Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Aroma See Sensory Evaluation: Sensory Characteristics of Human Foods; Food Acceptability and Sensory
Evaluation; Practical Considerations; Sensory Difference Testing; Sensory Rating and Scoring Methods;
Descriptive Analysis; Appearance; Texture; Aroma; Taste
Aroma Compounds See Flavor (Flavour) Compounds: Structures and Characteristics; Production
Methods
ARSENIC
Contents
Properties and Determination
Requirements and Toxicology
Properties and Determination
N Hata, I Kasahara, S Taguchi and K Goto, Toyama
University, Toyama, Japan
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Arsenic is located in group 5A of the periodic table
and has an atomic number of 33 with an atomic
weight of 74.922. The electron configuration is
(1s)
2
(2s)
2
(2p)
6
(3s)
2
(3p)
6
(3d)
10
(4s)
2
(4p)
3
.
Chemical Properties
0002 The structures of some of several important arsenic
compounds and their acid dissociation constants
(pK
a
) are shown in Figure 1.
0003 Inorganic arsenic (As) occurs in four oxidation
states, As(V) as in arsenate (AsO
3
4
), As(III) as in
arsenite (AsO
3
3
), As(0), and As(-III) as in arsine
(AsH
3
). In aerated water, arsenate is a stable form.
Since As(III) is considerably more toxic than As(V),
the form in which arsenic exists, whether as arsenate
or arsenite, is important. Doses of 70–180 mg of
arsenic trioxide (As
2
O
3
) are fatal. Arsenic acid is a
fairly strong acid, while arsenous acid behaves in
water practically as a weak, monobasic acid (see
Figure 1).
0004Arsenic forms a variety of organoarsenic com-
pounds. Arsenic compounds of the types, R
3
As,
RAs(OH)
2
,R
2
AsOH, RAsO(OH)
2
,R
2
AsO(OH),
and R
4
As
þ
X
are called arsines, arsenous acids, arsi-
nous acids, arsonic acids, arsinic acids, and arsonium
salts, respectively. It is believed that organoarsenic
compounds are much less toxic than the inorganic
arsenic compounds. Biochemical conversion of inor-
ganic arsenic compounds to organoarsenic com-
pounds has been known for more than 100 years.
Occurrence
0005Arsenic is distributed everywhere in the environment.
The use of arsenic is decreasing gradually but is still
important. Commercial elemental arsenic is mainly
employed as an additive for alloys. High-purity ar-
senic (at least 99.999%) is employed in electronics as
gallium arsenic (GaAs) or indium arsenic (InAs). Fur-
thermore, arsenic has been used in wood preserva-
tion, medical supplies (Salvarsan), pigment (Scheele’s
Green: CuHAsO
4
, Paris’s Green: 3Cu(A-
sO
2
)
2
Cu(CH
3
COO)
2
), agricultural chemicals, food
additives (arsanilic acid, H
2
NC
6
H
4
AsO(OH)
2
), and
poison gas (lewisite). British anti-lewisite (BAL),
which was developed as an antidote for lewisite, is
also one of the antidotes for heavy metal poisoning.
0006Arsenic occurs naturally in the earth’s crust (1.5–
2mgkg
1
). Hot-spring and well waters sometimes
contain appreciable quantities of arsenic, mostly in
304 ARSENIC/Properties and Determination
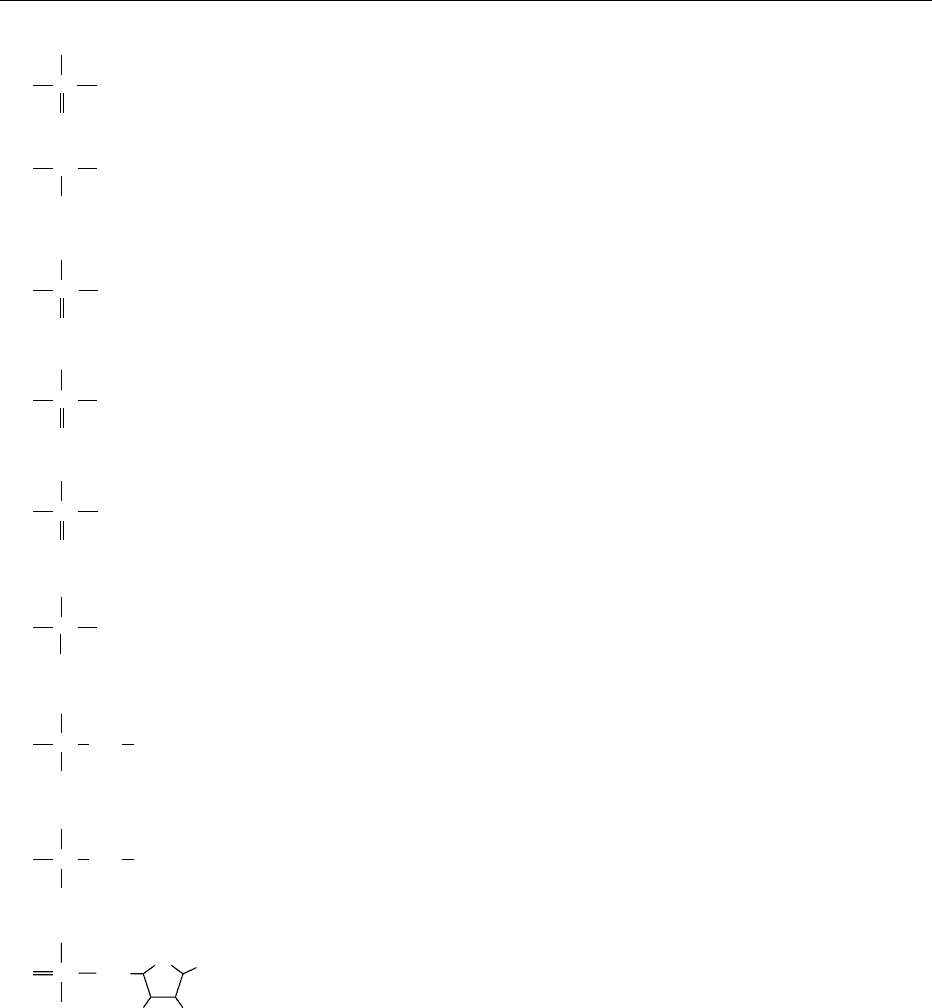
the form of arsenate. Arsenic is associated with sulfide
ores. Waste water discharged from sulfide mines
and ore-dressing plants often contains arsenic in
appreciable quantities. Arsenic contamination caused
by flue gases from lead and copper smelters has been
frequently reported. Most authorities adopt a value of
10 mgl
1
, measured as total arsenic, as the maximum
permissible concentration in drinking water.
0007The arsenic levels in ocean water are around
2 mgl
1
, and although arsenate is the stable form
in aerated waters, ocean water sometimes contains
appreciable proportions of arsenite. It is believed that
some bacteria are able to reduce arsenate to arsenite.
0008Because arsenic compounds have been used as
insecticides, herbicides, and animal-feed additives,
some soils, vegetation, swine, and poultry may be
contaminated with arsenic. The value of 1 mg kg
1
as As
2
O
3
has been the maximum allowable limit for
fruits and vegetables in Japan. Soil containing more
than 15 mg of arsenic per kilogram is considered
unsuitable for agriculture.
0009Edible marine organisms, such as lobsters, shrimps,
and brown kelps, contain considerable concentra-
tions of arsenic frequently in the mg kg
1
range, oc-
casionally at concentrations as high as several tens of
mg kg
1
on a wet-weight basis. Fortunately, however,
the arsenic is mostly in the form of organoarsenic
compounds, which are rapidly excreted and therefore
relatively nontoxic to humans. On average, about
80% of the arsenic in marine organisms is found in
the water-soluble fraction. Mostly, arsenobetaine
(AsB) is the predominant arsenic species in marine
animals, with arsenosugars in marine algae and
some marine bivalves. Concentrations in freshwater
plants and animals, and terrestrials are usually lower
than in marine species. Arsenic has been found in
mushrooms (Agaricus bisporus): As(III), 0.07
mg kg
1
; As(V), 0.14 mg kg
1
; monomethylarsonic
acid, 0.05 mg kg
1
; dimethylarsinic acid, 0.28 mg
kg
1
; and AsB, 0.46 mg kg
1
.
0010In 1900, there was an outbreak of arsenic
poisoning among beer-drinkers in England. The
cause of poisoning was traced to the use of invert
sugar produced using arsenic-contaminated sulfuric
acid. In 1955, there was an outbreak of unusual
disease and fatalities in Okayama and Hiroshima
Prefectures in Japan among infants drinking arsenic-
contaminated milk. The arsenic content of the
powdered milk that caused this disaster was esti-
mated to be 15–24 mg kg
1
as As. The dose of arsenic
in the infants was estimated to be 1.3–3.6 mg day
1
and the source of arsenic was traced to arsenic-
contaminated sodium phosphate added as a stabilizer.
The sodium phosphate had been produced from
waste bauxite processing liquor and renamed by
resale, but it contained about 3% arsenic.
0011Recently, a large number of fatal arsenic intoxica-
tions were traced from drinking water contaminated
OH
As
HO OH
O
As
HO OH
OH
As
H
3
COH
O
OH
As
H
3
COH
O
CH
3
As
+
H
3
COH
CH
3
CH
3
As
+
H
3
CCH
3
CH
3
CH
3
As
+
H
3
C
CH
3
CH
3
As
+
H
3
C
CH
3
CH
3
CH
2
COOH
CH
2
CH
2
OH
As
+
OCH
2
CH
3
CH
3
O
HO
OH
OCH
2
CH(OH)CH
2
R
(a) Arsenic acid
pK
1
= 9.2
pK
2
= 12.1
pK
3
= 13.4
(b) Arsenous acid
pK
1
= 2.3
pK
2
= 6.8
pK
3
= 11.6
(c) Monomethylarsonic acid,
MMAA (methanearsonic acid)
pK
1
= 3.6
pK
2
= 8.2
(d) Dimethylarsonic acid,
DMAA (cacodylic acid)
pK
n
= 6.2
(g) Arsenobetaine, AsB
(h) Arsenocholine, AsC
( i ) Arsenosugars
pK
a
= 2.2
(e) Trimethylarsine oxide,
TMAO
( f ) Tetramethylarsonium ion
pK
a
= 3.6
(as (CH
3
)
3
As
+
OH)
fig0001 Figure 1 Structure of some important arsenic compounds
and their acid dissociation constants. (i1) R ¼ -SO
3
H, 2-hydroxy-
3-sulfopropyl-5-deoxy-5-(dimethylarsinoyl)-b-ribofranoside; (i2)
R ¼-OH, 2,3-dihydroxypropyl-5-deoxy-5-(dimethylarsinoyl)-b-
ribofranoside; (i3) R ¼-OPO
3
HCH
2
CH(OH)CH
2
OH, 3-‘glyceropho-
sphoryl’-2-hydroxy-1-[5-deoxy-5-(dimethylarsinoyl)-b-ribofrano-
syloxy] propane; (i4) R ¼-OSO
3
H, (2S)-3-[-5-deoxy-5-
(dimethylarsinoyl)-b-
D-ribofranosyloxy]-2-hydroxypropyl hydro-
gen sulfate.
ARSENIC/Properties and Determination 305

by inorganic arsenic species. The main sources of
arsenic contamination of drinking water are natu-
ral, and high concentrations of arsenic in drinking
water are found in various parts of the world. In
Taiwan, patients with blackfoot disease are found in
areas where drinking water contains arsenic. In Ban-
gladesh and West Bengal in India, arsenic poisoning
became evident in the 1980s, and tens of millions of
people are estimated to be at risk. The survey is not
complete because the survey area is so large. The
source of arsenic is geological.
Speciation of Arsenic in Food and Water
0012 Various arsenic species show large differences in
their toxicity. Their acute toxicity decreases in the
following order: As(-III) (AsH
3
) >> arsenite
{As(III)} > arsenate {As(V)} >> dimethylarsinate > mo-
nomethylarsonate >> arsenobetaine and arsenocho-
line (organoarsenic compounds in seafoods).
Therefore, speciation of arsenic is required for reli-
able toxicological estimation. Arsenic in seafoods has
been well studied and is mostly in the form of orga-
noarsenic compounds, including arsenobetaines and
arsenosugars.
0013 Extraction and purification are needed, especially
for speciation of arsenic. For the speciation of orga-
noarsenic compounds in seafoods, the samples are
usually extracted with methanol or a methanol–
water mixture. The samples are then purified with
aC
18
cartridge, centrifugation, and/or filtration.
The purified sample is subjected to gel-permeation
chromatography, ion-pair chromatography, ion-
exchange chromatography, and preparative thin-layer
chromatography. Buffered ion-exchange chromatog-
raphy, close to neutrality, is necessary to prevent de-
composition of the compounds to dimethylarsinic
acid occurring at extremes of pH.
0014 Arsenic compounds extracted with methanol from
sea animals (cockle, trough shell, tuna, crab, sea cu-
cumber, squid, etc.) have been separated by liquid
chromatography. No single column has been found
to be satisfactory for separating all organoarsenic
compounds present in the seafoods. Four compounds
(Figure 1f, g, i2, i3 )havebeenfoundinanimals.
Arsenobetaine (Figure 1g) was found in all sea
animals tested. There is considerable evidence that
arsenocholine and arsenobetaine are rapidly excreted
and almost nontoxic to humans.
0015 Van Elteren and S
ˇ
lejekovec investigated the stabil-
ity of several arsenic compounds in food treatment
procedures. Under usual food treatments (micro-
wave treatment of 300 W of power for 120 min
and boiling on a hot plate for 100 min), aqueous
solutions of monomethylarsonic acid (MMAA),
dimethylarsinic acid (DMAA), arsenobetaine (AsB),
and tetramethylarsonium ion remained stable. Using
g-irradiation treatment with a dose of 10 kGy, 5% of
AsB decomposed to trimethylarsineoxide (TMAO),
and 1.5% of MMAA and 1% of DMAA decom-
posed to inorganic arsenic. After dry heating for
30 min at 160
C, 10% of AsB decomposed to
TMAO, 9% of MMAA to As(III), 6% of DMAA
to MMAA, and tetramethylarsonium ions remained
stable. Under unusually harsh treatments, however,
decomposition of some compounds was found.
Sample Preparation
0016To determine total arsenic, the sample must be
brought into solution. Care must be taken during
the destruction of the sample to ensure that no arsenic
is lost by vaporization of trivalent arsenic halides.
Loss can usually be avoided by oxidizing the arsenic
at an earlier stage by boiling with nitric acid under
reflux.
0017In determining arsenic at trace levels in food, the
Association of Official Analytical Chemists (AOAC;
Article 9.1.01 D) recommends that a sample be
digested with HNO
3
(heated in an oven at 150
C
oven for 2 h) in a closed system. In the closed system,
a sample is placed in a digestion vessel (or cylinder),
usually constructed of a fluorinated polymer such as
polytetrafluoroethylene (PTFE) or perfluoroalkoxy
(PFA). After the addition of digestion reagents, the
cylinder is tightly sealed. The microwave-assisted
alkali digestion with tetramethylammonium hydrox-
ide ((CH
3
)
4
NOH, TMAH), which is used as a tissue
solubilizer, is useful for the pretreatment of various
biological samples prior to analysis. A mixture of
nitric, sulfuric, and perchloric acids is also used for
wet ashing of food samples. Monomethylarsonate,
dimethylarsinate, arsenobetaine, and phenylarsonate
are decomposed to inorganic arsenate. Dry ashing at
600
C with magnesium nitrate for the destruction of
meat and poultry is also used. The ash is dissolved in
dilute hydrochloric acid.
0018To examine arsenic contamination of soils in
Japan, arsenic is extracted by shaking the soil with
1 M hydrochloric acid (50 ml per 10 g of sample) for
30 min at 30
C. The mixture is filtered through dry
paper and subjected to hydride generation atomic
absorption spectrometry.
0019Low levels of arsenic in water can be enriched by
coprecipitation with ferric hydroxide after being
oxidized to the pentavalent state with potassium
permanganate. Triton X-100 has been used to aid
the formation of large precipitates. It is claimed that
both arsenate and arsenite are coprecipitated with
iron hydroxide.
306 ARSENIC/Properties and Determination
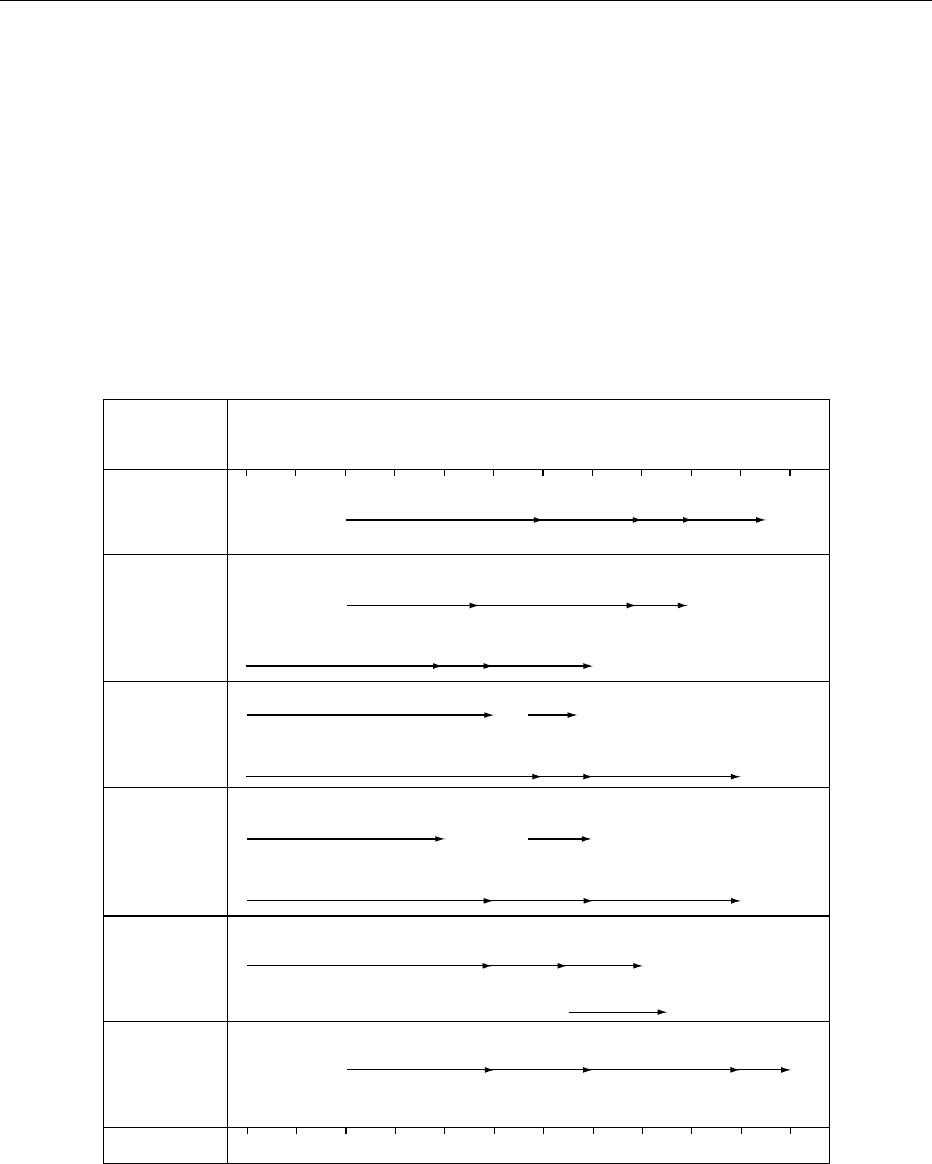
Methods of Analysis
0020 There are several methods available for the determin-
ation of low levels of arsenic, and various analytical
methods for determining arsenic in various concen-
trations are shown in Figure 2.
High-performance Liquid chromatography
0021 High-performance liquid chromatography (HPLC)
separation is most frequently employed for the speci-
ation of arsenic species. Gas chromatography (GC)
separation can also be used but requires compli-
cated analyte derivatization steps to form volatile
derivatives. Ion-pair chromatography in reverse
mode, ion-exchange chromatography, and gel-perme-
ation chromatography are often used. In reverse
mode, C
18
columns and polymer resin columns such
as Hamilton polymer reversed-phase (PRP) are com-
monly used. Ion-pair chromatography is based on the
distribution of ion-pairs between the aqueous mobile
phase and the hydrophobic stationary phase. A qua-
ternary ammonium ion such as tetrabutylammonium
ion is often adopted for the separation of the anionic
species. An alkyl sulfonate such as hexanesulfonate is
often adopted for the separation of cationic species.
Detection can be by UV absorption, conductivity, or
Species
As
(Liquid sample)
Flame AAS
XRF
GDS
Redox titration
Coulometric iodimetry
Magnesium ammonium
arsenate gravimetry
Molybdenum blue (Sp)
Iodimetry
TCD
HPLC/Flame-AAS HPLC/ICP-AES HPLC/ICP-MS
Cold trap/Fractional
volatilization/DC-AES
FPD
TC sensor
Controlled-potential electrolysis
Ozone chemiluminescence
HPLC/Conductivity detector HPLC/ICP-MS
HPLC/ICP-AES HPLC/ICP-MS
Ag-DDC (Sp)
(depth resolution: 0.01 µm) (space: 0.1 µm) (space: 0.1 µm, depth: 0.005 µm)
EPMA SIMS
GD-MS
Radio activation analysis
(sample several mg)
ICP-AES HG-AAS
GF-AAS
ICP-MS
Organoarsenic
compound
(Inorganic
arsenic
compound)
As
(Solid sample)
AsO
3−
AsO
3−
AsH
3
Analytical method (%)
10
2
10
1
10
0
10
−1
10
−2
10
−3
10
−4
10
−5
10
−6
10
−7
10
−8
10
−9
10
2
10
1
10
0
10
−1
10
−2
10
−3
10
−4
10
−5
10
−6
10
−7
10
−8
10
−9
4
3
fig0002 Figure 2 Analytical method for arsenic in various concentrations. From Mochizuki T (1993) Table of determination methods in
seventeen elements for various concentrations (in Japanese). Bunseki: 262 with permission. AAS, Atomic absorption spectrometry;
Ag-DDC, silver diethyldithiocarbamate; DC-AES, direct current ‘discharge’ atomic emission spectrometry; EPMA, electron probe
microanalysis; FPD, flame photometric detector; GD-MS, glow discharge mass spectrometry; GDS, glow discharge emission spec-
trometry; GF-AAS, graphite furnace atomic absorption spectrometry; HG-AAS, hydride generation atomic absorption spectrometry;
HPLC, high-performance liquid chromatography; ICP-AES, inductively coupled plasma atomic emission spectrometry; ICP-MS,
inductively coupled plasma mass spectrometry; SIMS, secondary-ion mass spectrometry; Sp, spectrophotometry; TCD, thermal
conductivity detector; XRF, X-ray fluorescence.
ARSENIC/Properties and Determination 307

hyphenated techniques (atomic absorption and mass
spectrometry).
Hydride Generation Method
0022 There are two approaches for hydride generation
(HG). The first is a selective HG for speciation
followed by detector and is limited to the determin-
ation of the hydride-forming arsenic species. Differ-
entiation of several arsenic species is possible in the
HG technique with sodium borohydride as a reduc-
tant. Inorganic arsenic, monomethylarsonic acid, and
dimethylarsinic acid are reduced, in acid solution, to
arsine, monomethylarsine, and dimethylarsine,
respectively, and collected in a cold trap. Differenti-
ation of these arsenic compounds is possible by frac-
tional volatilization of the arsines collected in the cold
trap, because there are great differences in boil-
ing point between arsine (AsH
3
, 55
C), mono-
methylarsine (CH
3
AsH
2
,2
C) and dimethylarsine
((CH
3
)
2
AsH, 36
C). The choice of a proper packing
material in the cold trap is important for the mutual
separation of these arsines. A silanized diatomaceous
earth impregnated with phenylmethylsilicon (15%)
or hydrofluoric-acid-etched glass beads (0.3 mm) has
been used. Arsenite (As(III)) (pH 6) and arsenate
(As(V)) (pH 1) can be separated by pH-selective
HG. Hydride generation for differentiation of arsenic
species is becoming obsolete because very stable
organoarsenic compounds such as arsenobetaine
cannot form arsine.
0023 The second removes total arsenic from the matrix
and is generally combined with HPLC followed by
decomposition (microwave digestion or UV decom-
position) as a postcolumn prior to detector. The gen-
erated arsines are often concentrated by introduction
to a cold trap before detection. The HG technique is
used in combination with many different detection
systems, including atomic absorption spectrometry
(flame, quartz furnace, and graphite furnace), atomic
emission spectrometry, mass spectrometry, atomic
fluorescence spectrometry, spectrophotometry, and
colorimetry. The HG technique is incorporated into
the Gutzeit method and silver diethyldithiocarbamate
(AgDDC) method.
0024 Arsenic is reduced in acid solution by zinc or
sodium borohydride to form arsine (AsH
3
), which is
volatile and is stripped by the hydrogen generated
during the reduction; it can be collected by a trap
cooled in liquid nitrogen or by a dilute iodine or
bromine solution. With zinc as the reductant, prior
reduction of arsenic to arsenite is necessary for the
conversion of arsenate into arsine. A mixture of po-
tassium iodide and tin(II) chloride also helps the re-
duction of zinc. In a strongly acid solution, both
arsenate and arsenite are converted into arsine by
sodium borohydride.
Gutzeit Method (Colorimetry)
0025The Gutzeit method is becoming obsolete, although it
is highly sensitive and has been employed for many
years for the detection and semiquantitative deter-
mination of traces of arsenic in foods, soils, and fer-
tilizers. The sample is brought into solution by wet
ashing or by other means. Arsenic is reduced to
arsenite with potassium iodide and tin(II) chloride
and further to arsine with zinc in acid solution. The
arsine is stripped by the hydrogen formed during the
reduction with zinc, and passed through a tube con-
taining a strip of HgBr
2
-impregnated paper. The
paper darkens on reaction with arsine, and the length
of the darkened area is proportional to the amount of
arsenic.
0026In the field kit based on this method, the paper
turns yellow on reaction with arsine, the concentra-
tion of arsenic is determined by comparison on a
color chart, and its detection limit is sub-mg kg
1
levels.
Spectrophotometry
0027Spectrophotometric methods used for the determin-
ation of arsenic are based on either the formation of
heteropolymolybdate, e.g., the molybdenum blue
method, or the formation of arsenic diethydithiocar-
bamate. Only arsenic(V) reacts with molybdic acid to
form heteropolymolybdate. For the determination of
total inorganic arsenic, prior oxidation of arsenic(III)
to arsenic(V) is required.
0028In the molybdenum (Mo) blue method, arsenic(V)
reacts with molybdic acid in the presence of a
reducing agent to form heteropolymolybdenum
blue. Since this method is interfered with phosphate
and silicate, it is used after isolation of arsenic by the
hydride generation method. First, arsenic is converted
into arsine. The arsine is then passed through an
arsine trap solution containing sodium hypobromite.
An ammonium molybdate–sulfuric acid solution is
added to the arsine trap solution to react as follows:
AsO
3
4
þ 12MoO
2
4
þ 24H
þ
! AsMo
12
O
3
40
þ 12H
2
O:
After reduction with hydrazinium sulfate, absorbance
at 845 nm is measured. The color develops slowly
(* 75 min) at room temperature but is stable.
0029The silver diethyldithiocarbamate ((C
2
H
5
)
2
NCSSAg, AgDDC) method involves arsine gener-
ation and bubbling the arsine formed through a trap-
ping solution in pyridine. Figure 3 shows an example
of the apparatus used in this method. The glass wool
in the sulfide scrubber is moistened with lead acetate
308 ARSENIC/Properties and Determination
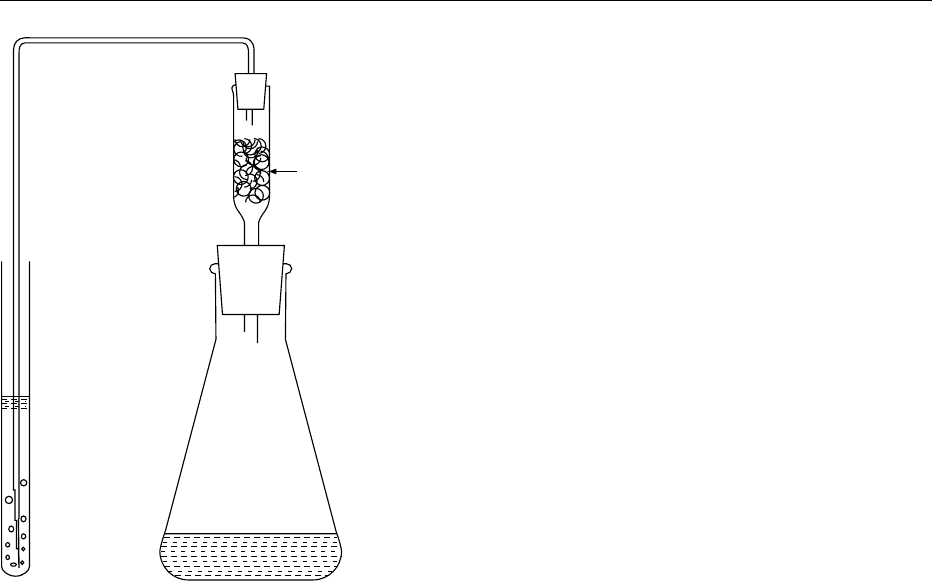
solution, and AgDDC–pyridine solution is placed in
the arsine trap.
0030 An appropriate volume of sample solution is placed
into an arsine generator flask, and hydrochloric acid,
potassium iodide, and tin(II) chloride are added to
the flask. The mixture is allowed to stand for more
than 15 min at room temperature. Zinc metal is
added, and the generator flask is immediately sealed
and the tube connected as illustrated. The solution
is then allowed to react for 30 min. The trap is dis-
connected, and the trapping solution is mixed by
gently drawing back and forth five times with
an aspirator assembly. The absorbance based on an
intense red color is measured at about 522 nm. The
wavelength for the absorption maximum changes
slightly with the composition of the trapping
solution. If AgDDC–brucine–chloroform solution is
used as a trapping solution, to prevent the production
of foul-smelling pyridine, an appropriate volume of
chloroform should be added to the trapping solution
at the end of the trapping operation to compensate for
the loss of chloroform evaporation.
Atomic Absorption Spectrometry
0031 A hollow cathode lamp or an electrodeless discharge
lamp can be employed as a light source. The primary
absorption line is 193.7 nm.
0032Direct nebulization of extracts into a flame atomic
absorption spectrometry is impractical at low concen-
trations because the flame strongly absorbs radiation
at the wavelengths of sensitive absorption lines.
Better sensitivity is obtained by the hydride gener-
ation technique, in which arsenic (as arsine vapor)
is introduced into a hydrogen–argon reducing flame
or into a heated tube for measurement of atomic
absorption.
0033Graphite furnace atomic absorption spectrometry
(GF-AAS) can also be employed. Arsenic sublimes at
613
C and arsenic trichloride (AsCl
3
) boils at 130
C,
but nickel arsenide melts at 968
C. Successful results
are obtained when nickel nitrate is added as a modi-
fier to prevent loss of arsenic during the ashing pro-
cess and to reduce interference. Similarly, magnesium
nitrate, palladium and magnesium nitrates, and zir-
conyl nitrate can also be used as modifiers.
0034Instead of GF-AAS, quartz furnace atomic absorp-
tion spectrometry coupled with HG is also employed.
The temperature in the quartz furnace is elevated
to 900
C.
Inductively Coupled Plasma Atomic Emission
Spectrometry
0035Inductively coupled plasma atomic emission spec-
trometry (ICP-AES) is a simultaneous multielement
analysis technique with a dynamic range. In ICP-
AES, arsenic can be measured simultaneously in vari-
ous emission lines (188.979, 180.042, 193.696,
197.192, or 228.812 nm) with different sensitivities.
Atomic emission at 193.7 nm is less subject to inter-
ference, giving a detection limit of c. 0.02 mg l
1
.At
228.8 nm, cadmium interferes. Atomic emission at
188.979 nm, which is recommended in recent years,
is giving a detection limit of mg1
1
levels.
0040At 228.812 nm cadmium interferes. Arsenic (V) is
converted into an arsenomolybdate, which is sorbed
by the filter as the ion-associate with tetrapentylam-
monium ion, and the filter and the arsenomolybdate
sorbed are dissolved in concentrated sulphuric acid,
diluted with water and nebulized into the plasma
torch. An emission line at 228.812 nm can be used.
Interference from cadmium is eliminated because
cadmium is not collected on the filter. Arsenic (III)
dose not form arsenomolybdate and is not deter-
mined.
0041The sensitivity of conventional direct nebulization
ICP-AES for arsenic is high but not sufficient to de-
termine traces of arsenic. The sensitivities of ultra-
sonic nebulization ICP-AES for arsenic are about ten
times better than that of conventional direct nebuli-
zation ICP-AES, but matrix (salt concentration) effect
becomes larger. The sensitivity of ICP-AES can be
improved by using HG techniques because of a
Sulfide scrubber
(glass wool wet with
lead acetate solution)
Generation flask
Arsine trap
fig0003 Figure 3 Apparatus for silver diethyldithiocarbamate method.
ARSENIC/Properties and Determination 309

more efficient sample introduction and matrix re-
moval. A continuous hydride generator is convenient.
The detection limits of HG-ICP-AES for arsenic are
about two orders in magnitude better than that of
conventional direct nebulization. ICP-AES is possible
to use a continuous monitoring. Do et al. investigated
the speciation of arsenic in urine by HPLC-HG-ICP-
AES. The limits of quantification were found to be
36 mgl
1
for AS(III), 9 mgl
1
for DMA, 57 mgl
1
for
MMA, and 101 mgl
1
for As(V), applying a 100 ml
sample injected for analysis.
Inductively Coupled Plasma Mass Spectrometry
0036 Inductively coupled plasma mass spectrometry (ICP-
MS) uses an inductively coupled plasma as an ion
source and is one of the most sensitive methods for
elemental determination. Arsenic is a monoisotopic
element, that is, 100% of arsenic is
75
As.
0037 The determination of arsenic using ICP-MS is inter-
fered with by any molecular ion having a mass/charge
ratio (m/z) equal to
75
As. Thus, the
75
As
þ
ion is
interfered with by
40
Ar
35
Cl
þ
formed by the com-
bination of plasma gas and chloride ions, and so
separation of the arsenic species from chloride ions
is often required. The removal or reduction of argon
from the excitation source by the addition of nitrogen
gas or helium gas also eliminates, or significantly
reduces, the formation of
40
Ar
35
Cl
þ
. Before ICP-
MS, an HG method or a high performance liquid
chromatography (HPLC) coupled with ICP-MS is
required for the determination of arsenic species.
The concentration of organic solvents must be lower
because the introduction of organic solvents destabil-
izes the inductively coupled plasma.
Other Methods
0038 Atomic fluorescence spectrometry (AFS) combined
with HG is also available for trace arsenic determin-
ation. With LC (anion and cation exchange, liquid
chromatography)–UV (decomposition)–HG–AFS
system, six arsenic species (As(III), DMAA, MMAA,
As(V), AsB, tetramethyarsonium ion) can be separ-
ated, with detection limits of about 0.5 mg of As per
liter (100 ml injected). The limits of determination for
arsenic in AFS, neutron activation without chemical
separation, and pulse polarography are 0.8, 0.02, and
0.004 mg l
1
, respectively.
Certified Reference Materials
0039 For quality control, the adoption of certified refer-
ence materials (CRM) is the best choice. A number
of CRM for total arsenic are available, although only
a few are available for speciation. Several easily
available CRM for arsenic, relating to foods, are as
follows: NIES CRM 9 Sargasso (certified value:
115 + 9mgkg
1
), BCR CRM 422 cod muscle
(21.1 + 0.5 mg kg
1
), NIST SRM 1566 oyster tissue
(14.0 mg kg
1
), BCR CRM 279 Ulva lactuca (sea
lettuce) (3.09 + 0.20 mg kg
1
), NIST SRM 1548
total diet (0.20 + 0.020 mg kg
1
), NIST SRM 1570a
spinach leaves (0.068 mg kg
1
), BCR CRM 185
bovine liver (0.024 + 0.003 mg kg
1
), NIST SRM
8433 corn bran (0.002 mg kg
1
). CRM are produced
by several institutions:National Research Council of
Canada, Community Bureau of Reference EC, Brus-
sels, Belgium (Standards, Measurements and Testing
Programme), International Atomic Energy Agency,
Vienna, Austria, National Institute of Environmental
Studies, Tsukuba, Japan, and National Institute of
Standards and Technology, Gaithersburg, Maryland,
USA.
See also: Arsenic: Requirements and Toxicology;
Chromatography: High-performance Liquid
Chromatography; Mass Spectrometry: Principles and
Instrumentation; Applications; Spectroscopy: Atomic
Emission and Absorption; Visible Spectroscopy and
Colorimetry
Further Reading
Benramdane L, Bressolle F and Vallon JJ (1999) Arsenic
speciation in humans and food products: a review.
Journal of Chromatographic Science 37: 330.
Cullen WR and Reimer KJ (1989) Arsenic speciation in the
environment. Chemical Reviews 89: 713.
Do B, Alet P, Pradeau D, Poupon J, Gulley-Gaillot M and
Guyon F (2000) On-line reversed-phase liquid chroma-
tography hydride generation emission spectrometry:
speciation of arsenic in urine of patients intravenously
treated with As
2
O
3
. Journal of Chromatography B 740:
179.
Ferguson JF and Gavis J (1972) A review of the arsenic cycle
in natural waters. Water Research 6: 1259.
The Geological Society of Japan (1998) Environmental
Problems about Arsenic (in Japanese) Tokyo: Tokai
University Press.
Gerhartz W (ed.) (1985) Ullmann’s Encyclopedia of
Industrial Chemistry, 5th edn, vol. A3, pp. 113–141.
Weinheim: VCH.
Guerin T, Astruc A and Astruc M (1999) Speciation of
arsenic and selenium compounds by HPLC hyphenated
to specific detectors: a review of the main separation
techniques. Talanta 50: 1.
McIver KA (ed.) (1996) Official Methods of Analysis of the
Association of Official Analytical Chemists, 16th edn.
Arlington, VA: Association of Official Analytical
Chemists.
Mochizuki T (1993) Table of determination methods in
seventeen elements for various concentrations: Arsenic
(in Japanese). Bunseki: 262.
310 ARSENIC/Properties and Determination

Morita M and Shibata Y (1987) Speciation of arsenic com-
pounds in marine life by high performance liquid chro-
matography combined with inductively coupled argon
plasma atomic emission spectrometry. Analytical
Science 3: 575.
Nriagu JO (ed.) (1994) Arsenic in the environment, Part I:
Cycling and characterization In: Advance in Environ-
mental Science and Technology, vol. 26. New York:
John Wiley.
US National Research Council, Committee on Medical and
Biologic Effects of Environmental Pollutants (1977)
Arsenic. Washington, DC: National Academy of Science.
Van Elteren JT and S
ˇ
lejekovec Z (1997) Ion-exchange sep-
aration of eight compounds by high-performance liquid
chromatography–UV decomposition–hydride generat-
ion–atomic fluorescence spectrometry and stability
tests for food treatment procedures. Journal of Chroma-
tography A 789: 339.
Van Elteren JT, S
ˇ
lejekovec Z and Byrne AR (1998) A dual
arsenic speciation system combining liquid chromato-
graphic and purge and trap-gas chromatographic separ-
ation with atomic fluorescence spectrometric detection.
Analytica Chimica Acta 358: 51.
Requirements and Toxicology
G I Rehner, Institut fu
¨
r Erna
¨
hrungswissenchaft,
Giessen, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Arsenic in the Environment
0001 Arsenic is widely distributed in the natural environ-
ment. Virtually all soils and waters contain a variety
of different arsenic compounds. As a byproduct of
melting copper, lead, and several other metals, as
well as from coal-fired power plants, high quantities
of this metalloid are emitted into the atmosphere.
Arsenic compounds are also used by the metallurgical
and chemical industry. Phosphate fertilizer, as well
as many herbicides, fungicides, wood preservatives,
insecticides, and rodenticides, contains arsenic. Thus
agriculture contributes significantly to arsenic pollu-
tion. Legislation in countries with developed conser-
vation systems attempts to minimize the quantity of
these pesticides as well as other sources of arsenic
pollution.
0002 Both the trivalent (arsenite) and the pentavalent
(arsenate) state of arsenic can be detected in bio-
logical material. The biochemically important forms
of arsenic are however methylated compounds:
dimethylarsenic acid, methylarsonic acid, trimethyl-
arsine oxide, trimethylarsonium ion, arsenocholine,
arsenobetaine, and unstable arsenyl esters.
0003Arsenic accumulated in the environment can be
subject to various biotransformations – reduction,
oxidation, and methylation – performed by micro-
organisms. Methylation of inorganic arsenic in
methanogenic bacteria under anaerobic conditions
may be a detoxification mechanism for arsenic. Also
marine algae transform arsenate into nonvolatile
methylated arsenic compounds (methanoarsenic
acid and dimethylarsenic acid) in seawater. These
less toxic compounds are concentrated in higher
marine organisms consuming the plankton. Several
fungi are able to transform aerobically inorganic
and organic arsenic compounds into very toxic vola-
tile methylarsines.
0004Owing to the ubiquity of arsenic in the environ-
ment, foodstuffs of plant and animal origin as well as
drinking water also contain arsenic. The maximally
permissible arsenic concentration in drinking water,
vegetables, fruits, and meat is regulated by law in
most industrially developed countries. The US drink-
ing water standard, for example, is fixed at 50 mgl
1
.
However, several springs and spa waters contain
0.5–1.3 mg of arsenic per liter.
0005The range of arsenic levels in foodstuffs in the
absence of significant pollution is as follows: cereals
0.05–0.4 mg kg
1
, fruits 0.03–1.0 mg kg
1
, vege-
tables 0.05–0.8 mg kg
1
; meat 0.05–1.4 mg kg
1
;
dairy products 0.01–0.23 mg kg
1
. Higher concentra-
tions – 1.5–15.3 mg kg
1
– are registered in seafoods
because marine organisms accumulate arsenic in the
form of nontoxic organic compounds. The average
daily intake of arsenic is estimated in the range of
12–40 mg. When diets contain substantial amounts
of seafood, as is usual in Japan, 70–170 mg could be
the realistic daily intake. (See Shellfish: Contamin-
ation and Spoilage of Molluscs and Crustaceans.)
Arsenic Metabolism
Intestinal Absorption
0006Arsenic is generally well absorbed by the gastrointest-
inal tract, although the solubility of the compounds
seems to correlate with the rate of absorption. Intes-
tinal absorption of most inorganic and organic com-
pounds of arsenic is a quick process. However, some
unusual compounds, such as Na-P-N-glycolylarseni-
late, given orally to humans, are poorly absorbed.
0007Some investigations in chicks, mice, rats, and
rabbits indicate that arsenate as well as arsenocholine
readily penetrates both the mucosal and the serosal
surfaces of the epithelial membrane and that arsenate
and phosphate do not share a common transport path-
way. Both arsenic compounds are apparently absorbed
by simple diffusion following a concentration
ARSENIC/Requirements and Toxicology 311

gradient. On the other hand, in vivo and in vitro
experiments in rats revealed a saturable transport
process for pentavalent arsenic which was signifi-
cantly reduced by phosphate. This result might indi-
cate that arsenic and phosphate share the same
secondary active carrier-mediated transport system.
Arsenic in Human Tissues
0008 Once absorbed, arsenic is quickly distributed to all
organs and tissues, probably as a complex with
a-globulins. Apart from inorganic arsenic, blood
also contains methylated metabolites formed in the
liver.
0009 If the uptake of arsenic is low, no significant accu-
mulation in the soft tissues occurs. However, in unex-
posed humans relatively high arsenic concentrations
are usually found in skin, nails, and hair. Because hair
levels correlate with arsenic in the air as well as with
renal arsenic excretion, hair concentration has often
been used as an index of contamination with toxic
amounts of arsenic. The median arsenic content of
human hair was found to be 0.51 mgg
1
. The hair of
workers at plants with arsenic-containing emissions
was found to be 10–31 mg of arsenic per g of hair.
Values greater than 3 mgg
1
indicate toxic levels of
uptake.
Arsenic Excretion
0010 In general, the biological half-life of arsenic is short:
the metalloid is excreted mainly in urine with a little
in feces. Some compounds, such as arsenobetaine,
which is the form found in marine animals, pass
through into the urine unchanged. For this reason,
despite the high arsenic content, intoxication by sea-
food is unlikely. Different methylated compounds are
the major urinary metabolites.
0011 Excretion via the bile is low after dietary intake of
arsenic, but can significantly increase when drugs
containing arsenic are administered intravenously.
The hepatobiliary transport of arsenic is gluta-
thione-dependent and associated with a profound
increase in biliary excretion of glutathione; thus, hep-
atic glutathione depletion and diminished glutathione
conjugation result.
Biotransformation of Arsenic
0012 Inorganic arsenic is methylated in the liver with S-
adenosylmethionine as methyl donator. As the first
step, arsenate is reduced to arsenite by using glu-
tathione. Arsenite is methylated to dimethylarsenic
acid with monomethylarsenic as a precursor. Investi-
gations with germfree animals have shown that
methylation is mainly performed by the liver cells,
with little contribution from the intestinal microflora.
0013The rate of methylation of inorganic arsenic varies
considerably between species and individuals. As
shown by studies in human volunteers exposed to
specific doses of inorganic arsenic, the rate of renal
excretion of arsenic increases with methylation effi-
ciency. It could be assumed that the variations in
interindividual susceptibility to arsenic in humans
could be due to differences in the capacity of arsenic
methylation.
0014Some organic compounds, especially arsenobe-
taine, are excreted unchanged. Arsenocholine can be
incorporated into phospholipids, replacing choline,
but the major part of this compound is excreted by
the kidney.
0015Some mold species are able to produce volatile
neurotoxic compounds by biotransforming arsenical
pigments contained especially in wallpapers. Some
lethal poisoning cases have been described resulting
from such toxic products of aerobic and nonaerobic
fungi as well as other primitive organisms.
Biochemical Function and Essentiality of
Arsenic
0016Although a specific function for arsenic on a molecu-
lar level is unknown, some evidence has been accu-
mulated indicating a distinct role for this element in
several metabolic processes.
0017This could be the case in CH
3
-group metabolism.
Almost all organic forms of arsenic produced in vivo
are methylated compounds. One of the trimethylated
ones is arsenocholine, which can be incorporated into
phospholipids, replacing choline. Choline is a methyl
donator for different methylation reactions in inter-
mediary metabolism. There is some evidence indicat-
ing that arsenocholine might have a similar function
in labile methyl metabolism. As some experiments
have shown that arsenic deprivation in the rat,
chick, and hamster affects labile methyl metabolism,
it could be speculated that the role of arsenocholine
cannot be fulfilled by choline.
0018Arsenic binds strongly to sulfydryl groups which
are known to participate in hundreds of enzymatic
reactions. In this context it behaves like heavy metals
that impair enzymatic catalysis. It is likely that the
biochemical basis is the inhibition of a wide range of
SH-enzymes by arsenic. But it cannot be ruled out
in advance that such an interaction is not only of
toxicological but also of biological significance.
0019Some investigators have reported that arsenic can
provide partial protection from chronic selenosis,
with the vitamin E level influencing the incorporation
of selenium into the tissues. It has been shown that
selenium toxicity for cultured mice fibroblasts can
also be counteracted by arsenic. Interactions between
312 ARSENIC/Requirements and Toxicology

arsenic and cadmium have also been shown. Con-
sidering the common mode of action that these elem-
ents have at the cellular level, especially through
interaction with SH-groups, these facts are not
surprising. (See Cadmium: Toxicology; Selenium:
Physiology.)
0020 Studies with chicks suggest that arsenic is closely
related to the metabolism of zinc. Depressed growth
caused by arsenic deprivation was alleviated when
dietary zinc was given in excess. It seems, however,
not only that interactions between arsenic and zinc
exist but also that the arginine status of the chicks
plays a distinct role in these complicated correlations
between the two trace elements and the amino acid.
There is support for the possibility that arsenic par-
ticipates – in a way that has yet to be clarified – in
utilizing amino acids for protein synthesis, or in pro-
tein degradation, as well as in uric acid metabolism.
(See Zinc: Physiology.)
0021 Effects of arsenic on carbohydrate metabolism
have been observed in experiments with guinea pigs.
The most prominent finding after repeated arsenic
(As
2
O
3
) administration was a marked decrease in
total carbohydrate content of the liver, mainly
owing to depletion of glycogen. (See Carbohydrates:
Digestion, Absorption, and Metabolism.)
0022 Some other biochemical facts in connection with
arsenic – used in physiological as well as toxicological
amounts – have been registered in the last few
years. We are far from being able to classify the
frequently conflicting facts and their significance for
metabolism.
0023 Since 1975, some conclusive evidence has been
published supporting the suggestion of nutritional
essentiality of arsenic. An element is considered es-
sential if a dietary deficiency consistently results in a
suboptimal biological function that is preventable or
reversible by intake of a physiological amount of that
element. Signs of arsenic deprivation were studied in
four animal species – chick, goat, miniature pig, and
rat. Safe and adequate intakes of arsenic for these
species are not precisely defined. However, a diet
containing less than 50 mg of arsenic per kg is denoted
as an arsenic-deficient ration in these animal experi-
ments, whereas 350–500 mgkg
1
is considered a
normal arsenic supply and 3.5–5mgkg
1
as a thera-
peutic dose.
0024 The essentiality of arsenic has been systematically
investigated in growing, pregnant, and lactating
goats, as well as in miniature pigs and their offspring.
Deficient rations contained less than 10 mg of arsenic
per kg of semisynthetic diet, and control rations
350 mgkg
1
.
0025 The following deficiency symptoms were de-
scribed: feed consumption was reduced and this was
correlated with diminished growth rates and milk
production; a high abortion rate for goats and
increased perinatal mortality for miniature pigs and
rats were also registered; intrauterine arsenic deple-
tion in goats, miniature pigs and rats resulted in sig-
nificantly reduced birth weight of the offspring. The
decreased milk-fat concentration could be a conse-
quence of lowered triglyceride concentration in the
blood serum of arsenic-deficient goats.
0026Histological changes, accompanied by ultrastruc-
tural alterations of the mitochondria, have been seen,
especially in skeletal muscles, the myocardium, and
liver tissue. Some modifications in the mineral com-
position of different organs with a remarkable reten-
tion of manganese were also connected to arsenic
deficiency.
0027Many recent findings from studies with sophisti-
cated new techniques have revealed possible sites of
essential action of arsenic. It seems to have an import-
ant role in the conversion of methionine to different
metabolites, e.g., S-adenosylmethionine, S-adenosyl-
homocysteine, and taurine. Additionally, arsenic
affects the formation of the polyamines spermine,
spermidine, and putrescine from arginine and the
metabolism of labile methyl groups.
0028As shown in recent studies, arsenic induces the
cellular synthesis of certain heat shock or stress pro-
teins. This event is controlled at the transcriptional
level and may involve changes in the methylation of
core histones. Thus, arsenic seems to regulate gene
expression. Arsenic also enhances DNA synthesis in
unsensitized and in stimulated human lymphocytes.
0029Beneficial effects of arsenic preparations have been
known – or at least believed – for centuries. Arsanilic
acid used to be incorporated into pig and poultry feed
in pharmacological doses as a growth-promoting
agent. Intake of relatively high amounts of inorganic
arsenic salts by humans and horses was reported from
several parts of Europe. ‘Arsenic eaters’ were con-
vinced that these preparations had protective effects
against diseases, and increased physical condition and
virility. Regular daily intakes of 0.5 g of arsenic have
been reported; this kind of dose can only be tolerated
once you are accustomed to arsenic for a long time.
(See Trace Elements.)
0030Nevertheless, there is as yet no convincing evidence
that arsenic is essential for humans, but the possibility
cannot be ruled out. For definitive judgment of essen-
tiality of arsenic, further critical investigations are
necessary.
0031Only data from animal studies are available for
estimating the approximate amount of arsenic re-
quired by humans, if it is essential for humans at all.
Cautious assessments support a daily requirement of
about 12–15 mg for persons consuming 8.4 MJ per
ARSENIC/Requirements and Toxicology 313
