Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

then pregnancy entails no appreciable additional
energy (food) cost. It could be argued that the
fetus is remarkably protected from the influence of
maternal malnutrition, and that women exposed
to generations of deprivation have simply adapted
to a limited food supply. Small stature is often
cited (incorrectly) as an example of adaptation.
Studies of the dietary habits of women living in afflu-
ent circumstances, however, have shown that this is
not so.
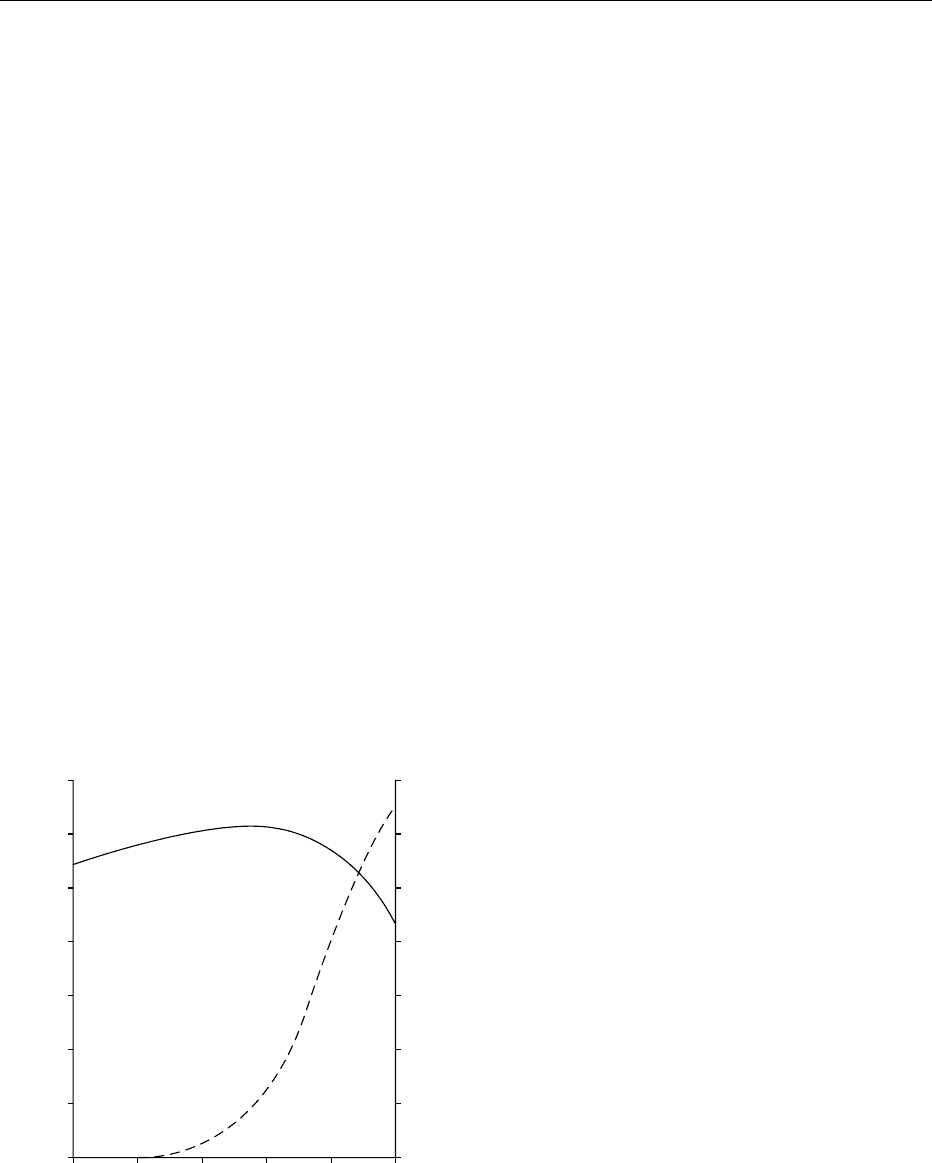
0009 As long ago as 1953, analyses of the diets of 2300
well-nourished women in Nashville, USA failed
to show any association between maternal food
intake and birthweight. Furthermore, on average, no
increase in food consumption during pregnancy was
apparent (Figure 1). During the second trimester, an
increase of around 251 kJ (60 kcal) per day was
noted, but during the last trimester, when a marked
acceleration in fetal growth occurs, energy intake fell
by 753 kJ (180 kcal) per day. This surprising discov-
ery, ignored for 30 years, has been amply confirmed
by studies in the 1980s in Paris, Cambridge, and
Glasgow.
0010 How, then, is the obvious requirement for increased
nutrition during pregnancy (Table 1) to be reconciled
with the mother’s failure, even under the most favour-
able environmental circumstances, to increase food
intake? Only a small fraction of the fat stored in
the first two trimesters of pregnancy is mobilized
before term, and there exists no ad-hoc store of
minerals and water-soluble vitamins. The answer to
this question is to be found in the complex mechanisms
whereby nutrients are transported from the maternal
circulation to the fetus, and in the adjustments that
occur in the maternal physiology in response to the
gravid state.
Role of the Placenta
0011The role of the placenta is discussed in Pregnancy:
Role of Placenta in Nutrient Transfer. It is primarily
an organ for nutrition of the fetus, but functions also
as an endocrine gland throughout the greater part of
gestation, regulating nutrient utilization by the
mother.
0012Glucose provides the energy for fetal growth, the
fetus being unable to oxidize fatty acids. Transfer of
glucose across the placenta is by ‘facilitated diffu-
sion,‘ and is therefore influenced by the maternal
blood glucose concentration. A prolonged low blood
glucose concentration, as may occur in mothers in the
developing world engaged in hard physical work, or
an abnormally high concentration associated with
gestational diabetes can therefore lead to growth
retardation, or to excessive growth and fat deposition
(macrosomia). From early pregnancy, the fasting
blood glucose concentration is reduced, a change
that may favor transfer to the fetus since the placenta
is particularly effective at extracting nutrients from
low concentrations in the blood. Glucose tolerance
following a glucose load is also progressively relaxed
so that the glucose concentration remains elevated for
a longer period, again facilitating placental uptake.
Whether or not the metabolic response to carbohy-
drate consumed as part of a meal is similarly affected
is unclear. (See Glucose: Maintenance of Blood Glu-
cose Level; Glucose Tolerance and the Glycemic (Gly-
caemic) Index.)
0013Fat-soluble vitamins also cross the placenta by
diffusion, maternal blood levels being maintained
by dietary input and mobilization of tissue stores.
In contrast, amino acids, minerals, and the water-
soluble vitamins are actively transported, the concen-
trations in the fetal circulation being much higher
than in the maternal circulation. Furthermore, there
is evidence that some vitamins are chemically altered
by the placenta to a form that is unable to diffuse
back into the maternal bloodstream, so reinforcing
the one-way transport mechanism. Thus, the fetus
has prior claim on the nutrient supply, much of
which is maintained, by homeostatic regulation,
within narrow concentration limits in the maternal
plasma. In adverse nutritional circumstances, it is the
mother, not the fetus, who is ‘at risk.‘ (See Amino
0
1600
1700
1800
1900
2000
2100
2200
0.0
1.0
2.0
3.0
816
Stage of pregnancy (weeks)
Weight of fetus (kg)
Maternal energy intake (kcal per day)
24 32 40
fig0001 Figure 1 Diagram showing changes in maternal energy intake
(—) and weight of the fetus (– – –) at different stages of pregnancy.
Reproduced from Pregnancy: Metabolic Adaptations and Nutri-
tional Requirements, Encyclopaedia of Food Science, Food Technol-
ogy and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds),
1993, Academic Press.
PREGNANCY/Metabolic Adaptations and Nutritional Requirements 4725

Acids: Metabolism; Minerals – Dietary Importance;
Vitamins: Overview.)
Metabolic Adjustments in Pregnancy
0014 Under normal circumstances, the integrity of the
tissues is assured by homeostatic regulation of the
absorption, excretion and catabolism of the nutrients.
During reproduction, these control mechanisms are
adjusted, under the influence of hormones secreted by
the placenta, in order to make available an additional
nutrient supply for growth of the conceptus.
Absorption of the Nutrients
0015 The macronutrients, fat, carbohydrate, and protein,
are almost completely digested and absorbed in the
small intestine, so that no appreciable change occurs
in pregnancy. The absorption of minerals, however,
is carefully regulated. Most Western diets provide a
considerable excess of calcium, and no more than
around 30% is normally absorbed. From early in
pregnancy, the blood concentration of the physiolo-
gically active form of vitamin D (1,25-dihydroxy-
cholecalciferol) is elevated, leading to an increased
uptake of calcium from the gut, thus allowing the
mother to satisfy the needs of her developing fetus
without recourse to her own skeletal calcium or the
need to increase her dietary intake. Only when her
customary calcium intake or the biosynthesis of vita-
min D is severely restricted is calcification of the fetal
skeleton compromised. (See Calcium: Physiology;
Cholecalciferol: Physiology.)
0016 As with calcium, the proportional absorption of
iron from a diet providing 12–14 mg per day is very
small (about 10%), but as gestation advances, iron
uptake increases progressively and may exceed 40%.
This adjustment in absorption, in association with a
reduction in iron loss resulting from the cessation of
menstruation, is sufficient to satisfy the iron cost of
pregnancy. Judged by normal standards, the charac-
teristic fall in hemoglobin concentration that is
noted in pregnancy would indicate a state of anemia.
Blood volume, however, is considerably increased so
that the total amount of hemoglobin in circulation,
and consequently the oxygen-carrying capacity of
the blood, is actually raised. Supplementation of
the diet of women who begin pregnancy in an iron-
sufficient state may therefore be injurious as it has
been shown to increase red cell size and could ad-
versely affect blood flow in the small capillaries. (See
Iron: Physiology.)
0017 Other minerals, such as copper and zinc, may also
show improved absorption. (See Copper: Physiology;
Zinc: Physiology.)
Urinary Excretion of Nutrients
0018The glomerular filtration rate is increased during preg-
nancy. It is suggested that the presence of traces of
glucose in the urine at some stages of pregnancy in
most women merely indicates that more glucose has
been filtered from the plasma than can be reabsorbed
by the kidney tubules at that time. Some women, how-
ever, may lose large amounts of glucose in their urine,
particularly those who develop gestational diabetes.
0019The urinary excretion of most nutrients is altered,
but whether or not this reflects specific modifications
in kidney function is unclear since the picture is ob-
scured by withdrawal of nutrients from circulation by
the placenta, which changes with time. The pattern of
excretion of amino acids is most consistent. In non-
pregnant women, about 1% of total nitrogen excre-
tion is accounted for by free amino acids, but urinary
loss rises in early pregnancy, reaching a plateau in
midpregnancy at a level approximately double that
found in the nongravid state. Most of the increase is
accounted for by the nonessential amino acids, pos-
sibly reflecting selective uptake by the placenta. Since
the plasma amino acid concentrations show little
change, the large increase in urinary excretion cannot
be attributed to kidney overload and is most likely the
result of altered hormonal status affecting kidney
function. The return to normal levels of excretion
during lactation supports this conclusion.
0020One example of altered kidney function that favors
conservation has clearly been identified. The amino
acid taurine, a derivative of the nonessential amino
acid cystine, shows a dramatic and sustained reduc-
tion in excretion from early pregnancy. It has been
suggested that taurine may act as a membrane stabil-
izer, as an inhibitory neurotransmitter or neuro-
modulator, and as a growth modulator in fetal
tissues. Tissue concentrations are particularly high
in the fetus, and the suppression of urinary excretion
of taurine during pregnancy is seen as a means of
satisfying the needs of the developing fetus, which
lacks the ability to synthesize taurine de novo.
Metabolism of Protein
0021The rate of accretion of nitrogen by the fetus and
maternal reproductive tissues is known to rise 10-
fold between the first and last quarters of gestation.
Since, on average, no appreciable change in food
intake, and thus protein intake, occurs in pregnancy,
one would expect to find a positive nitrogen balance,
rising continuously throughout pregnancy. In the few
nitrogen-balance studies carried out on pregnant
women, however, no differences have been found
between the values for nitrogen retention measured
4726 PREGNANCY/Metabolic Adaptations and Nutritional Requirements

at different stages of gestation. This apparent para-
dox was resolved by the study of reproduction in the
rat. (See Protein: Digestion and Absorption of Protein
and Nitrogen Balance.)
0022 During the first 2 weeks of pregnancy, when com-
petition between the dam and her fetuses for nutrients
is minimal, a substantial reserve of protein is built up
in the muscles (the ‘anabolic phase‘). During the final
week, the period of rapid fetal growth, the protein
reserve is broken down, the amino acids released
being taken up by the placenta for growth of the
conceptus. This ‘catabolic phase‘ is not influenced
by the protein content of the maternal diet, and
is controlled by the hormone progesterone. (See
Hormones: Steroid Hormones.)
0023 Evidence for a similar biphasic system of protein
metabolism in women was provided by measurement
of the excretion of the amino acid 3-methylhistidine.
This amino acid is found predominantly in muscle.
Histidine undergoes methylation after its incor-
poration into the peptide chains of the contractile
proteins actin and myosin, and, in the course of muscle
protein turnover, the 3-methylhistidine released is not
reutilized, but is quantitatively excreted in the urine.
In pregnancy, the excretion of 3-methylhistidine rises
sharply during the last trimester, indicating the
hydrolysis of an amount of protein approximating
the estimated protein cost of pregnancy.
0024 Such a redistribution of amino acids from maternal
to fetal tissues would not be detected in measure-
ments of nitrogen balance.
0025 This cyclic course of protein metabolism has im-
portant nutritional implications. The protein cost of
pregnancy is distributed over the whole gestational
period, and the influence of acute or chronic maternal
undernutrition on fetal growth is minimized.
0026 Nitrogen retention occurs when the intake of pro-
tein-nitrogen exceeds nitrogen losses from the body,
largely the products of amino acid catabolism in the
urine (mostly urea) and undigested dietary protein in
the feces along with cells shed from the gut epithe-
lium. If, during pregnancy, the catabolism of amino
acids were in part suppressed, the fraction spared
could be used for fetal protein synthesis. In the case
of a woman existing on 40 g of protein per day, as in
many developing world populations, a reduction of
less than 9% in amino acid oxidation would spare
enough protein to cover the entire estimated require-
ment for pregnancy.
0027 The hypothesis was tested in the rat. The activities
of two rate-limiting enzymes (alanine aminotransfer-
ase and argininosuccinatesynthetase), which regulate
the oxidation of amino acids and the conversion of
amino-nitrogen to urea, were measured in the livers at
different stages of gestation. The activities of both
enzymes were markedly depressed by the end of the
first week, and declined even further (by around
50%) as pregnancy advanced. A parallel change in
plasma urea was also noted.
0028Evidence for a similar adjustment in amino acid
catabolism in pregnant women was later obtained in
a metabolic study using urea labeled with a stable
isotope of nitrogen. Measurements made in the last
trimester and in the postpartum postlactational
period showed a reduction of 30% in the rate of
urea synthesis in late pregnancy and a similar fall in
the plasma urea concentration.
0029The mechanism for protein sparing, which oper-
ates progressively throughout pregnancy, combined
with the biphasic system of early storage and later
breakdown of stored protein, ensures a supply of
amino acids commensurate with the demands of the
growing fetus. The suppression of hepatic amino acid
oxidation was also shown to be induced by the
placental hormone progesterone. The fetoplacental
unit thus indirectly controls its supply of amino
acids as well as its energy needs.
Metabolism of Energy
0030As stated earlier, the dietary energy supply is the
major determinant of fetal growth. In healthy preg-
nant women, energy balance becomes positive during
the first trimester, probably in response to the rising
secretion of progesterone. The purpose of the aug-
mented fat reserve, amounting, on average, to some
4 kg of fat, is primarily to subsidize the high energy
cost of lactation, but a small amount is mobilized in
late pregnancy to provide an alternative fuel for use
by the maternal tissues and enhance the availability of
glucose for use by the fetoplacental unit. The human
fetus derives its energy almost exclusively from the
oxidation of glucose. (See Energy: Energy Expend-
iture and Energy Balance.)
0031The discrepancy between the measured energy
intakes of healthy pregnant women and the values
predicted for energy expenditure has yet to be
explained. There is no doubt that some saving is
made in energy expenditure from a reduced level of
physical activity, but this is difficult to quantify.
0032The alterations in carbohydrate metabolism that
are characteristic of pregnancy could also lead to the
sparing of energy. In pregnancy, there is an increased
output of insulin in response to a glucose stimulus,
and reduced glucose uptake by the peripheral tissues
(muscle and adipose tissue). Insulin antagonism has
been attributed to the action of placental lactogen.
Consequently, more of the ingested carbohydrate
is directed to the liver, the organ that maintains the
blood glucose concentration. The conversion of
PREGNANCY/Metabolic Adaptations and Nutritional Requirements 4727

carbohydrate to fat is a very costly process. Approxi-
mately 20% of the energy that is available from the
direct oxidation of glucose is lost as heat if the glucose
is first converted to fat, and the fat is later oxidized to
produce energy. This adjustment in carbohydrate util-
ization, therefore, not only conserves energy for ana-
bolic purposes, but also safeguards the fetal energy
supply. (See Carbohydrates: Digestion, Absorption,
and Metabolism.)
Dietary Recommendations for Pregnancy
0033 Growth of the fetus is little affected by transient or
prolonged moderate undernutrition of the mother.
Relatively small deficits in birthweight may be ac-
counted for by a lower proportion of body fat resulting
from maternal dietary energy restriction in late preg-
nancy, from the diversion of glucose (the fetal fuel) to
the muscles should the mother be engaged in hard
physical work throughout pregnancy, or from mal-
function of the placenta.
0034 The security of the fetus is provided by the pla-
centa, an organ designed not only to ensure a priority
claim on all nutrients present in the maternal circula-
tion, but also, by the secretion of hormones, to modu-
late the homeostatic regulation of nutrient utilization
at all levels – absorption, excretion, and catabolism,
in order to augment the nutrient supplies. No govern-
ment committee responsible for devising dietary
guidelines would be so incautious as to suggest that
women during pregnancy require no more food than
when in the nongravid state, although all evidence
points very clearly to that conclusion. One obvious
a priori condition would be that nutritional status
should be satisfactory before conception and
throughout pregnancy.
0035 Attention has been focused on the latter half of
pregnancy, when fetal growth is most rapid, the
need for nutritional input is at its greatest, and the
effects of maternal food deprivation most apparent.
These considerations led a Joint FAO/WHO (Food
and Agriculture Organization, World Health Organ-
ization) Ad Hoc Expert Committee in 1973 to pro-
pose an additional 628 kJ (150 kcal) per day for the
first trimester, rising to 1464 kJ (350 kcal) per day for
the second and third trimesters, the stage of preg-
nancy when women, unencumbered by professional
advice, would spontaneously reduce their food con-
sumption. In the light of continuing research, how-
ever, estimates of energy and nutrient requirements
are being revised in a downward direction. In 1974,
the US report on Recommended Daily Allowances
proposed an additional daily supplement of 30 g of
protein, 400 mg of calcium and 30–60 mg of iron for
the pregnant woman, acknowledging that such a high
intake of iron could not be met by the iron content of
habitual US diets. The implication was that preg-
nancy was a clinical condition that required thera-
peutic intervention. One decade later, the proposed
increments in energy and calcium were little changed,
but the supplement of iron was reduced to 15 mg, and
the protein allowance was changed to anticipate the
pattern of accretion by the fetus, rising from 1.2 g per
day in the first trimester to 10.7 g in the final trimester
of pregnancy. (See Dietary Requirements of Adults.)
0036The UK dietary recommendations for pregnancy
have consistently been on a less generous scale. Over
a similar period, the daily allowance for energy has
fallen from 1004 kJ (240 kcal) in the second and third
trimesters to 837 kJ (200 kcal) during the third
trimester only. Protein is unchanged at 6 g per day
throughout pregnancy, but the recommendation for
calcium has fallen from 700 mg per day in the third
trimestertozero.Likewise,norecommendationwas
made for an increase in iron intake in the 1991 report
on Dietary Reference Values, compared with the small
increase of 1 mg per day in the earlier 1979 edition.
0037There is no doubt that as scientific opinion changes,
other values will also be reduced, and nutritional
guidelines for pregnancy ultimately will correspond
to the dietary practices of healthy women satisfying
their natural appetites on a well-balanced diet.
See also: Anorexia Nervosa; Calcium: Physiology;
Carbohydrates: Digestion, Absorption, and Metabolism;
Dietary Requirements of Adults; Energy: Energy
Expenditure and Energy Balance; Fats: Requirements;
Hormones: Steroid Hormones; Iron: Physiology;
Lactation: Human Milk: Composition and Nutritional
Value; Malnutrition: Malnutrition in Developed Countries;
Minerals – Dietary Importance; Premenstrual
Syndrome: Nutritional Aspects; Protein: Digestion and
Absorption of Protein and Nitrogen Balance; Vitamins:
Overview
Further Reading
Campbell DM and Gilmer DG (eds) (1983) Nutrition in
Pregnancy. London: Royal College of Obstetricians and
Gynaecologists.
Department of Health (1991) Dietary Reference Values for
Food Energy and Nutrients for the United Kingdom.
London: The Stationery Office.
Hytten F and Chamberlain GVP (eds) (1980) Clinical
Physiology in Obstetrics. Oxford: Blackwell Scientific.
Naismith DJ (1983) Maternal nutrition and fetal health. In:
Chiswick ML (ed.) Recent Advances in Perinatal
Medicine, vol. I, pp. 21–39. Edinburgh: Churchill
Livingstone.
National Research Council (1989) Recommended Daily
Allowances 10th edn. Washington, DC: National
Academy Press.
4728 PREGNANCY/Metabolic Adaptations and Nutritional Requirements

Role of Placenta in Nutrient
Transfer
Y Kudo
y
and C A R Boyd,UniversityofOxford,
Oxford, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 During intrauterine life, the developing fetus effect-
ively receives all of its nutrition across the placenta,
which, in the human, has a chorionic villus structure in
which maternal blood directly superfuses the external
surface, the trophoblast of the placenta. This tropho-
blast forms an unusual epithelium separating the ma-
ternal plasma from the fetal extracellular space of the
villus core through which the fetal capillary circula-
tion flows. For the fetus, the trophoblast is thus
equivalent to the epithelium lining the small intestine
of the newborn, since, from a nutritional perspective,
it must absorb those molecules required for both
growth and maintenance of the organism. The tropho-
blast also has an important role in acting as the lung for
the fetus (since all gas exchange between mother and
baby must occur across this surface), as the fetus’s
kidney (since excretion from the conceptus to the
mother occurs across this structure), as well as being
an important endocrine tissue secreting peptide and
steroid hormones into the mother. The placenta itself
also plays a very substantial role in intermediary me-
tabolism, so that it cannot be assumed that there is no
metabolism of absorbed nutrients during solute move-
ment from mother to fetus.
0002 As an epithelium, the trophoblast has two surfaces,
the one facing the mother and the other facing the
fetus. These surfaces differ structurally; for example,
microvilli are found projecting into the maternal
bloodstream at the apical surface, where they form a
brush border, whereas the basal surface facing the
fetus does not have this surface specialization. From
the functional point of view, it is the differences in
the distribution of transport membrane proteins
(channels, carriers, and pumps) that determine the
overall transport of nutrients from mother to baby,
at least as far as water-soluble molecules are con-
cerned. In the placenta, all transport appears to be
across the trophoblast since, uniquely, the cells that
compose this structure are fused to form a syncytium;
this is in contrast to other epithelia where transport
between adjacent epithelial cells allows a functional
paracellular route to lie in parallel to the route
through the epithelial cells themselves (the transcel-
lular route). The question in the human as to
whether there is a special route available for the
transport of large molecules (MW 5000) is not yet
resolved: certainly, at term, the human placenta does
appear to be able to permit the transport of larger
molecules at a slow rate from baby to mother.
This route is unlikely to be of nutritional significance,
since the rate of transport is low, but it may be
important with regard, for example, to immuno-
logical sensitization.
0003The placenta, together with the growing fetus, has
a very substantial metabolic energy requirement, and
in the human, ATP synthesis appears to be met by the
very substantial rate of glucose delivery from the
mother. The glucose transporter systems that are
found in both the apical and basal surfaces of the
human trophoblast appear to be the type named
GLUT1; in other words, they are sodium-independent
facilitated transporters that are not regulated by insu-
lin. The Michaelis constant (K
m
) for glucose transport
at both surfaces is relatively high (approximately
30 mM), and the maximal transport rate (V
max
)is
very substantial. The result of this is that glucose
delivery across the brush-border membrane of the
placenta will be in a direction and rate that are
dependent solely on the chemical driving force from
mother to baby (maternal–fetal plasma glucose con-
centration). It seems likely that this fundamental
property is the basis for the macrosomia (‘large-for-
dates‘) found in the babies of mothers with elevated
plasma glucose concentration, as typically found in
diabetes mellitus. Transport of glucose, which is
stereospecific, may be inhibited by glucose analogs
that share the chemical structure of d-glucose at
carbon 1; for example, both 3-0-methylglucose and
2-deoxyglucose are transported, whereas a-methyl-
glucoside (with a methyl group on carbon 1) is not.
The transport of other monosaccharides has been
studied rather little; in the human, fructose is trans-
ported much more slowly than glucose itself (in con-
trast to other nonprimate species). The question of
regulation of carbohydrate delivery across the pla-
centa is not fully resolved since some glucose trans-
porters may be regulated by phosphorylation, and
the gradient for transplacental glucose delivery will
also depend upon factors regulating glucose utiliza-
tion and production by the placenta itself; little is
known of the physiological regulation of either of
these processes. (See Carbohydrates: Digestion,
Absorption, and Metabolism; Glucose: Maintenance
of Blood Glucose Level.)
0004In contrast to glucose transport, amino acid trans-
port can be powered. The overall gradient of amino
acid between maternal plasma and fetal plasma varies
y
Deceased.
PREGNANCY/Role of Placenta in Nutrient Transfer 4729

for individual amino acids. Typically, individual
amino acid concentrations are twice as high in fetal
as in maternal plasma. Current understanding of the
mechanisms responsible for this relates to the distri-
bution of the membrane transport proteins between
the two faces of the trophoblast and in particular to
the distribution of sodium-coupled transporters that
are found predominantly (although not exclusively)
in the brush border. Recent work using isolated mem-
branes that reseal to form artificial structures (ves-
icles) has been useful in establishing the numbers and
properties of such transporters. In addition to the
direct effect of sodium ions in moving amino acids
into the trophoblast across the brush-border mem-
brane against a concentration gradient, these trans-
porters are often electrogenic and are thus also driven
physiologically by the membrane potential. One
example of such a process is the transport system
called ‘A,‘ which uses alanine, serine, and proline
as transported substrates and accumulates these
amino acids in the trophoblast against a concentra-
tion gradient. These amino acids then leave the
trophoblast across the basal membrane by a different
transport system. Other amino acids may be trans-
ported via tertiary active transport; for example, leu-
cine is found in higher concentrations in fetal plasma
than in maternal plasma, but it is not itself a substrate
for sodium-coupled transport; rather, it appears to
exchange with amino acids, such as alanine, that
have been accumulated in the trophoblast as just
described.
0005 The cationic and anionic amino acids are unusual
in that, having their own charge, they will be acceler-
ated or retarded by the membrane potential in cross-
ing each of the plasma membrane surfaces of the
trophoblast. For cationic amino acids (lysine, argin-
ine, histidine), entry into the placenta appears to be
largely by system y
þ
(Na
þ
-independent), whereas exit
into the fetus involves an electroneutral system (y
þ
L),
which exchanges the cationic amino acid for a neutral
amino acid (e.g., leucine) and a sodium ion, thus
effectively solving the problem of permitting posi-
tively charged amino acids to exit against an inside-
negative membrane potential. For anionic amino
acids (glutamate and aspartate), very high intratro-
phoblast concentrations are achieved by a transport
system that is coupled to K
þ
efflux as well as Na
þ
entry. Essential amino acid requirements for the fetus
are different from those of adults. However, it is not
clear whether transport of specific amino acids across
the placenta ever becomes rate-limiting for fetal
growth. In the human, intrauterine growth retard-
ation not associated with other disease has been
shown to be associated with reduced placental deliv-
ery of amino acids through specific systems, e.g.,
associated with decreased function of system A. IGF
(insulin-like growth factor) and IGFBP (insulin-like
growth factor binding protein) are now recognized as
having an important role in either normal or abnor-
mal fetal growth via controlling placental amino
acids and glucose transport (e.g., IGF-I (insulin-like
growth factor-1) selectively enhances system A activ-
ity). In certain unusual metabolic disorders (e.g., ma-
ternal phenylketonuria (PKU)) maternal levels of one
particular amino acid may be elevated; this results in
competition between this amino acid and others that
share the same transporters. Some of the abnormal-
ities found in the developing babies of such mothers
may be a consequence of nutritional deprivation of
tyrosine, for example, owing to competition by raised
maternal phenylalanine levels for the delivery of this
amino acid across the placenta. The fact that amino
acid transport across the placenta involves a family
of transport proteins with overlapping substrate
(amino acid) specificities means that the nutritional
consequences for the fetus of changing the level of
one amino acid in the mother will be complex.
This follows because, in contrast to placental glucose
transporters, the K
m
and V
max
of the amino acid
transporters are relatively low.
0006Lipid transport across the placenta in relation to
human nutrition has been studied less rigorously,
in part because it is likely to be perfusion- rather
than membrane-limited, since the lipid-soluble nature
of such a substrate allows ready transmembrane
transport. Nutritionally, the nervous system of the
developing fetus requires substrate delivery of precur-
sors for myelin synthesis. Studies suggest that placen-
tal binding proteins may provide a pool of essential
fatty acids for fetal utilization. (See Amino Acids:
Metabolism; Fatty Acids: Metabolism.)
0007Transport of the inorganic cations of sodium and
potassium involves both channels and transporters.
Sodium transport into the trophoblast is coupled to
the entry of those solutes (which include both organic
and inorganic molecules) powered by secondary
active transport. The extrusion of sodium across the
basal surface of the trophoblast is likely to be a result
of sodium pumping by Na
þ
/K
þ
ATPase activity. In
contrast, potassium, accumulated in the trophoblast,
as in other epithelia by the sodium pump, requires
channel-mediated release to account for its move-
ments between mother and baby. Potassium channels
have recently been shown to be sensitive to modula-
tion in this tissue (e.g., by G proteins, by arachidonic
acid, and by pH). These regulatory factors may them-
selves be controlled by circulating factors in both
mother and fetus. It is clear that fetal plasma potas-
sium is carefully regulated by control of placental
transport of this cation.
4730 PREGNANCY/Role of Placenta in Nutrient Transfer

0008 The divalent cation of calcium is found in higher
concentrations in fetal than in maternal plasma.
Active transport processes are therefore involved in
placental calcium metabolism, and these are unusual
in that regulation of transport is clearly precisely
controlled. It seems likely that calcium extrusion
across the basal surface of the trophoblast is ATP-
driven and regulated by calmodulin. Entry of calcium
across the brush border is now known to be via ECaC
(epithelial calcium channels) that are regulated: mem-
brane potential hyperpolarization activates, and
intracellular calcium inhibits, these channels. Fetal
parathyroid hormone and vitamin D are likely to
regulate all of these events.
0009 Iron is also transported from mother to fetus in a
regulated fashion, and again, there is a greater con-
centration of iron in the fetal circulation. In some
species, transport of iron has been shown to be active
in that it is inhibited by anoxia. The role of the
transferrin receptor found in the human placental
microvillus membrane is now known to be related
to the mechanisms of iron entry into the cytosol.
From this compartment, iron has to leave, but the
mechanisms responsible for this are not fully under-
stood. (See Calcium: Physiology; Hormones: Thyroid
Hormones; Iron: Physiology; Potassium: Physiology;
Sodium: Physiology.)
0010 Anion entry into the placenta has been studied
using isolated membrane preparations. These studies
show that for monovalent ions (chloride and other
halides), two routes are available, an exchange
and a conductive route, the latter likely to be via
channels. The anion exchange system appears to
be functionally linked to the transport of organic
anions (bicarbonate, lactate) from placenta into the
maternal circulation. Phosphate transport is also
regulated and appears to involve a sodium-dependent
cotransporter at the maternal-facing surface and an
efflux mechanism (possibly driven by the membrane
potential) at the basal surface of the tissue. As for
calcium transport, phosphate delivery is regulated
by parathyroid hormone concentration in the fetus.
0011 Trace-element delivery across the placenta also in-
volves specific placental binding proteins analogous
to those found in adult liver; however, membrane
transport is also required to allow such ions to gain
access to and from the trophoblast. The nature of
such transporters varies greatly, although the role
of the divalent cation transporter (DCT-1) in the
placenta may be more generally important. For
zinc, there is evidence that the histidine amino
acid transporter is responsible for delivery of a histi-
dine–zinc complex, whereas for transition-metal
oxides, it appears that anion exchange is important.
The transport of iodide across the human placenta is
also likely to be by anion exchange since SCN can
inhibit it, but the mechanisms responsible for the
concentration of this element in the fetal compart-
ment are not clear. Selenate appears to share a path-
way with sulfate for entry across the brush border,
and the transport of both of these ions is inhibited by
blockers of anion exchange. This pathway appears to
be shared with those available for transport of the
trace elements chromium and molybdenum as chro-
mate and molybdate. (See Trace Elements.)
0012Vitamin transport also is highly specific for indi-
vidual substrates; thus, for ascorbic acid, a sodium-
independent transporter for the reduced from of this
nutrient has been described in the brush-border mem-
brane; this transporter appears to be functionally
coupled to a placental system that maintains ascor-
bate in this chemical form. The Na
þ
-dependent
multivitamin transporter that transports panto-
thenate, biotin, and lipoate is expressed in human
placenta.
See also: Amino Acids: Metabolism; Calcium:
Physiology; Carbohydrates: Digestion, Absorption, and
Metabolism; Fatty Acids: Metabolism; Glucose:
Maintenance of Blood Glucose Level; Hormones: Thyroid
Hormones; Iron: Physiology; Potassium: Physiology;
Sodium: Physiology; Trace Elements
Further Reading
Bain MD, Copas DK, Taylor A et al. (1990) Permeability of
human placenta in vivo to four non-electrolytes. Journal
of Physiology 431: 505–513.
Boyd CAR and Kudo Y (1990) Placental amino acid trans-
port. Biochimica et Biophysica Acta 121: 169–174.
Hoenderop JGJ, van der Kemp AW, Hartog A et al. (1999)
Molecular identification of the apical Ca
2þ
channel in
1,25-dihydroxyvitamin D
3
-responsive epithelia. Journal
of Biological Chemistry 274: 8375–8378.
Kudo Y and Boyd CAR (1996) Placental tyrosine transport
and maternal phenylketonuria. Acta Paediatrica 85:
109–110.
Parkkila S, Waheed A, Britton RS et al. (1997) Association
of the transferrin receptor in human placenta with HFE,
the protein defective in hereditary hemochromatosis.
Proceedings of the National Academy of Sciences, USA
94: 13198–13202.
Shennan DB and Boyd CAR (1988) Trace element transport
in placenta. Placenta 9: 333–343.
Sibley CP, Birdsey TJ, Brownbill P et al. (1998) Mechan-
isms of maternofetal exchange across the human pla-
centa. Biochemical Society Transactions 26: 86–91.
Wang H, Huang W, Fei YJ et al. (1999) Human placental
Na
þ
-dependent multivitamin transporter. Journal of
Biological Chemistry 274: 14875–14883.
PREGNANCY/Role of Placenta in Nutrient Transfer 4731

Safe Diet
R B Fraser and F A Ford, University of Sheffield,
Sheffield, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Would-be pregnant and pregnant women have
heightened concerns about their diet, which can be
summarized by the common question, ‘Will it harm
my baby?‘ Because the question is so common, many
sources of advice are available in the lay press, not all
as scrupulously researched and scientifically based as
they might be. Another source of potentially mislead-
ing advice is the food industry and written media, the
agents of which possess a lack of objectivity that may
not be obvious to the casual reader. Articles prolifer-
ate with titles such as ‘How to have a beautiful baby‘
and ‘How to have a perfect baby‘. These tend to
contain a mixture of dietary and lifestyle advice
with the implication that, if followed, the undesirable
outcomes of pregnancy such as miscarriage, congeni-
tal malformation, and fetal death are avoidable. Pro-
fessionals in the field of nutrition must be aware of
this for the following reason. If a woman learns
of such advice only after her pregnancy has failed in
some way there is a potential for a lifelong burden of
guilt. For this reason purveyors of advice should re-
strict themselves to that which has been demonstrated
in scientifically valid experiments. In particular, they
should be guarded in their extrapolations from obser-
vational studies and animal experiments.
0002 This article highlights the principal areas in which
women have dietary concerns. Some of these are
dealt with in more detail elsewhere and are cross-
referenced.
Periconceptional Nutrition
0003 In an ideal world, pregnancies would be planned but
in the real world a high proportion are not. It is
important to remember that many women who have
not planned their pregnancies and have not subse-
quently followed the advice outlined below may
need reassuring that they have probably not caused
harm to their baby.
0004 The work of Frisch in the USA identified the link
between body composition and ovulation in the
human female. She suggested that fat must comprise
at least 22% of body weight for the maintenance of
ovulatory cycles and also observed that in normal
postpuberty women fat is about 28% of body weight.
It is well recognized that the relationship is to the fat
content of the body rather than absolute body weight,
as trained athletes of average or above-average body
weight may have very low body fat content and may
be oligo- or amenorrheic. Frisch observed that
amongst trained athletes who became fit after a
normal menarche, 60% continued regular cycles,
but 40% had irregular cycles and presumably associ-
ated subfertility. Other causes of secondary amenor-
rhea leading to infertility include psychological stress,
thyrotoxicosis, and the various malabsorption syn-
dromes. Eating disorders such as anorexia nervosa
and bulimia nervosa which may have been under-
diagnosed in the past are now been seen as contrib-
uting to the problem. Particularly for bulimics, a
remission in the disorder may allow the body fat to
build up to a stage where ovulation and conception
can take place, but the disorder may relapse during
the pregnancy, leading to serious nutritional deficien-
cies for the mother and possibly for the fetus.
0005The Nurses Health Study in the USA has also pro-
duced some interesting information about the relative
risk of menstrual cycle irregularity, not only in under-
weight but also in overweight women. For women
with a body mass index (BMI) below 20, at the age of
18 years, ovulatory infertility was found with a rela-
tive risk of about 1.2 compared to women with a BMI
between 20 and 25. Interestingly, however, the rela-
tive risk of ovulatory infertility was 1.5 in those with
a BMI of 28 and more than 2 in the obese group with
a BMI above 30. About half of the risk is associated
with polycystic ovarian syndrome (PCOS) in which
ovulatory infertility and obesity coexist but there is
still a doubling in relative risk of ovulatory infertility
in women with a BMI above 30 who do not have
ultrasonically detectable polycystic ovaries.
Vitamin Intakes and Congenital
Malformations
Folic Acid
0006In 1991, the Medical Research Council (MRC) vita-
min trial reported that a significant reduction in the
recurrence of neural tube defects (NTD) had been
obtained by a daily dietary supplement of 4 mg of
folic acid in women who were at high risk. Women
at risk of a recurrence of NTD should be advised to
take a folic acid supplement of 5 mg (5000 mg) per day
when planning a pregnancy and continue with it until
12 weeks’ gestation. A later study reported that first
occurrence of NTD could also be prevented by daily
supplements containing 800 mg of folic acid. In the
UK, in line with many other nations, women are
advised to take a prepregnancy supplement of
0.4 mg (400 mg) of folic acid as a daily medicinal
supplement from when they begin trying to conceive
4732 PREGNANCY/Safe Diet

until 12 weeks’ gestation, in addition to eating a
folate-rich diet and breads and breakfast cereals
fortified with folic acid.
0007 The mechanism for the effect of folic acid in redu-
cing the risk of NTD is not clear. The most likely
explanation is that it overcomes genetically deter-
mined defects in folate metabolism that interfere
with normal neural tube development. One other
hypothesis is that it might decrease the likelihood of
an affected pregnancy surviving, and there is some
concern that folic acid supplementation could be
associated with a higher risk of fetal death. How-
ever, the generally accepted explanation for this is
that folic acid might initially permit the survival of
affected or nonviable pregnancies to a point where
they are recognized as spontaneous abortions.
0008 Educational campaigns conducted since 1995 have
had a substantial effect in increasing the number of
women in the UK who are aware of the link between
folic acid and NTD. However, such education can
only have a limited effect because it is estimated that
only 50% of all pregnancies are planned. NTD arise
very early in gestation and by the time a woman
realizes that she is pregnant it is usually too late to
prevent an NTD by taking supplements. For this
reason it has been suggested that the most effective
way of reducing NTD in the UK would be to fortify
the food supply with folic acid so that even women
with unplanned pregnancies would be less likely
than at present to have offspring with NTD. The
Department of Health Committee on Medical
Aspects of Food and Nutrition Policy (COMA) has
recently recommended universal fortification of flour
at 240 mg 100 g
1
, which would reduce the risk of
NTD by 41% without resulting in unacceptably
high intakes in any group of the population.
Retinol
0009 It has been known for a long time from animal studies
that high levels of vitamin A in the form of retinol
are teratogenic in the periconceptional period. This
led to the Department of Health in 1990 advising
women to avoid food sources containing high levels
of the retinal form of vitamin A in the periconcep-
tional period and indeed during pregnancy. The
Department of Health advises that very high intakes
of retinol, i.e., more than 10 times the recommended
dietary allowance either in liver/liver products or
vitamin/fish liver oil supplements, can damage the
developing embryo. This advice remains current and
all women of child-bearing age should avoid liver and
its products and should not take supplements con-
taining more than four times the recommended daily
amount of retinol.
0010The same risks do not seem to be present for vita-
min A derived from b-carotene. Manufacturers of
multivitamin supplements recommended for preg-
nancy have generally recognized this and switched
the source of vitamin A to b-carotene.
Nutritional Management of Common
Symptoms in Pregnancy
Heartburn
0011Heartburn is thought to be caused by gastroesopha-
geal reflux. Although occasionally experienced in the
first trimester, it is generally more common in the last
trimester and occurs in 30–50% of women.
0012Small, frequent meals or snacks are usually toler-
ated better than large, well-spaced meals. Common
foods cited as causing heartburn as spicy and fatty
foods, fizzy drinks, citrus fruits, fruit juices, and
cucumber. Milk and milk products can help to relieve
symptoms but antacids are frequently used.
Nausea and Vomiting
0013Psychological factors, changing hormone levels,
hunger, altered carbohydrate metabolism, and vita-
min deficiencies have all been proposed as possible
causes for nausea and vomiting, but none has been
confirmed.
0014Symptoms may start before the woman knows she
is pregnant or in later pregnancy but commonly they
are worst between weeks 6 and 10, and subside by
about 13 weeks. Nausea is experienced at any time of
day or night and can be either slight or severe. It often
becomes worse when the stomach is empty, and
eating small, frequent meals based on starchy carbo-
hydrates may relieve it. Morning sickness is common
and consuming dry biscuits or toast before getting up
can relieve this.
0015Nausea can also be triggered by traveling, fried and
spicy foods, and smells such as coffee, perfume, and
cigarette smoke.
0016Some women just feel nauseated while others actu-
ally vomit as well; this may cause minor weight loss
but rarely causes nutrient deficiency. Women need
reassurance that not eating proper cooked meals or
losing some weight and their taste alterations will
cause no problems for their developing fetus. The
more severe cases of pregnancy vomiting (hyper-
emesis gravidarum) require hospital admission, intra-
venous fluids and, sometimes, parenteral nutrition.
Constipation
0017Constipation is common at all stages of pregnancy. It
may be related to a general reduction in motility in
the gastrointestinal tract, with prolonged transit
PREGNANCY/Safe Diet 4733

times and increased water resorption from the stool.
General advice about constipation is also suitable for
pregnancy, i.e., increased intake of fiber, particularly
cereal fiber, and increased fluid intake.
0018 Constipation may be aggravated by the consump-
tion of iron tablets; if it is not appropriate to reduce or
stop them, bulking agents may be prescribed.
Qualitative Aspects of Diet
0019 The following section refers to common questions of
dietary safety; some arise because of suspicion of
harm when items are included in the diet, others for
the paradoxical reason that their omission from the
diet might be dangerous.
Alcohol
0020 As far as alcohol is concerned, the picture is confus-
ing. There is no doubt that a heavy intake of alcohol
can damage the unborn baby and cause miscarriage.
Many women choose to give up alcohol and this
seems to be a sensible practice but there is no evidence
of harm from occasional drinking or the consumption
of less than 2 units per day (Table 1). Despite this,
many women do give up alcohol when trying to con-
ceive or whilst pregnant, and this seems a sensible but
not mandatory practice. (One unit is 15 g of absolute
alcohol, e.g., 0.28 l of beer, one glass of wine, or one
measure of spirits.)
0021 A well-defined group of anomalies referred to as
the fetal alcohol syndrome is now recognized. The
major defects of affected infants are weight and
length below the 10th centile for gestational age,
and microcephaly. Microcephaly is a condition of
small head size associated with an underdeveloped
brain. Such children are likely to be mentally retarded
and their physical growth is stunted. The syndrome
has been reported in up to 40% of the infants of
women drinking more than 6 units of alcohol per day.
0022 Definite harm has been recorded to the offspring of
women drinking between two and six units of alcohol
per day and long-term growth retardation and mental
retardation to offspring of those drinking more than 6
units a day (fetal alcohol syndrome).
0023A recent study showed that a woman’s alcohol
intake is associated with decreased fertility, even
among women with a weekly alcohol intake corres-
ponding to five or fewer drinks. This finding needs
further corroboration, but it seems reasonable for the
moment to encourage women to avoid alcohol if they
are having trouble conceiving.
Caffeine
0024Concern about the detrimental effects of caffeine
during pregnancy is not new but to some extent the
literature is conflicting. Caffeine is present in tea,
coffee, cola drinks, and many over-the-counter
remedies for colds and allergic symptoms. Animal ex-
periments with high doses have shown that some
congenital malformations may be induced but there is
no evidence of harm in humans. Current recommenda-
tions are that pregnant and breast-feeding women
should limit their caffeine intake to 300 mg day
1
,
which is equivalent to 4 cups ordinary-strength coffee
per day (or 6 cups of tea or 7 cans of cola). A recent
review from the UK Committee on Toxicity of Chem-
icals in Food, Consumer Products and the Environment
(DoH 2001) issued a statement on the reproductive
effects of caffeine in October 2001. A meta-analysis
of studies of maternal caffeine intake during pregnancy
and the risk of spontaneous abortion or low birth
weight, compared maternal caffeine intakes during
pregnancy of more than 150 mg/day with less than
150 mg/day. Calculated odds ratios were significantly
increased for spontaneous abortion (miscarriage) (odds
ratio ¼1.36; 95% confidence interval, 1.29–1.45) and
low birth weight (odds ratio ¼1.51; 95% confidence
interval, 1.39–1.63), for low birth weight (< 2.5 kg).
0025Two studies, which examined the links between
caffeine intake and pregnancy in SIDS, received a
huge amount of publicity and caused a lot of worry
for women. However, the results were conflicting.
Peanuts
0026Peanut allergy is increasing in children, although the
cause is unclear. The use of peanuts and peanut oil in
the British diet has increased rapidly over the last few
years and it is thought that being exposed to peanuts
at a young age may cause the allergy. However, it is
not known if this can happen as a result of a mother
eating peanuts when she is pregnant or breast-
feeding. Current advice is that women, their partners,
and existing children who have a family history of
atopic disease should avoid peanuts.
Over-the-Counter Remedies
0027As a general rule, self-prescribing in pregnancy
should be kept to a minimum. Those drugs that
tbl0001 Table 1 Congenital malformation rates and maternal alcohol
consumption
DrinksperdayMalformationrates(per1000)
a
None 78
Less than one 77
One to two 83
a
No statistically significant difference.
From Mills JL and Graubard BI (1987) Is moderate drinking during
pregnancy associated with an increased risk for malformations? Pediatrics
80: 309–314.
4734 PREGNANCY/Safe Diet
