Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0031 Ascorbic acid has a number of chemical properties
responsible for the biological reactions that charac-
terize vitamin C. Usually, these characteristics are
positively associated with the benefits provided by
this vitamin. Paradoxically, these properties provide
difficulties in the analysis of ascorbic acid in foods
and physiological samples.
See also: Antioxidants: Natural Antioxidants; Browning:
Nonenzymatic; Cancer: Diet in Cancer Prevention;
Chromatography: High-performance Liquid
Chromatography; Controlled-atmosphere Storage:
Effects on Fruit and Vegetables; Food Fortification;
Oxalates; Oxidation of Food Components; Storage
Stability: Mechanisms of Degradation; Parameters
Affecting Storage Stability; Tocopherols: Properties and
Determination
Further Reading
Behrens WA and Made
`
re R (1994) Ascorbic acid, isoascor-
bic acid, dehydroascorbic acid and dehydroisoascorbic
acid in selected food products. Journal of Food Compos-
ition and Analysis 7: 158–170.
Budavari S (1996) The Merck Index, 12th edn. Whitehouse
Station, NJ: Merck.
Hodiroglou N, Made
`
re R and Behrens W (1998) Electro-
chemical determination of ascorbic acid and isoascorbic
acid in ground meat and in processed foods by high
pressure liquid chromatography. Journal of Food Com-
position and Analysis 11: 89–96.
Kall MA and Andersen C (1999) Improved method for
simultaneous determination of ascorbic acid and
dehydroascorbic acid, isoascorbic acid and dehydroi-
soascorbic acid in food and biological samples. Journal
of Chromatography B 730: 101–111.
Lykkesfeldt J (2000) Determination of ascorbic acid and
dehydroascorbic acid in biological samples by high-
performance liquid chromatography using subtraction
method: Reliable reduction with Tris[2-carboxyethyl]-
phosphine hydrochloride. Analytical Biochemistry 282:
89–93.
Martell AE and Hancock RD (1996) Metal complexes in
aqueous solutions. In: Fackler JP (ed.) Modern Inorganic
Chemistry. New York: Plenum Press.
Martell AE and Smith RM (1977) Critical Stability Con-
stants, vol. 3. New York: Plenum Press.
Packer L and Fuchs J (eds) (1997) Vitamin C in Health and
Disease. New York: Marcel Dekker.
Saxholt E and M
Øller A (1996) The Composition of Food,
4th edn. Copenhagen: Danish Veterinary and Food
Administration, Gyldendal.
Speek AJ, Schrijver J and Schreurs WHP (1984) Fluoro-
metric determination of total vitamin C and total
isovitamin C in foodstuffs and beverages by
high-performance liquid chromatography with precol-
umn derivatization. Journal of Agricultural and Food
Chemistry 32: 352–355.
Vanderslice JT and Higgs DJ (1991) Vitamin C content of
foods: sample variability. American Journal of Clinical
Nutrition 51: 1323S–1327S.
Vanderslice JT, Higgs DJ, Hayes JM and Block G (1990)
Ascorbic acid and dehydroascorbic acid content of food
as eaten. Journal of Food Composition and Analysis 3:
105–118.
Physiology
G F M Ball, Wembley, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Ascorbic acid (vitamin C) has many diverse functions
as a reducing agent in the body. It keeps the bound
iron or copper ions of several hydroxylating enzymes
in the necessary reduced state, and aids the intestinal
absorption of iron by forming soluble ferrous–ascor-
bate complexes. Vitamin C helps in the fight against
cancer by reducing harmful free radicals to harmless
nonradical species, and inhibiting the formation of
Time (min)
0
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
123456789
Fluorescence response (mV)
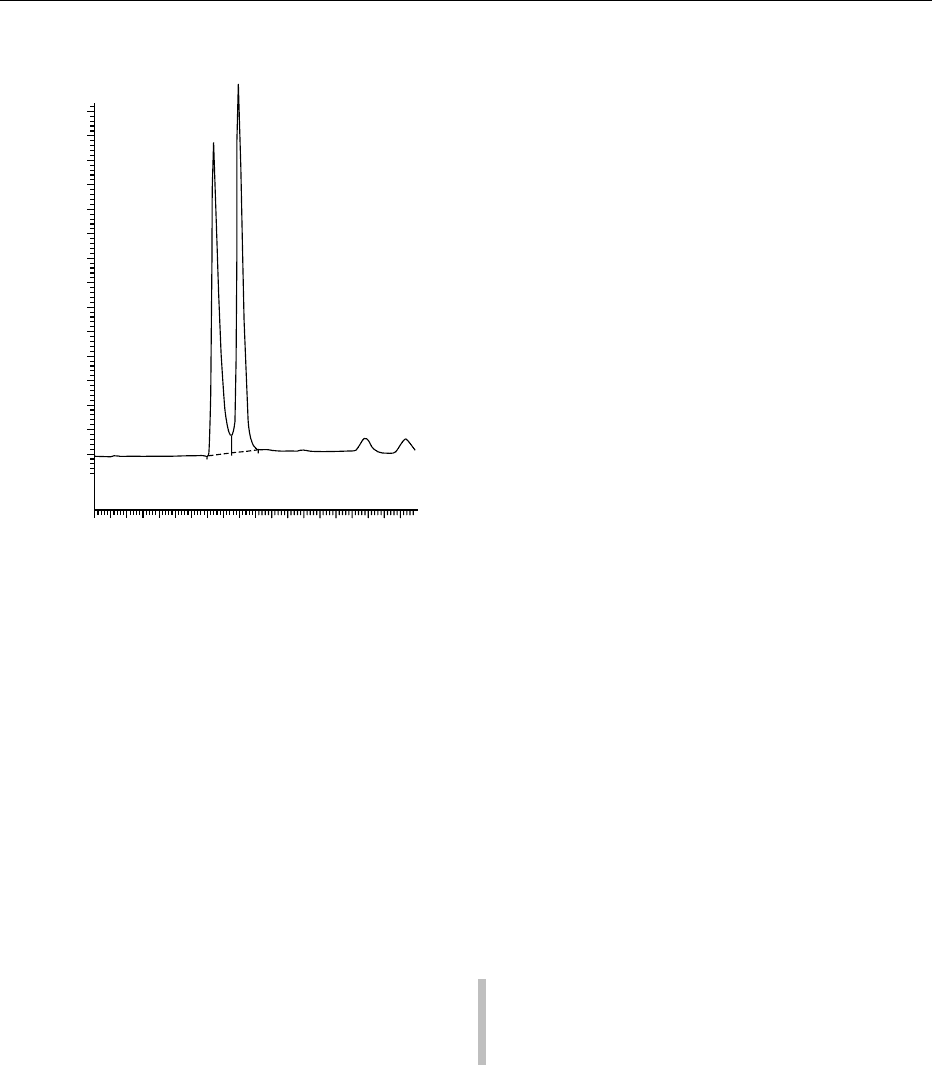
DHIAA
DHAA
fig0006 Figure 6 Separation of DHAA and DHIAA on a C18 column
followed by postcolumn derivatization and fluorometric detec-
tion. From Kall MA and Andersen C (1999) Improved method for
simultaneous determination of ascorbic acid and dehydroascor-
bic acid, isoascorbic acid and dehydroisoascorbic acid in food
and biological samples. Journal of Chromatography B 730:
101–111, with permission.
324 ASCORBIC ACID/Physiology
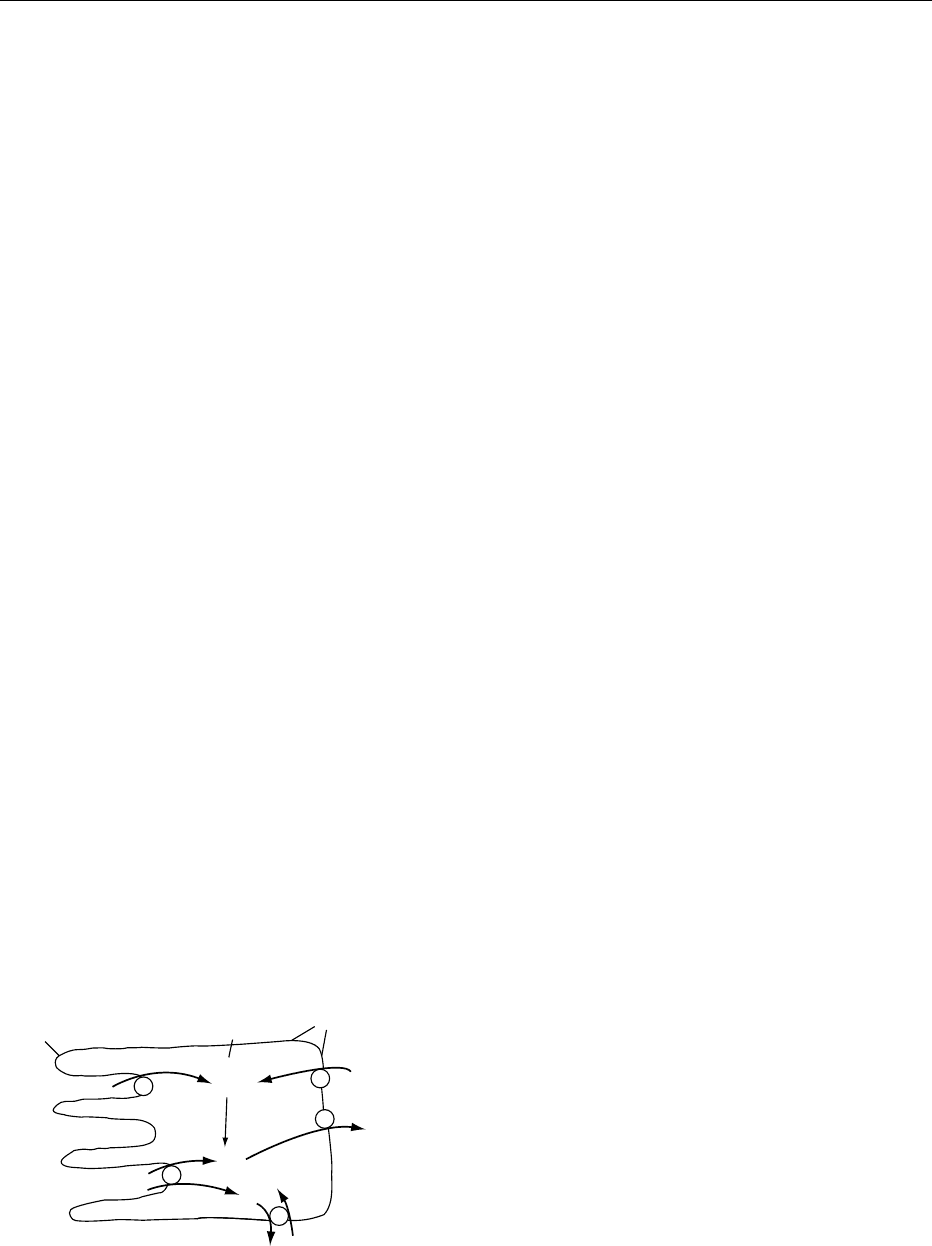
carcinogenic N-nitroso compounds in the stomach.
There is increasing evidence that vitamin C is in-
volved in the human immune system and in the regu-
lation of prostaglandin synthesis.
Metabolism
0002 Humans and other primates, guinea-pigs, and fruit-
eating bats lack the enzyme gulonolactone oxidase,
which catalyzes the final step in the biosynthesis of
ascorbic acid, and thus rely on their diet to provide
the vitamin. Other animal species can synthesize as-
corbic acid from glucose and have no need for dietary
vitamin C.
Intestinal Absorption
0003 The overall system of intestinal transport and metab-
olism in humans is shown in Figure 1. Ascorbic acid is
ionized within the pH range of the intestinal contents,
and therefore, it is the ascorbate anion (specifically
l-ascorbate) that is transported. At physiological
intakes, ascorbate must move across the brush-border
membrane of the intestinal epithelium from a region
of lower concentration in the lumen to one of higher
concentration in the cytoplasm of the absorptive cell
(enterocyte). Such ‘uphill’ movement requires active
transport – a mechanism that depends ultimately
upon the expenditure of metabolic energy, i.e., the
energy released from the hydrolysis of adenosine tri-
phosphate produced during cellular metabolism. The
precise mechanism of ascorbate absorption is second-
ary active transport in which a transmembrane pro-
tein (inappropriately called a carrier) mediates the
sodium-coupled transfer of ascorbate across the
brush-border membrane. The carrier spans the mem-
brane in a weaving fashion and effects solute transfer
through a conformational change in its molecular
structure. The immediate energy source for the
transport mechanism is the concentration gradient
of sodium across the brush-border membrane. The
gradient is maintained by the constant extrusion
of sodium from the enterocyte by the action of the
sodium pump at the basolateral membrane. The
sodium pump is driven by metabolic energy and is
the primary driving force for ascorbate absorption.
0004An electrical potential difference ranging from 30
to 90 mV (cell interior negative) typically exists
across cell membranes. This membrane potential in-
fluences the active transport of charged solutes across
membranes. Using isolated brush-border membranes
from the intestines of guinea-pigs, it has been shown
that ascorbate transport is unaffected by changes in
the membrane potential. The transport system is
therefore electroneutral, indicating a 1:1 cotransport
of Na
þ
and ascorbate
by the same carrier. l-
Ascorbic acid leaves the enterocyte by sodium-
independent facilitated diffusion at the basolateral
membrane.
0005Dehydro-l-ascorbic acid is taken up from the
bloodstream across the basolateral membrane of
enterocytes as well as being absorbed across the
brush-border membrane. The transport mechanism
in both cases is sodium-independent facilitated diffu-
sion driven by the steep concentration gradient
maintained by the intracellular reduction of dehy-
droascorbic acid.
0006The efficiency of vitamin C absorption of foods
over a range of usual intakes (20–120 mg per day)
is approximately 90%. Absorption by the sodium-
coupled secondary active transport mechanism
reaches its maximum rate at a relatively low luminal
concentration. The higher concentrations resulting
from ingestion of vitamin C supplements are absorbed
additionally by passive diffusion, which proceeds at a
very low rate. Absorption therefore becomes progres-
sively less efficient with increasing dose levels, the
efficiency falling from 50% of a single 1.5-g dose to
16% of a single 12-g dose. Absorption efficiency is
increased by the ingestion of several spaced doses
throughout the day, rather than the ingestion of a
single megadose. Ingesting the vitamin in a sustained
release form also improves absorption efficiency. As-
corbic acid bioavailability from fruits and vegetables
is not significantly different to that from synthetic
ascorbic acid, indicating that the bioavailability in
these natural food sources is high.
0007Intestinal absorption of ascorbate is adaptively
regulated in a transient and reversible manner by
the level of dietary ascorbate. The mechanism of
regulation is an increase or decrease in the number
of carriers at both brush-border and basolateral mem-
branes in response to low or high concentrations of
ascorbate in the blood. The rationale for adaptive
Brush-border membrane
of microvillus
Enterocyte
Basolateral membrane
DHAA
DHAA
DHAA
AA
−
AA
−
AA
−
Connective
tissue
K
+
Na
+
Na
+
[H]
Intestinal
lumen
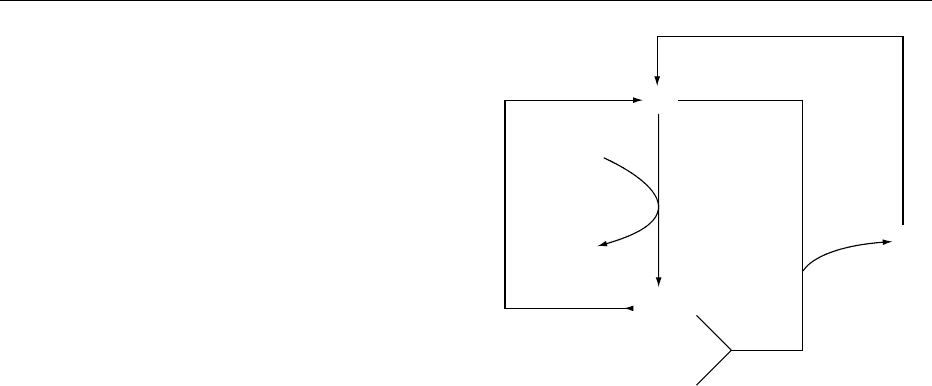
fig0001 Figure 1 Model of intestinal transport of the L-ascorbate anion
(AA
) and uncharged dehydro-L-ascorbic acid (DHAA) in vitamin
C-dependent animals. Thick arrowed lines indicate directional
pathways; [H] signifies enzymatic reduction.
ASCORBIC ACID/Physiology 325

regulation is that carriers are most needed at low
dietary ascorbate levels; at excessive levels, the re-
quired amount of ascorbate can be absorbed by
fewer carriers, aided by passive diffusion. As ascor-
bate does not provide metabolizable energy, there is
nothing to gain from the cost of synthesizing and
maintaining carriers when the vitamin supply is in
excess.
Postabsorptive Metabolic Events
0008 Vitamin C circulates in the bloodstream mainly as
free (nonprotein-bound) ascorbic acid. The kidney
actively reabsorbs ascorbate present in the glomerular
filtrate, thereby maximizing vitamin C conservation
in the body and helping the intestine to maintain the
circulating vitamin in its useful, reduced state. Con-
trary to the intestinal transport system, the renal
system is present also in species for which ascorbate
is not a vitamin (e.g., rat, rabbit). Renal uptake of the
l-ascorbate anion at the brush-border membrane of
the absorptive cell of the proximal convoluted tubule
is a sodium-coupled, secondary active transport
system. Unlike the corresponding intestinal transport
system, the renal system is affected by experimental
changes in the membrane potential, indicating an
electrogenic mechanism with a Na
þ
/ascorbate
coupling ratio of 2:1. As the loaded carrier bears a
net positive charge, its transport is accelerated by the
negative membrane potential. Rapid renal reabsorp-
tion of ascorbate is important, as the transit time in
the proximal tubule is only about 10 s.
0009 Many cells and tissues can accumulate ascorbate
against a concentration gradient. Particularly high
concentrations of the vitamin are found in leukocytes
(white blood cells), lung tissue, the adrenal and pitu-
itary glands, and compartments of the eye. Skeletal
muscle contains much of the body’s pool of ascorbate,
although the concentration is relatively low. The ul-
timate metabolic fate of vitamin C is urinary excre-
tion of ascorbic acid or metabolites.
Effect upon Absorption of Inorganic Iron
0010 The iron present in natural foodstuffs exists in two
forms, heme iron and inorganic iron. Heme iron is
obtained from the hemoglobin and myoglobin pre-
sent in meat, fish, and poultry. Inorganic iron is found
primarily in plant foods; it provides 85–90% of iron
in a mixed diet and is the only source of iron in a
vegetarian diet. The mechanisms of absorption of
heme iron and inorganic iron differ. Unlike heme
iron absorption, absorption of inorganic iron is
affected by the presence of vitamin C and other con-
stituents of the diet.
0011Dietary inorganic iron is largely in the ferric (Fe
3þ
)
state, which is poorly soluble in the alkaline environ-
ment of the small intestine where absorption of iron
takes place. Ferrous iron (Fe
2þ
), however, is soluble at
alkaline pH. The amount of inorganic iron absorbed
by the intestine is influenced by stimulatory and
inhibitory factors of either dietary or endogenous
origin. Ascorbic acid both reduces and chelates iron,
forming ferrous–ascorbate complexes that are readily
absorbed. Other weak organic acids (e.g., citric acid),
certain sugars, and sulfur-containing amino acids
have similar properties, but are less effective. Meat,
fish, and poultry also enhance the absorption of inor-
ganic iron, apparently because of the binding of iron
by cysteine-containing peptides that result from the
digestion of muscle tissues. Phytates that occur in
cereals (especially in the bran fraction) bind inorganic
iron and retard its absorption. The same is true for
tannins (polyphenols) that are found in some vege-
tables and in tea.
0012Supplemental ascorbic acid consistently enhances
absorption of inorganic iron from single meals, as
indicated by incorporation of iron into red blood
cells. In one study, 500 mg of ascorbic acid taken
with the meal increased iron absorption about six-
fold. The most pronounced effects of ascorbic acid
are found in meals containing a high content of phy-
tates and/or tannins. This finding has led to the sug-
gestion that ascorbic acid enhances iron absorption
by counteracting the influence of inhibitory sub-
stances.
Biochemical Functions
0013Ascorbic acid acts as a cofactor for eight mammalian
enzymes (Table 1) by keeping enzyme-bound iron or
copper ions in the necessary reduced state. Other
reducing agents are far less effective as cofactors
tbl0001Table 1 Ascorbic acid-dependent mammalian enzymes
Enzyme Function
Prolyl-4-hydroxylase (EC 1.14.11.2) Collagen synthesis
Prolyl-3-hydroxylase (EC 1.14.11.7) Collagen synthesis
Lysyl hydroxylase (EC 1.14.11.4) Collagen synthesis
e-N-Trimethyllysine hydroxylase (EC
1.14.11.8)
Carnitine synthesis
g-Butyrobetaine hydroxylase (EC
1.14.11.1)
Carnitine synthesis
Dopamine b-hydroxylase (EC 1.14.17.1) Noradrenaline
synthesis
Peptidylglycine a-amidating
monooxygenase (EC 1.14.17.3)
Peptide amidation
4-Hydroxyphenylpyruvate hydroxylase
(EC 1.13.11.27)
Tyrosine metabolism
326 ASCORBIC ACID/Physiology
in vitro, and so these enzymes are considered to be
ascorbic acid-dependent. The roles of such enzymes
in four biochemical processes are discussed below.
Biosynthesis of Collagen
0014 Collagen is the major macromolecule of most con-
nective tissues. It forms a triple helix and is highly
cross-linked to give a rigid and inextensible structure.
The amino acid composition is unusual among
animal proteins, with an abundance of proline and
4-hydroxyproline and a few residues of 3-hydroxy-
proline and hydroxylysine. The hydroxyproline resi-
dues are necessary for proper structural conformation
and stability; hydroxylysine residues take part in
cross-linking and facilitate subsequent glycosylation
and phosphorylation.
0015 Free hydroxyproline and hydroxylysine are not in-
corporated into procollagen during its synthesis. The
presence of these two unusual amino acids in pro-
collagen (the precursor of collagen that contains
additional ‘propeptides’) arises through the post-
translational hydroxylation of particular proline
and lysine residues in the polypeptide chain. Within
the cisternae of the rough endoplasmic reticulum, the
newly synthesized procollagen chains encounter three
hydroxylating enzymes. Two of these enzymes, pro-
lyl-4-hydroxylase and prolyl-3-hydroxylase, convert
proline residues to 4-hydroxyproline or 3-hydroxy-
proline, respectively, and the third, lysyl hydroxylase,
converts lysine residues to hydroxylysine. Each of
these enzymes contains an iron ion (maintained in
the ferrous state by ascorbate) and requires molecular
oxygen and a-ketoglutarate as cosubstrates (Figure 2).
In addition to the posttranslational modifications of
procollagen, ascorbate stimulates transcription of the
type I procollagen gene. The absence of wound
healing is one of the features of scurvy that can be
attributed to impaired collagen synthesis arising from
lack of vitamin C.
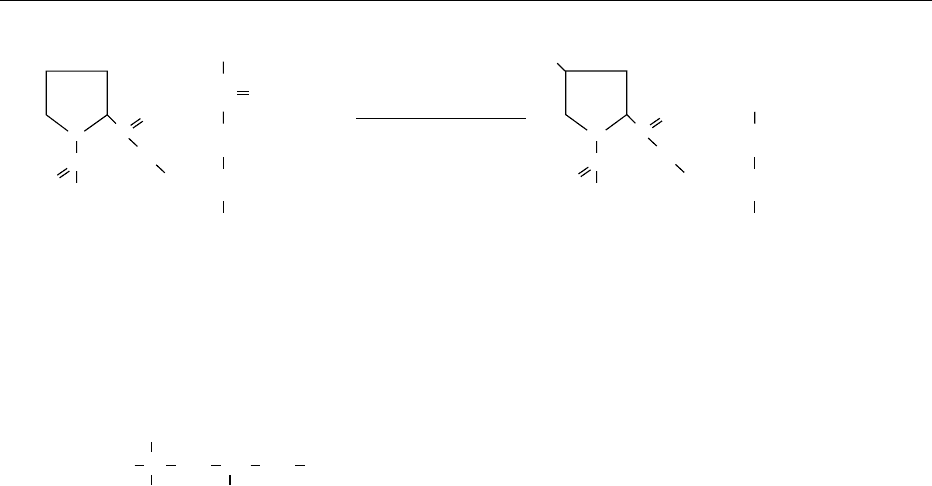
Biosynthesis of Carnitine
0016Carnitine (3-hydroxy-4-N-trimethylaminobutyric acid,
Figure 3) is essential for the b-oxidation of long-chain
fatty acids, producing energy in the mitochondria.
Neither free fatty acids nor fatty acid coenzyme As
can penetrate the inner membranes of mitochondria,
but acylcarnitine can readily do so. The translocation
of fatty acids from the cytosol to the b -oxidation site
in the matrix of the mitochondrion is therefore
dependent upon carnitine.
0017The synthesis of carnitine commences with the
conversion of specific peptide-linked lysine residues
to trimethyllysine within certain proteins. Proteins in
which trimethyllysine has been found include his-
tones, myosin, calmodulin, and cytochrome c. Free
trimethyllysine released by proteolytic cleavage is
converted to carnitine in a number of steps, which
include two hydroxylations. The enzymes responsible
for these hydroxylations are e-N-trimethyllysine
hydroxylase and g-butyrobetaine hydroxylase. Like
the hydroxylases involved in collagen biosynthesis,
both enzymes contain ferrous ion and require molecu-
lar oxygen and a-ketoglutarate as cosubstrates.
0018Vitamin C deficiency results in a variable decrease
in carnitine levels of skeletal muscle, heart muscle,
liver, and kidney. Carnitine status depends on dietary
carnitine intake, a modest rate of carnitine biosyn-
thesis, and efficient conservation of carnitine by renal
reabsorption. The rate of carnitine synthesis in
scorbutic guinea-pigs is much higher than the rate
N
C
C
R
R'
O
O
NH
COOH
COOH
CH
2
CH
2
C
O
++
O
2
N
C
C
R
R'
O
O
NH
COOH
COOH
CH
2
CH
2
++
CO
2
Prolyl-4-hydroxylase
Fe
2+
, ascorbate
HO
Peptide-bound
proline
Peptide-bound
hydroxyproline
α-Ketoglutarate
Succinate
fig0002 Figure 2 Hydroxylation of proline.
CH
3
CH
3
CH
2
N
+
CH CH
2
OH
CH
3
COO
−
fig0003 Figure 3 L-Carnitine.
ASCORBIC ACID/Physiology 327
in normal guinea-pigs, and therefore impaired syn-
thesis is not responsible for carnitine depletion. The
responsible factor is, in fact, increased urinary excre-
tion resulting from inefficient renal reabsorption.
Biosynthesis of Noradrenaline
0019 As a neurotransmitter, noradrenaline (norepineph-
rine) is synthesized in the nerve terminals of post-
ganglionic sympathetic neurones, referred to as
adrenergic neurones. As a hormone, noradrenaline,
along with adrenaline, is synthesized in the chro-
maffin cells of the adrenal medulla. The final and
probably rate-limiting step in the biosynthetic path-
way – the conversion of dopamine to noradrenaline
(Figure 4) – is catalyzed by dopamine b-hydroxylase
in the presence of molecular oxygen. Dopamine
b-hydroxylase is a tetramer, each monomer contain-
ing two copper ions maintained in the cuprous (Cu
þ
)
state by ascorbate. The enzyme is localized within
storage vesicles in both adrenergic neurones and adre-
nomedullary chromaffin cells. Intravesicular ascor-
bate is maintained in the reduced state by electron
transport across the vesicle membrane, cytosolic
ascorbate being the most likely electron donor. Cyto-
solic semidehydroascorbate generated in the extra- to
intravesicular electron transfer reaction may be re-
duced by the outer mitochondrial membrane enzyme,
semidehydroascorbate reductase (EC 1.6.5.4), to
complete the ascorbate regeneration cycle. In this
manner, a stable rate of noradrenaline biosynthesis
can be maintained in the absence of exogenous ascor-
bate.
Amidation of Peptides to Hormones and Hormone-
releasing Factors
0020A large number of peptides act as hormones and
hormone-releasing factors. The vast majority of
these peptides are initially synthesized as larger, in-
active precursor molecules, which are converted to
their active forms through a series of posttrans-
lational modifications. One such final step of acti-
vation is carboxy-terminal a-amidation, which is
essential for biological activity. Examples of
a-amidated peptides include a- and g-melanotropins,
calcitonin, pro-ACTH, vasopressin, oxytocin, chole-
cystokinin, gastrin, gastrin-releasing peptide, and
releasing factors for growth hormone, corticotropin,
and thyotropin. Direct precursors to a-amidated pep-
tides have glycine as the carboxy terminal amino acid.
The enzyme responsible for the amidation is the
bifunctional peptidylglycine a-amidating monooxy-
genase (PAM), which is found in secretory granules
in many neuroendocrine tissues. PAM is actually a
precursor protein that undergoes endoproteolytic
cleavage to generate two enzymes, peptidylglycine
a-hydroxylating monooxygenase (PHM) and pepti-
dyl-a-hydroxyglycine a-amidating lyase (PAL). The
two-step nature of the amidation is shown in Figure
5. Step 1 requires molecular oxygen, cuprous ion, and
ascorbate as the reductant.
Antioxidant Role
0021Ascorbic acid helps to fight diseases such as cancer by
scavenging harmful free radicals. A free radical can be
CH
2
CH
2
NH
2
+
O
2
CH
2
CH
2
NH
2
+
H
2
O
dopamine β-hydroxylase
Cu
+
, ascorbate
OH
HO
HO
HO
HO
Dopamine Noradrenaline
fig0004 Figure 4 Hydroxylation of dopamine.
Step 1
Step 2
Peptidyl
Peptidylylycine Peptidyl-α-hydroxyglycine
NH
CH
2
C
O
OH Peptidyl NH CH C
OH O
OH
HCC
OO
OHPeptidyl NH Peptidyl NH
2
CH C
OH O
OH
+O
2
PHM
PAL
Cu
+
, ascorbate
+
α-Amidated peptide
Glyoxylate
fig0005 Figure 5 Amidation of peptidylglycine.
328 ASCORBIC ACID/Physiology
defined as an atom or molecule capable of an inde-
pendent existence that contains an unpaired electron
in its outer orbit. A superscript dot is used to denote
free radical species; the superoxide anion radical with
its two unpaired electrons is usually represented by
O
2
.
. Owing to their unstable electronic configuration,
free radicals are much more reactive than nonradi-
cals. They readily extract electrons from other mol-
ecules with which they collide, and these molecules in
turn become free radicals. Thus, a chain reaction is
propagated.
002 2 Free radicals can be generated anywhere within the
cell by a variety of processes: spontaneous autoxida-
tion reactions (e.g., flavin oxidation), phagocytosis
during infection, metabolic processing of foreign
compounds (e.g., constituents of tobacco smoke,
drugs, pesticides, solvents, and pollutants), ultravio-
let irradiation of the skin, and ionizing radiation.
Among free radicals of biological importance are
the hydroxyl (HO
.
), peroxyl (ROO
.
), superoxide
(O
2
.
), and nitric oxide (NO
.
) radicals.
002 3 Many, or perhaps most, free radicals that are gen-
erated in the body are metabolized to nonradicals by
enzymes such as superoxide dismutase (EC 1.15.1.1),
which catalyzes the reduction of the superoxide rad-
ical to hydrogen peroxide and water (1).
O
:
2
þ O
:
2
þ 2H
þ
! H
2
O
2
þ O
2
:ð1 Þ
002 4 Although hydrogen peroxide is relatively unreact-
ive, it can traverse biological membranes and cause
intracellular damage through the production of hy-
droxyl radicals catalyzed by ferrous or cuprous
ions (2).
Fe
2þ
ðor Cu
þ
ÞþH
2
O
2
!Fe
3þ
ðor Cu
2þ
Þ
þ HO
:
þ OH
:ð2 Þ
002 5 Two enzymes remove hydrogen peroxide in mam-
malian cells: catalase (EC 1.11.1.6) (eqn (3)) and
glutathione peroxidase (EC 1.11.1.9) (eqn (4)),
whichuseshydrogenperoxidetooxidizereducedglu-
tathione(GSH)tooxidizedglutathione(GSSG).
2H
2
O
2
! 2H
2
O þ O
2
ð3 Þ
H
2
O
2
þ 2GSH ! GSSG þ 2H
2
O :ð4 Þ
002 6 Enzymes do not completely prevent the formation
of free radicals, and various secondary antioxidants
fulfill the role of free-radical scavengers. Ascorbate is
one such antioxidant within the aqueous environ-
ment of the cytosol and extracellular fluids, and at
the water–lipid interface of cellular and subcellular
membranes. Figure 6 shows a possible scheme in
which ascorbate can be recycled after trapping a free
radical. Ascorbate (AH
) donates an electron to the
free radical (R
.
), forming detoxified product (R), and
is itself oxidized to the ascorbyl free radical (A
.
), also
known as semidehydroascorbate. Pairs of ascorbyl
free radicals disproportionate to form one molecule
each of dehydroascorbic acid (A) and ascorbate. The
high disproportion rate constant of the ascorbyl free
radical prevents substantial interaction with other
substances. Dehydroascorbic acid can be reduced to
ascorbate by either a glutathione-dependent or an
NADPH-dependent reductase.
0027In the lipid environment of cellular and subcellular
membranes and plasma lipoproteins, vitamin E (toco-
pherol, TOH) protects the lipids from peroxidation
by reacting with peroxyl radicals, forming the toco-
pheroxyl radical (TO
.
) and lipid hydroperoxide
(ROOH), thereby terminating the chain reaction
(eqn (5)). The potentially harmful lipid hydroperox-
ide can be converted enzymatically to a fatty acid
alcohol. Like the ascorbl radical, the tocopheroxyl
radical has a low reactivity and can be converted
back to tocopherol by enzyme systems.
ROO
:
þ TOH ! ROOH þ TO
:
:ð5Þ
0028In model in-vitro systems, ascorbate regenerates
vitamin E by reducing the tocopheroxyl radical back
to tocopherol, producing the ascorbyl radical (eqn
(6). However, any vitamin E sparing effect exerted
by vitamin C in vivo is negligible in comparison
with other metabolic processes.
TO
:
þ AH
! TOH þ A
:
: ð6Þ
0029Ascorbate can exert pro-oxidant properties in vitro
through interaction with iron or copper ions and
A
−
.
A
−
.
AH
−
R
R
.
(a)
(d)
A
(b)
(c)
fig0006Figure 6 Recycling of ascorbate during the process of free
radical scavenging. AH
, ascorbate; A
.
, ascorbyl free radical
(semidehydroascorbate); A, dehydroascorbic acid; R
.
, free rad-
ical species; R, detoxified species. (a) Single-electron transfer;
(b) semidehydroascorbate reductase; (c) nonenzymatic dismuta-
tion of ascorbyl free radical; (d) dehydroascorbic acid reductase.
ASCORBIC ACID/Physiology 329
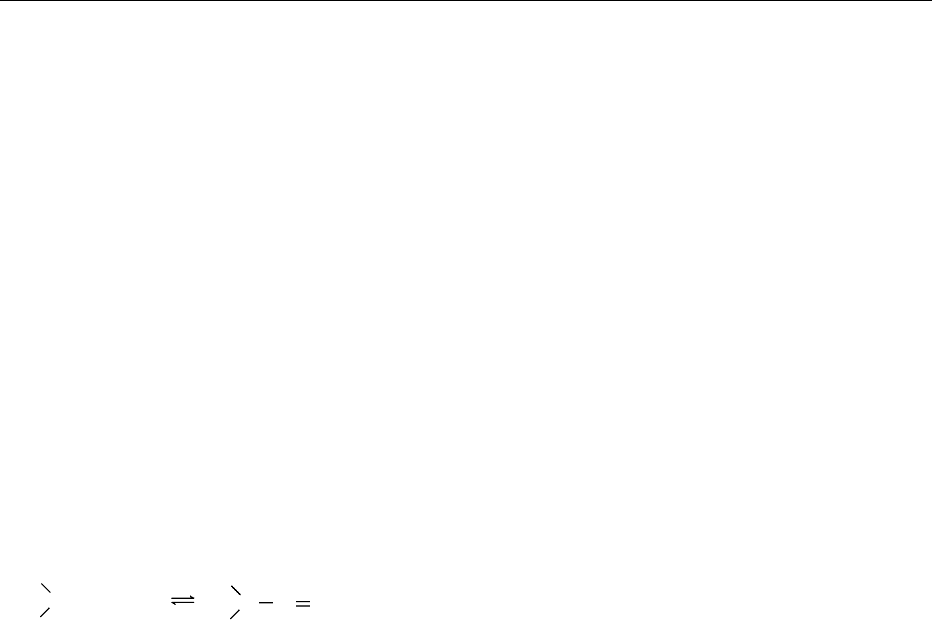
hydrogen peroxide to produce hydroxyl radicals. In
the healthy human body, these metal ions are largely
sequestered in forms unable to catalyze free radical
reactions, and so the pro-oxidant properties of ascor-
bate would not normally be expected to be biologic-
ally significant.
Inhibition of
N
-nitroso Compound
Formation
003 0 Nitrosating agents, for example nitrous anhydride
(N
2
O
3
), are derived from nitrite (eqns (7)–(9))
NO
2
þ H
þ
Ð HNO
2
ð7Þ
HNO
2
þ H
þ
Ð H
2
NO
þ
2
ð8Þ
H
2
NO
þ
2
þ NO
2
Ð N
2
O
3
þ H
2
O :ð9Þ
0031 In the acidic contents of the stomach, nitrosation
reactions take place between nitrosating agents and
secondary amines or N-substituted amides to form N-
nitrosamines (eqn (10)) or N-nitrosamides, collect-
ively called N-nitroso compounds.
NH + N
2
O
3(10)
R
1
R
2
N
N
O + HNO
2
.
R
1
R
2
0032 N-Nitroso compounds are implicated in cancer of
the stomach, esophagus, and nasopharynx. Nitrite is
present naturally in some foods and is added as a
preservative in cured meats. Nitrosatable amines
and amides may be ingested as drugs, food additives,
or natural constituents of foods. Ascorbic acid is
added to the curing brine in the production of bacon
and other cured meats in order to prevent the subse-
quent formation of N-nitroso compounds in the
stomach. Presumably, the vitamin acts by reducing
nitrous anhydride to nitric oxide (eqn (11)), which is
not a nitrosating agent.
AH
þ N
2
O
3
Ð A þ 2NO þ H
2
O :ð11Þ
0033 For ascorbic acid to be effective, it must be present
in sufficient quantity, since nitric oxide can be oxi-
dized to nitrogen dioxide, and these two species can
combine to form nitrous anhydride (eqn (12)).
NO þ NO
2
Ð N
2
O
3
:ð12Þ
Immune Function
003 4 Vitamin C possesses antihistamine activity and is im-
plicated in a variety of immune functions, including
neutrophil chemotaxis, T lymphocyte proliferation,
natural killer cell activity, and activation of the com-
plement component C1q. Neutrophils and lympho-
cytes (types of leukocytes) concentrate vitamin C
at levels up to 100 times higher than in plasma,
suggesting that the vitamin has a physiological role
in these immune-system cells.
Antihistamine Activity
0035In the initial stages of an immune response, histamine
amplifies immunoresponsiveness by increasing capil-
lary permeability and smooth muscle contraction,
enhancing the flow of immune factors to the site of
injury or infection. Subsequently, histamine sup-
presses the immune response by inhibiting neutrophil
lysosomal enzyme release. Excess histamine has a
negative effect, and antihistamine therapy is well
known for alleviating conditions such as hay fever
and inflammatory skin disorders. Ascorbic acid de-
grades histamine in vitro. In humans, chronic oral
administration of vitamin C results in a lowering of
blood histamine levels, whereas low blood levels of
ascorbate are associated with increased histamine
levels.
Neutrophil Chemotaxis
0036Neutrophils (polymorphonuclear leukocytes) destroy
pathogenic organisms by phagocytosis (engulfment)
and intracellular enzymatic degradation. If foreign
materials are too large to be engulfed, enzymes and
reactive oxygen species are released extracellularly.
Neutrophil chemotaxis refers to the response of
neutrophils to chemical signals produced at sites of
infection. Histamine lowers the chemotactic respon-
siveness of neutrophils by elevating intracellular
levels of cyclic adenosine 3
0
,5
0
-monophosphate (cyclic
AMP). A rise in cyclic AMP is associated with de-
pressed neutrophil motility. Data from a study using
healthy human subjects suggest that vitamin C at
doses of 2 g per day for 2 weeks may indirectly en-
hance neutrophil chemotaxis through its antihista-
mine activity.
Proliferation of T Lymphocytes
0037Vitamin C regulates proliferation of T lymphocytes,
which are responsible for destroying viruses that
reside and multiply inside living cells of the body.
Specialized types of T lymphocyte exert immuno-
regulatory and effector functions through the secre-
tion of lymphokines such as interferon. Cytotoxic
T lymphocytes kill viruses by attaching to the infected
cell through recognition of viral antigens and releas-
ing granules whose contents cause perforation of the
host cell membrane. The granules also contain sub-
stances that induce apoptosis – genetically pro-
grammed cell death by fragmentation of nuclear
DNA. Since death by apoptosis does not result in
the release of the cell’s contents, killing by this mech-
anism may prevent the spread of infectious virus into
other cells.
330 ASCORBIC ACID/Physiology

Natural Killer Cell Activity
0038 Natural killer cells are large nonphagocytic leuko-
cytes that kill tumor cells by cell membrane perfor-
ation and apoptosis. The cytotoxic activity of killer
cells is spontaneous, unlike that of cytotoxic
T lymphocytes, which only develops after an immune
response has been initiated. An in-vivo effect of as-
corbic acid on enhancement of human natural killer
cell activity has been reported at a dosage of 60 mg
per kilogram of body weight.
Regulation of the Complement Component C1q
0039 Complement is a collective term for at least 25
plasma and cell membrane proteins that are import-
ant in the host’s immune system. Following initial
activation by antigen–antibody complexes, the vari-
ous complement components interact, in a highly
regulated enzymatic cascade, to generate various
reaction products. Some of these products induce
localized vasodilation and attract phagocytic cells
chemotactically. Other products coat the foreign
particle, enabling it to be recognized by phagocytic
cells that bear receptors for these complement prod-
ucts. Finally, the terminal components of the comple-
ment system generate the membrane–attack complex,
which lyses bacteria and membrane-enveloped
viruses.
0040 The first complement component to be activated in
the classical cascade pathway is the C1 complex, in
which C1q is one of three proteins. When guinea-pigs
were fed tissue-saturating amounts of vitamin C, C1q
concentrations were significantly higher than in those
animals fed only enough ascorbate for adequate
growth and for the prevention of scurvy. C1q is a
hydroxyproline-containing protein, and so the ob-
served effect is consistent with the known role of
vitamin C in hydroxyproline synthesis.
Prostaglandin Synthesis
0041 The prostaglandins (PG) are a group of hormone-like
lipids formed in the body from derivatives of essential
fatty acids, particularly arachidonic acid (20:4n-6).
Prostaglandins have a vast range of effects and
modulate cardiovascular, pulmonary, immune, and
reproductive functions. Vitamin C stimulates the for-
mation of PGE
1
from dihomo-g-linolenic acid in
human platelets. This effect occurs over the physio-
logical range of vitamin C concentrations and is de-
sirable because PGE
1
is an inhibitor of platelet
aggregation. PGE
1
is also required for T lymphocyte
formation, regulation of collagen and cholesterol me-
tabolism, and regulation of responsiveness to insulin.
There are sufficient data to suggest that some of
vitamin C’s biological actions could be explained by
a regulatory effect on prostaglandin synthesis.
Dietary Intake and Supplementation
Recommended Dietary Allowance (RDA)
0042In the USA, the 1989 RDA for vitamin C is 60 mg per
day for adults. At this level, saturation of tissue bind-
ing and maximal rates of metabolic and renal tubular
absorption seem to be approached. The RDA of
100 mg per day for smokers is higher because the
plasma concentration of vitamin C is lowered by the
use of cigarettes. Plasma ascorbate levels of women
decrease during pregnancy, and the RDA includes a
10 mg per day increment for pregnant women. A
daily increment of 35 mg is recommended during the
first 6 months of lactation and 30 mg thereafter. As-
sessments of human vitamin C status are based upon
measurement of ascorbate concentrations in plasma
and leukocytes.
Deficiency
0043Scurvy, the classical vitamin C deficiency disease, can
occur under circumstances of poor diet, such as arises
in chronic alcoholism.
Toxicity
0044Ascorbic acid is generally regarded as being nontoxic
at high intakes. Once the plasma concentration of
ascorbate reaches the renal threshold, it is excreted
more or less quantitatively with increasing intake.
Excessive daily intakes of vitamin C can cause an
increased production of oxalic acid in some individ-
uals, leading to an increased risk of kidney stone
formation.
Rebound Scurvy
0045Theoretically, the absorption of ascorbic acid could
be impaired on resumption of normal vitamin C
inputs following megadosing (> 1 g per day), because
of insufficient carriers in the enterocyte cell mem-
branes. Based on experiments with guinea-pigs, it is
considered likely that, in humans, renewed synthesis
of carriers will take place well before the onset of
scurvy. During megadosing, reduced ascorbate ab-
sorption is accompanied by increased rates of ascor-
bate catabolism. In adult guinea-pigs, the accelerated
catabolism is not reversible after more than 2 months
on subnormal uptake of ascorbate. Guinea-pigs are
thus susceptible to a systemic conditioning effect
known as rebound scurvy, caused by an induction of
ascorbic acid-metabolizing enzymes by high dietary
vitamin C. The body stores of vitamin C are depleted
more rapidly in juvenile guinea-pigs than in adults,
ASCORBIC ACID/Physiology 331

increasing the likelihood of rebound scurvy in juven-
iles. In human infants, rebound scurvy could possibly
occur, despite an adequate daily intake of vitamin C,
if the mother has received large supplements of the
vitamin during pregnancy. Reported cases of rebound
scurvy in adult humans are rare.
Treatment of the Common Cold
0046 Vitamin C supplementation has been suggested for
the prevention of the common cold and the allevi-
ation of its symptoms. Reviews of numerous studies
generally conclude that vitamin C megadoses have no
consistent effect on reducing the incidence of colds in
people in general. However, in four studies with Brit-
ish male schoolchildren and students, a statistically
high significant reduction in common cold incidence
was found in vitamin C-supplemented groups. This
could be interpreted as a correction of a marginal
deficiency in the study subjects, rather than an effect
of the high dosage. Placebo-controlled studies have
consistently shown that high doses of vitamin C alle-
viate common cold symptoms, although the validity
of some of these studies has been questioned.
See also: Adaptation – Nutritional Aspects;
Antioxidants: Natural Antioxidants; Bioavailability of
Nutrients; Cancer: Diet in Cancer Prevention; Dietary
Reference Values; Immunology of Food;
Nitrosamines; Prostaglandins and Leukotrienes;
Scurvy
Further Reading
Hallberg L, Brune M and Rossander L (1986) Effect of
ascorbic acid on iron absorption from different types
of meals. Human Nutrition: Applied Nutrition 40A:
97–113.
Halliwell B (1996) Vitamin C: antioxidant or pro-oxidant
in vivo? Free Radical Research 25: 439–454.
Harris JR (ed.) (1996) Subcellular Biochemistry. Vol. 25.
Ascorbic Acid: Biochemistry and Biomedical Cell Biol-
ogy. New York: Plenum Press.
Hemila
¨
H (1997) Vitamin C intake and susceptibility to the
common cold. British Journal of Nutrition 77: 59–72.
Institute of Medicine (2000) Dietary Reference Intakes for
Vitamin C, Vitamin E, Selenium, and Carotenoids.
Washington, DC: National Academy Press.
Jacob RA (1999) Vitamin C. In: Shils ME, Olson JA, Shike
M and Ross AC (eds) Modern Nutrition in Health and
Disease, 9th edn. Philadelphia, PA: Lippincott Williams
& Wilkins.
Johnston CS, Martin LJ and Cai X (1992) Antihistamine
effect of supplemental ascorbic acid and neutrophil
chemotaxis. Journal of the American College of Nutri-
tion 11: 172–176.
Karasov WH, Darken BW and Bottum MC (1991) Dietary
regulation of intestinal ascorbate uptake in guinea pigs.
American Journal of Physiology 260: G108–118.
Packer L and Fuchs J (eds) (1997) Vitamin C in Health and
Disease. New York: Marcel Dekker.
Siegel BV (1993) Vitamin C and the immune response in
health and disease. In: Klurfeld DM (ed.) Nutrition and
Immunology Series. Human Nutrition: A Comprehen-
sive Treatise. New York: Plenum Press.
ASPARTAME
M B A Glo
´
ria, Federal University of Minas Gerais,
Belo Horizonte, Minas Gerais, Brazil
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Aspartame is an intense nutritive sweetener dis-
covered accidentally in 1965 by the chemist James
Schlatter from G.D. Searle and Co. It was intro-
duced in 1981 and has been assigned the INS number
of 951. At present, it is available under the brand
names of Nutrasweet
1
, Equal
1
, and Canderel
1
.
Aspartame is a caloric substance because it is a
dipeptide that is completely digested after consump-
tion. However, its intense sweetness allows function-
ality to be achieved at very low levels, providing very
few calories.
0002Aspartame has been approved for food, beverage,
pharmaceutical, and tabletop sweetener use in more
than 100 countries. Currently, it is the most widely
consumed high-intensity sweetener. It is used in ap-
proximately 6000 different products and consumed
by hundreds of millions of people in countries around
the world.
0003The synthesis of aspartame can be performed by
chemical or chemoenzymatic methods. There are
over 70 patents on the manufacturing process. It can
be made from pure amino acids and is not extracted
from any food, and so it is kosher. It is also parve,
which means that it contains neither dairy nor meat
products. Although kosher and parve, other ingredi-
ents in a product containing aspartame must be
checked for their kosher status. Aspartame sold for
commercial use must meet all requirements of the
Food Chemical Codex.
332 ASPARTAME

Physical and Chemical Characteristics
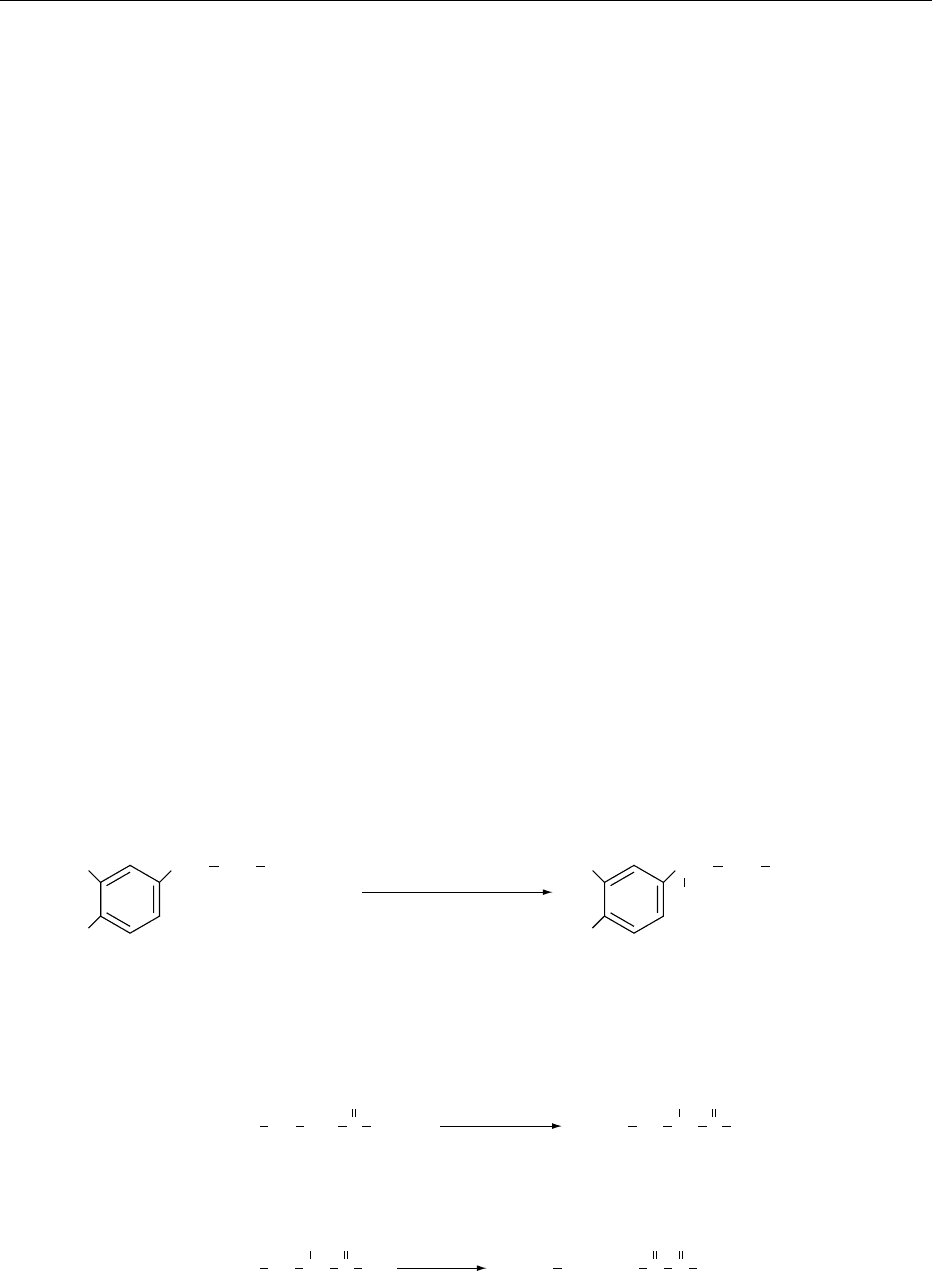
0004 Aspartame is a dipeptide composed of two amino
acids, l-aspartic acid and l-phenylalanine methyl
ester. Its chemical structure is shown in Figure 1.
Chemically, aspartame is N-l-a-aspartyl-l-phenyl-
alanine methyl ester or 3-amino-N-(a-carboxy-
phenethyl)succinamic acid N-methyl ester. It has a
molecular formula of C
14
H
18
O
5
N
2
and a molecular
weight of 294.30. It is a white, odorless, crystalline
powder, with a melting point of 246–247
C and
[a]
22
d
¼ 2.3
(1 mol per liter of HCl).
0005 Aspartame is slightly soluble in water (about 1.0%
at 25
C), sparingly soluble in alcohol, and insoluble
in fats and oils. The solubility in water is affected by
temperature and pH. It increases as the pH is lowered
and the temperature is increased.
Sensory Characteristics
0006 Aspartame is described as having a sweet, clean taste
and sweetness profile similar to that of sucrose, with-
out any bitter or metallic aftertaste often associated
with other high-intensity sweeteners. At concentra-
tions of 0.10–0.89 g l
1
, aspartame has no off-tastes.
However, it displays a slow onset of sweetness
coupled with a lingering sweet taste.
0007 Aspartame has 100–200 times the sweetness of
sucrose, depending on the food system. For example,
its sweetness potency in flavored yogurt is 175–220
times that of sucrose. The relative sweetness is also
affected by pH and the amount of sucrose or other
sugars being replaced.
0008 The perception of aspartame sweetness increases
linearly with its concentration. However, increasing
the ethanol content causes a reduction in the per-
ceived sweetness.
0009 Aspartame extends and intensifies flavors, particu-
larly enhancing fruit flavors, such as orange, lemon,
grapefruit, cherry, and strawberry. This flavor-enhan-
cing property, as observed with chewing gum, pro-
duces a longer-lasting sweetness and flavor up to four
times longer. Such a characteristic is important in
many food applications.
0010Aspartame exhibits synergism, a superior taste pro-
file, and an improved stability when combined with
other sweeteners, such as saccharin, cyclamate, ace-
sulfame-K, and stevioside. The flavor-enhancement
quality of aspartame masks bitter flavors, even at
subsweetening levels. Its use is thus recommended
in blends with sweeteners that possess potentially
undesirable or more complex taste profile.
Food and Beverage Applications
0011Aspartame provides many opportunities for formu-
lating new products while lowering or limiting cal-
ories and sugar consumption. Other benefits of using
aspartame are cost reductions in packaging, shipping,
and storage. Furthermore, aspartame is generally
cheaper than powdered sugar. It has a lower cost per
unit sweetness than sucrose.
0012Aspartame is approved for use as a prepackaged
sugar substitute tablet. Its use as a sweetener and
flavor enhancer is also approved. It has a potential
for a wide range of food applications, including
carbonated soft drinks, fruit drinks, dry beverage
bases, instant tea, hot chocolate, gelatin, puddings,
fillings, yogurt, icecream, frozen novelty, hard candy,
confectionery, breath mint, breakfast cereals, chewing
gums, dairy products, and toppings.
0013Aspartame is widely used both as a dry powder and
as an aqueous solution. The largest application is in
soft drinks followed by dry mix products. The main
products in the dry mix market are sweetener tablets
and sachets, cold beverage mixes, and instant des-
serts. In these products, aspartame’s sweetness should
be released rapidly and evenly throughout the prod-
uct for maximum performance. In order to insure
uniformity of the final product, stability against seg-
regation, and proper reconstitution upon prepar-
ation, agglomeration processes are used either as a
re-wet or a straight through process (the dry mix is
dissolved and spray-dried). Another way to overcome
this problem would be by coating food ingredients
such as citric, fumaric, and lactic acids, sucrose, dex-
trose, fructose, oligo-fructose, and maltodextrin with
fine-grade aspartame.
0014Blends or combinations of sweeteners are often
used to achieve the desired level of sweetness in food
and beverage products that traditionally have been
sweetened with single sweeteners. In the USA, almost
every major soft-drink manufacturer now uses a com-
bination of aspartame and saccharin in diet drinks.
The blending of these two noncarbohydrate sweeten-
ers reduces the cost and helps to prolong the shelf-life
of the beverages compared with aspartame alone.
C
14
H
18
N
2
O
5
(FW 294.30)
N-
L-aspartyl-L-phenylalanine-1-methyl ester
ASPARTAME
N
O
HNH
2
COOCH
3
CH
2
COOH
fig0001 Figure 1 Chemical structure of aspartame.
ASPARTAME 333
