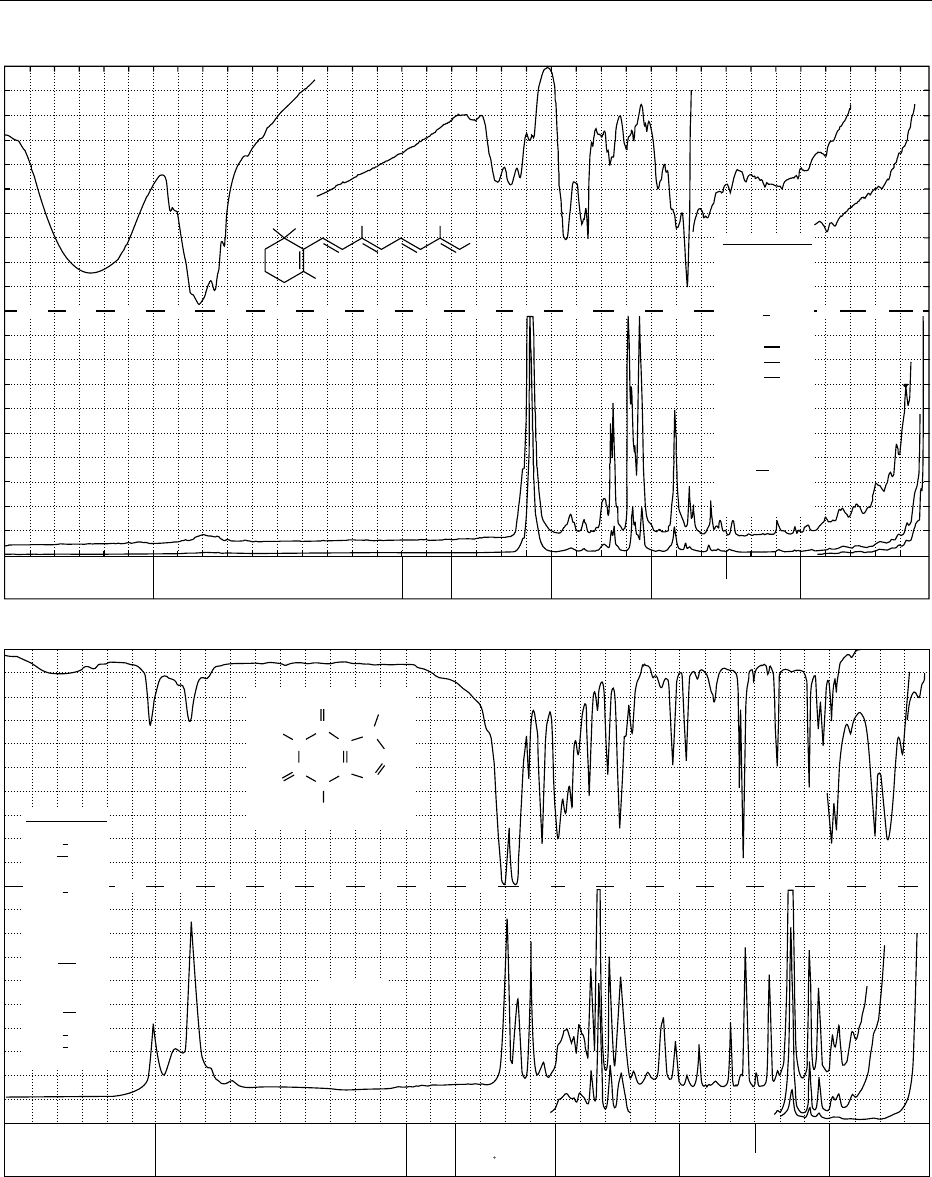
Figure 3 Continued
AA
3600 3400 3200 3000 2800 2600 2400 2200 2000 1800 1600 1400 1200 1000 400
Raman infrared
877
965
1004
1008
1078
1155
1193
1266
1270
1278
1359
1382
1448
1586
1627
1664
1717
1.5
2s
s
5
m
9.5
9.5
5.5
m
4.5 sh
s
s
s
48
m
m
m
200 0
III
II
I
H
3
CCH
3
CH
3
CH
3
All-trans-retinol
Vitamin A
(c)
CH
3
CH
2
OH
A
B
ν
~
IR:
FIR:
RA:
Perkin-Elmer 180
Perkin-Elmer 180
Cary 81
Solid:
Solid:
Solid:
ORD, −EXPS. 100
5830 A, 300 MW
DC, S20, 1050V, BBB
2
5
3/1
C
20
H
30
O
MW 286.46 MP 58−60
C4 − 01
FLUKA
cm
−1
cm
−1
cm
−1
3mm: III 0.1 II 0.2
A 2.0 mg / 200 mg KCI
B 10 mg / 80 mg PE
1 mm: I 1 ,
3200 3000 2800 2600 2400 2200 2000 1800 1400 1200 1000 800 600 400 200 01600
A
B/C
I
II
II
III
IR
FIR
Ra.
C
8
H
10
N
4
O
2
MW 194.19
I 14 − 02
m.p. 238
EGA
:
: Grubb Parsons IS 3
:
Perkin − Elmer 521
Cary 81 DC; S20; 1300; AAB
5 cm
−1
2 cm
−1
6/1 cm
−1
Bspl.: 6.25/25
µ
Solid B/C 20 mg/150 mg PE
Solid A 1 mg/400 mg KJ
Solid 3 mg I 1 II 0.4 ; 250 mg III 0.05 5145 A: 300 mW
C
O
O
CH
3
CH
3
N
H
3
C
N
N
N
CC
CH
C
ν
Raman infrared
~
445
483
557
611
644
743
760
974
1023
1239
1283
1324
1358
1487
1551
1601
1656
1699
2957
3115
M
M
M
W
S
M
M
M
S
M
W
S
S
S
M
VS
VS
M
M
3.5
0.5
4.5
SH
SH
1.5
4
5
3.5
SH
5.5
3.5
3
5
7
7
6
13.5
0.5
20
Caffeine
(d)
Continued
5414 SPECTROSCOPY/Infrared and Raman
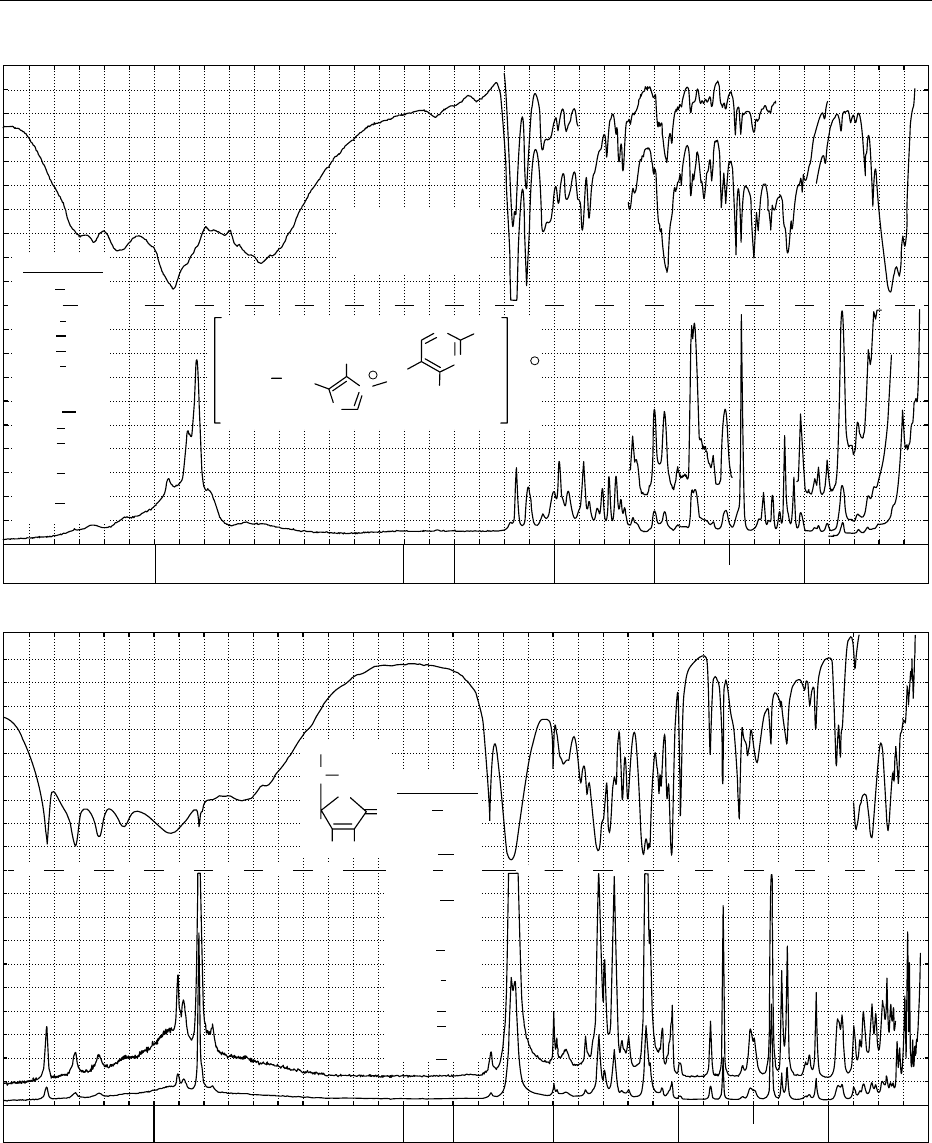
Figure 3 Continued
IR:
Solid:
Solid:
Solid:
Perkin−Elmer 180
Perkin−Elmer 180
Cary 81 **
FIR:
RA:
3000 2800 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 600 400 200 0
A 1.5 mg, B 4.0 mg / 400 mg KI
C 5 mg / 80 mg PE
1 mg: I 1 , II 0.25 2 mm: III 0.1
2 cm
−1
2 cm
−1
4/2 cm
−1
ORD.−EXPS. 100
5145 A, 2000 MW DC,S20,1120V,BBB
C
12
H
17
CLN
4
OS
MW 300.81
FLUKA
J8 − 11
I
C
A
B
0.7 mg
I
III
II
~
3200
106 24
9
7
544
w
347
6.5
36
751
937
1382
1252
10
1478
1608
10
1648 10
1661
2927
~3028
28
9.5
1043
1220
581
16
ν Raman Infrared
m
m
m
m
m
sh
sh
sh
s
s
vs
sh
Cl
CH
3
CH
3
CH
2
NH
2
CH
2
HOCH
2
−
+
N
N
N
Thiamine
Monohydrochloride
Vitamin B
1
(e)
S
IR: Solid:
Solid:
Pellet:
Perkin−Elmer 180
Perkin−Elmer 180
Cary 81 **
FIR:
RA:
(f)
A 0.8 mg / 400 mg KI
A
B
I
L-Ascorbic acid
II
B 10 mg / 80 mg PE
2 mg: I 1 , II 0.25
2 cm
−1
5 cm
−1
1 cm
−1
ORD.−EXPS. 130
5145 A, 790 MW DC, S20, 1168V, CCB
C
6
H
8
O
6
MW 176.14
MW 190 d
Merck
3600 3400 3200 3000 2800 2600 16002400 1400 1200 1000 800 600 400 200 0
81
23
3
8
448
367
3.5
628
756
1129
1026
12
1138
1256
10
1275
20
1319
1667
1673
2918
27
7.5
820
989
565
16
ν Raman Infrared
m
m
m
s
s
m
s
s
s
s
s
s
s
~
5.5
CH
2
OH
OH
OHHO
HC
O
H
O
Continued
SPECTROSCOPY/Infrared and Raman 5415
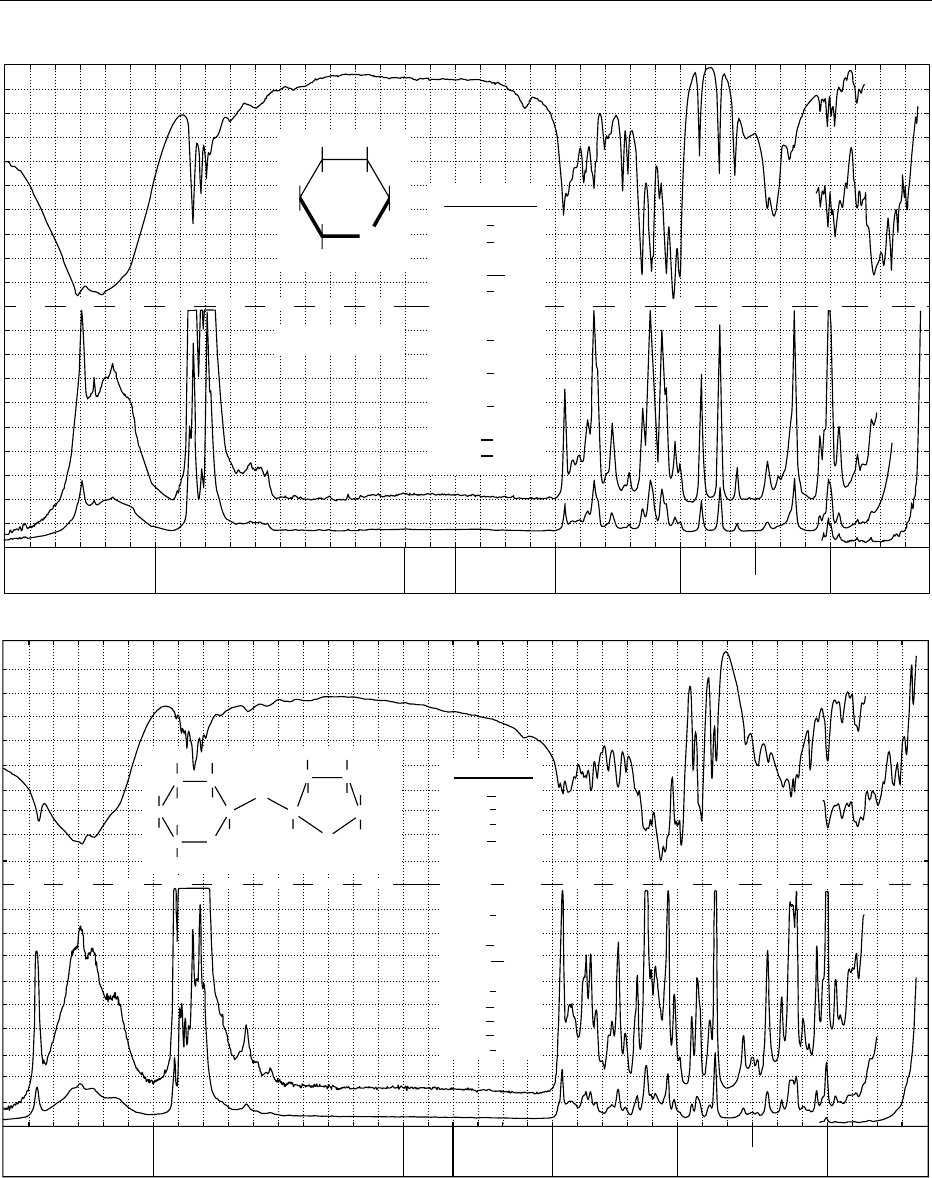
Figure 3 Continued
IR: Solid:
Solid:
Solid:
Perkin−Elmer 180
Perkin−Elmer 180
Cary 81 **
FIR:
RA:
(g)
A 1.0 mg / 400 mg KI
B 10 mg / 80 mg PE
6 mg: I 1 , II 0.25 2 mm: III 0.1
2 cm
−1
2 cm
−1
6/1 cm
−1
ORD.−EXPS. 150
5145 A, 830 MW DC, S20, 1138V, BBC
C
6
H
12
O
6
MW 180.16
MP 147−48
MERCK
III
I
B
A
II
3200 3000 2800 2600 2400 2200 1400 1200 1000 800 600 400 200 03400
3600
H
H
H
H
H
HO OH
OH
O
CH
2
OH
OH
α-D-(+)-glucose
404
619
540
913
1022
1117
1110
1148
1343
1457
2840
2945
~3310
1049
1071
839
ν Raman Infrared
m
m
m
s
s
sh
s
sh
s
s
s
s
s
9
7
31
7.5
2.5
4.5
3.5
8
8
8
5
36
~
B
I
III
A
Sucrose
II
IR: Solid:
Solid:
Solid:
Perkin−Elmer 180
Perkin−Elmer 180
Cary 81 **
FIR:
RA:
(h)
A 3.0 mg/400 mg KI
B 8 mg/80 mg PE
3 mg: I 1 , II 0.2 3 mm: III 0.02
2 cm
−1
2 cm
−1
5/2 cm
−1
ORD.−EXPS. 150
5145 A, 2000 MW DC, S20, 1170V, BBB
C
12
H
22
O
11
MW 342.30
MP 183−5
K2 − 01
PFEIFER U. LANGEN
3600 3400 3200 3000 2800 2600 2400 2200 1400 1200 1000 800 600 400 200 0
HO
HOH
H
OH H
H
C
C
CC
C
C
CC
O
CH
2
OH
CH
2
OH
HO
OH H
H
H
HOH
2
C
H
C
C
O
400
638
w
522
865
906
1067
1036
1122
1235
1280
10
1347
1460
920
987
846
ν Raman Infrared
m
m
m
m
m
m
m
m
s
s
sh
s
sh
s
s
sh
~
5
5
6
8
1
7
4.5
5.5
10
14
10
44
39
2912
2940
~3390
5416 SPECTROSCOPY/Infrared and Raman
