Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

different types of bran can vary widely. Rice bran
contains approximately 10% phytic acid, whereas
wheat bran and rye bran contain only 5%. The phytic
acid content of different cereals depends on the
botanic subfamily to which the grain belongs.
Wheat and rye are members of the Hordeae sub-
family, whereas rice is a member of the Oryzeae.
The phytic acid content of oat bran (subfamily:
Aveneae) is approximately 6%.
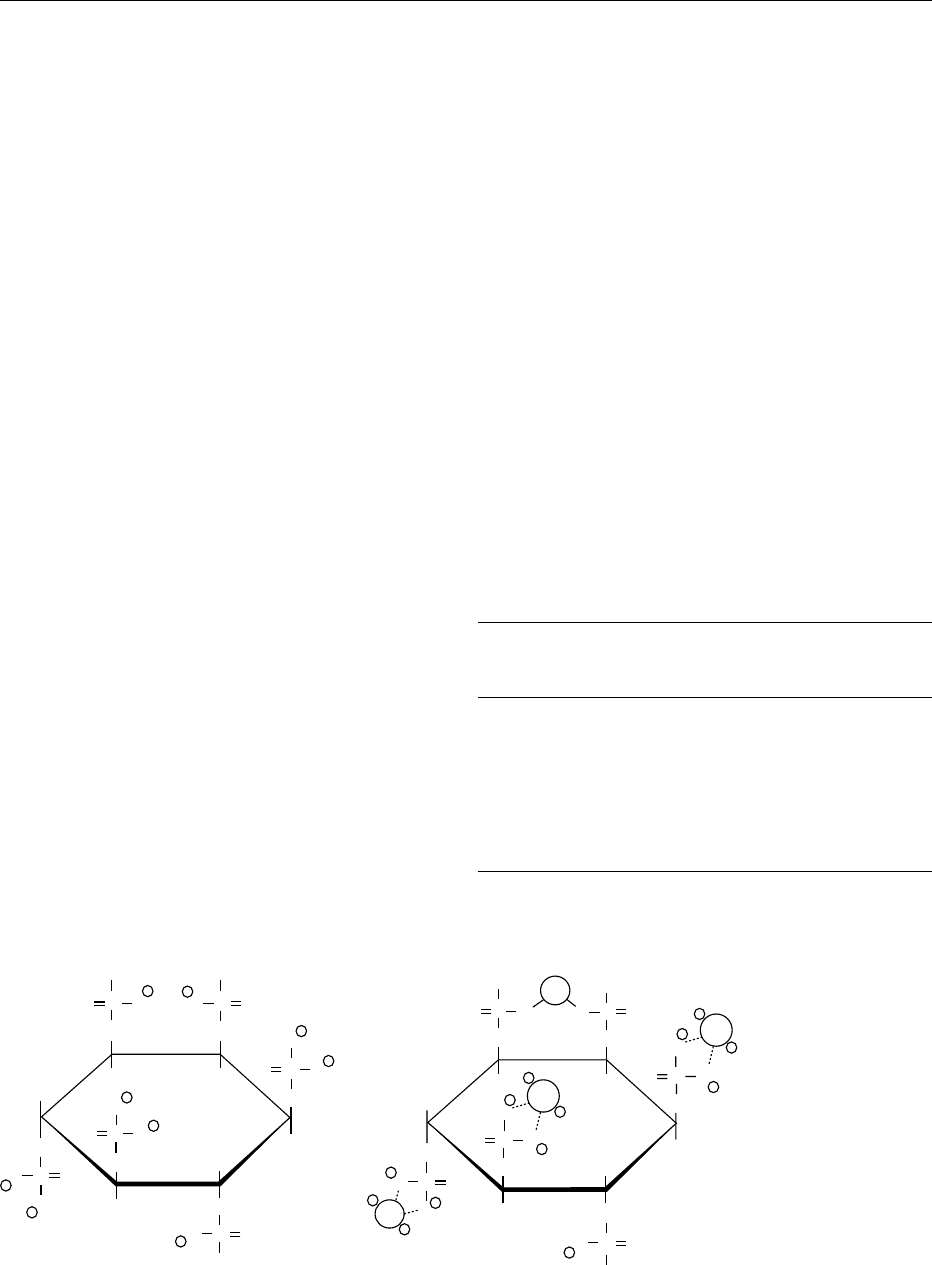
0009 Phytic acid can strongly chelate multivalent metal
ions, especially the positively charged ions zinc, cal-
cium, and iron. The chelates are named phytates.
Such chemical bonds with multivalent cations can
be formed intra- and intermolecularly by one or
several phosphate groups of the pyhtic acid molecule
(Figure 2). These complexes are insoluble.
0010 The distribution of phytic acid in different morpho-
logic layers of seeds can vary significantly. In cereal
grains from the Hordeae subfamily, phytic acid is
normally concentrated in the aleurone cells and, to
a lesser degree, in the germ. The aleurone layer of
wheat grain, for example, contains 85% of the phytic
acid, the embryo 13%, and the endosperm 2%. In
contrast, 80% of the phytic acid content of rice and
maize is concentrated in the pericarp and embryo,
respectively.
0011 Inositol-6-phosphate and inositol-5-phosphate
show the strongest inhibiting effect on mineral bio-
availability, whereas the effect of inositol-4-phosphate
and inositol-3-phosphate, two products of inositol-6-
phosphate hydrolysis, is markedly lower. Phytic acid is
hydrolyzed by the enzyme phytase. This enzyme was
first described in rice bran. In wheat bran, the highest
phytase activity is present in the aleurone layer, where
more than 40% of the phytase activity is located.
Approximately 34% is present in the endosperm,
15% in the scutellum, and 3% in the germ. In rye
bran, the phytase activity is 10–20 times higher than
in the rye flour. The total amount of cereal phytase
activity varies depending on the food origin: phytase
activity is high in wheat and rye grains, medium in
barley, and low to very low in oat and maize grain.
(See Phytic Acid: Properties and Determination;
Nutritional Impact.)
Lignans
0012Lignans are phenolic dimers possessing a 2,3-diben-
zylbutane structure. Such compounds are known to
exist as minor constituents of many plants, where they
form the building blocks for the formation of lignin in
the plant cell wall. The compounds occur mainly in
the glycosidic form. In rye, lignans are predominantly
present in the bran fraction (Table 2). The glycosides
are converted by fermentation in the proximal colon
to mammalian lignans. The two major mammalian
lignans, enterodiol and enterolactone, are the prod-
ucts of colonic bacterial metabolism of the plant lig-
nans secoisolariciresinol and matairesinol.
OH
H
O
O
O
O
O
O
O
O
O
O
O
P
P
OH
H
H
H
H
O
O
O
O
O
O
P
P
P
HO
Ca
−
−
−
−
Mg
+
+
O
O
O
O
P
H
−
−
−
Zn
+
+
Fe
OH
H
O
O
O
O
O
O
O
O
O
O
O
P
P
P
OH
H
H
H
H
O
O
O
O
OO
P
P
HO
−
−
−
−
−
−
−
−
−
O
O
O
OP
H
+
+
fig0002 Figure 2 Structure of phytic acid and a phytate chelate at neutral pH. From Ingelmann H-J, Rimbach G and Pallauf J (1993)
Phytinsa
¨
ure – ein antinutritiver Faktor? Ernhrungs^Umschau 40: 400–404, with permission.
tbl0002Table 2 Lignan content of whole grains and bran
a
Secoisolariciresinol
(mgper100g
dry weight)
Matairesinol
(mgper100g
dry weight)
Wheat bran (whole grain) 33 3
Wheat bran 110 0
Rye meal 47 65
Rye bran 132 167
Oat meal 13 0
Oat bran 24 155
Barley (whole grain) 58 0
Barley bran 63 0
a
Adapted from Mazur and Adlercreutz. Pure and Applied Chemistry
70: 1759–1776.
1846 DIETARY FIBER/Bran

Physiologic Effects of Bran
Stool weight
0013 Stool weight is significantly increased by adding
sources of insoluble fiber to the diet. The physio-
logical effect of dietary fiber is largely determined
by the size of the particles. One gram of fine wheat
bran increases the stool weight by 3.1 g, and 1 g
of coarse bran increases the stool weight by ap-
proximately 6.2 g. Barley bran is more effective in
increasing stool weight than fine wheat bran but
less effective than coarse wheat bran. Comparable
amounts of fiber provided by wheat and oat bran
have the same effect on daily stool output, even
though > 90% of wheat bran fiber and only 50–
60% of oat bran fiber are insoluble. Wheat bran
increases the fecal concentration of sugars and mass
of plant material more than oat bran, whereas oat
bran increases the fecal bacterial mass more. Oat bran
increases the stool weight by providing rapidly fer-
mented soluble fiber in the proximal colon for bacter-
ial growth, which is sustained until excretion by
fermentation of the insoluble fiber. Bacteria and lipids
are major contributors to the increase in stool weight
with oat bran consumption, whereas ingested plant
fiber is responsible for much of the increase in stool
weight with wheat-bran consumption.
0014 Constipated subjects should start increasing diet-
ary fiber by 2–4 g, e.g., with 1–2 tablespoons of bran.
Plenty of liquid should be taken with bran, as it may
cause irritations in the digestive tract. Intake can be
increased to three times a day with a meal. Bran
should be mixed with food in order to absorb water
and increase stool volume. Coarse bran holds water
better than fine bran. Depending on the severity of
constipation, improvement may take a few days to
several months.
Sterol Metabolism
0015 Bile acids Soluble dietary fiber can adsorb bile acids.
Accordingly, oat bran and rye bran increase fecal
excretion of total bile acids, whereas wheat bran has
no or only modest effects. Moreover, oat bran and rye
bran influence the metabolism of bile acids. Rye bran
increases the daily excretion and percentage of conju-
gated primary bile acids (cholic acid, chenodesoxy-
cholic acid) and lowers the percentage of free
secondary bile acids (desoxycholic acid, lithocholic
acid). Rye bran thus decreases the ratio of secondary
bile acids to primary bile acids. Rye bran, but not
wheat bran or barley bran, reduces the ratio of litho-
cholic acid to desoxycholic acid. Oat bran enlarges
the cholic acid pool, increases the relative proportion
of chenodeoxycholate in bile, and reduces the relative
proportion of desoxycholic acid, whereas cholate
remains unchanged.
0016Blood cholesterol levels Since the first study per-
formed in 1963 in humans with fiber-rich rolled
oats, several studies have reported a beneficial effect
of oat bran or oat meal-enriched diets on plasma
cholesterol levels in healthy subjects or mildly hyper-
lipidemic subjects. The daily intakes ranged from 17
to 100 g oat bran, and the duration was 2–12 weeks.
Oat bran and oat gum (80% b-glucan) has been
served in drinks and instant whips, or baked in
bread and muffins. The majority of controlled studies
performed in hyperlipidemic subjects showed a
decrease in plasma total cholesterol, with most de-
creases in the range of 4to10%. It is noteworthy
that decreases in plasma cholesterol are related to
decreases in LDL cholesterol, whereas while HDL
cholesterol does not generally noticeably change.
Consequently, oat bran causes a modest reduction in
blood cholesterol level. Larger reductions are seen in
subjects who have initially higher blood cholesterol
levels (> 5.9 mmol l
1
or 229 mg dl
1
). Studies have
shown clearly that soluble b-glucan in oat fiber has a
cholesterol-lowering effect in animals and humans.
About 3 g of b-glucan per day causes a 10% drop in
LDL cholesterol. In bread and muffins, the baking
process can decrease the solubility and/or molecu-
lar weight of b-glucans, resulting in a weak serum
cholesterol-lowering effect. Approximately 100 g of
oat bran should be ingested daily to have any effect on
plasma cholesterol levels. Oat b-glucan also improves
glucose and insulin regulation. The mechanisms of
action of viscous polysaccharides like b-glucans in
the human gastrointrestinal tract are not completely
understood. Increased luminal viscosity is believed to
cause delayed gastric emptying and reduce the rate of
absorption of nutrients in the small intestine, thereby
attenuating the postprandial glucose and insulin
response to an oral carbohydrate load. Viscous gums
alter bile acid- and cholesterol metabolism, reduce
and alter the site of lipid absorption, and undergo
fermentation in the colon to short-chain fatty acids.
All of these effects might contribute to a reduction in
serum cholesterol levels in humans. Full-fat rice bran
leads to a similar reduction in serum LDL cholesterol
compared with oat bran. Rice bran contains low
amounts of soluble fiber, but rice bran oil has a hypo-
lipidemic effect. Essentially all human studies per-
formed since 1969 have shown no effects of wheat
bran on fasting blood total cholesterol as well as
LDL cholesterol in healthy or hyperlipidemic subjects
following a chronic intake of 14–77 g of wheat bran
per day. Moreover, in rats, wheat bran essentially
does not change fasting blood lipid parameters.
DIETARY FIBER/Bran 1847

(See Cholesterol: Properties and Determination; Ab-
sorption, Function, and Metabolism.)
0017 Female sex hormones A daily intake of 15 g of
wheat bran can significantly reduce circulating levels
of estradiol and estrone in premenopausal women by
approximately 15–20%. The mechanism by which
wheat bran modulates serum estrogen levels most
likely involves their enterohepatic circulation. Estro-
gens are conjugated in the liver to form glucoronides
and sulfoglucoronides, and 20–50% of estrogen
metabolites are excreted in the bile as these polar
biologically inactive substances. Approximately
80% of the estrogens in the gut are reabsorbed, a
process that requires deconjugation by bacterial
enzymes. Wheat bran intake may lower fecal b-glu-
coronidase activity. An additional mechanism may be
the binding of unconjugated estrogens to fiber in the
gut, thus impeding their reabsorption. Oat bran
causes reductions in fecal b-glucuronidase activity to
a lesser extent than wheat bran. Maize bran has no
significant effect on this enzyme.
0018 Vitamin D A high intake of wheat bran can also
influence the metabolism of vitamin D, another ster-
oid substance: an additional intake of 20 g of dietary
fiber per day from wheat bran can reduce the plasma
half-life of tritium-labeled 25-hydroxyvitamin D by
approximately 30%. A dietary fiber-induced decrease
in vitamin D status may diminish active absorption of
dietary calcium. The occurrence of rickets and osteo-
malacia in Asians living in the UK has been partially
associated with a high intake of fiber-rich chapatties
(unleavened bread). However, such an adverse effect
may only be of clinical importance in subjects with
marginal or insufficient vitamin D status and low
dietary calcium intake. (See Vitamins: Overview.)
Effects of Bran on Mineral Metabolism
0019 It has been demonstrated in several human studies
that wheat bran and high-fiber diets decrease intes-
tinal Ca absorption. Wheat bran alters the usual
inverse relationship between calcium load and frac-
tional absorption in humans. This effect is the result
of the high Ca-binding capacity of wheat bran. At
pH 8.0, 1 g of wheat bran can bind approximately
0.45 mmol of calcium. This amount is slightly higher
than the calcium binding of rye bran (0.38 mmol of
Ca per gram of bran) and slightly lower than the
calcium binding of rice bran (0.49 mmol g
1
),
whereas soy bran only binds 0.19 mmol of calcium
per gram at pH 8.0. In wheat bran, phytic acid and
dietary fiber are both present in high quantities.
Theoretically, each mole of phytic acid can maximally
bind 6 moles of calcium. Phytic acid can form
insoluble calcium salts resulting in a low bioavailabil-
ity of calcium, but wheat bran can bind more calcium
in vitro than expected from the molar binding ratio
of bran phytate:calcium. In vitro calcium binding to
wheat bran is linear over a wide range. Thus, the
amount of calcium binding by wheat bran cannot be
explained by the content of phytic acid alone. Other
bran constituents may influence calcium bioavaila-
bility. Although neutral cellulose has little affinity to
cations, lignin strongly binds calcium. The lignin in
wheat bran is indigestible during the gastrointestinal
transit. However, as the result of an adaptation pro-
cess, wheat bran intake is also associated with a re-
duced renal calcium excretion. Consequently, a high
bran intake does not necessarily increase the risk of a
negative calcium balance.
0020Intakes of high (> 15 g) amounts of bran (wheat,
oat, barley) can reduce zinc absorption and decrease
iron availability. Measures to reduce the phytate con-
tent of cereals like malting and soaking (activation
of endogenous phytase activity) can significantly im-
prove zinc and iron absorption. Nevertheless, in diets
that provide sufficient amounts of trace elements,
decreased absorption does not necessarily influence
the trace element balance and status of human sub-
jects. A reduced endogenous fecal excretion can com-
pensate for the lower absorption. However, if zinc
and iron intakes are marginal or inadequate, the
additional consumption of a fiber concentrate may
contribute to the development of trace element
deficiencies.
0021In animal diets, supplemental phytases of microbial
and cereal sources improve dietary phytate phos-
phorus utilization. Such a measure is environmentally
advantageous in order to replace the content of inor-
ganic phosphorus in those diets.
Beneficial Health Effects of Bran and its
Components
0022In animals, wheat bran causes a significant reduction
in incidence of mammary cancer. A suggested protect-
ive effect of wheat bran on breast cancer in humans
may be due, at least in part, to the effect on circulat-
ing estrogen concentrations and their bioavailability.
Moreover, the lignans especially present in the aleur-
one layer of some cereals (Table 2) may exert anti-
estrogenic effects in humans.
0023Wheat bran and its component phytic acid have
both been shown to decrease early markers of colon
carcinogenesis like certain indices of cell proliferation
and certain aberrant cryt foci parameters. Wheat
bran, dephytinized wheat bran, and phytic acid
induce cell apoptosis, increase cell differentiation,
1848 DIETARY FIBER/Bran
and favorably affect colon morphology, thus indicat-
ing that phytic acid as well as other components of
bran may be preventive against colon cancer. A lower
concentration of the co-carcinogenic secondary bile
acids and a high bacterial production of butyrate may
contribute to a normal metabolism and proliferation
of the colon epithelial cells after bran intake.
0024 Phytic acid inhibits in vitro and in vivo the enzym-
atic degradation of starch and reduces the glycemic
index owing to complexation of the starch molecule,
amylase, and calcium ions, which are essential for the
amylase activity. The reduced rate of glucose absorp-
tion and reduced postprandial increase in blood
glucose may both be beneficial in prophylaxis and
treatment of diabetes mellitus. Protective effects of
phytic acid against lead toxicity have been demon-
strated in the presence of calcium ions. Acute toxic
effects of 1 g of lead per kilogram of diet can be
reduced by 20 g of calcium phytate per kilogram.
Commercial Products
0025 Both fine wheat bran and coarse wheat bran are avail-
able for the consumer. One tablespoon of bran contains
approximately 2 g of dietary fiber. Bran may be used
alone as a cereal, mixed with other cereals, sprinkled on
cereals or included in bran muffins, hamburgers, etc.
Bran is legally regarded as a food. Moisture should not
exceed 14%, and the amount of starch in wheat bran
should not exceed 15% of the dry weight. Since espe-
cially the bran fraction of wheat can accumulate rela-
tively high amounts of cadmium, the cadmium content
should not exceed 50–60 mgkg
1
. Wheat bran tablets
are also obtainable – one tablet equals approximately
2 g of dietary fiber. Moreover, breakfast cereals with a
bran content up to 85% are in the market (such as
Kellogs All-Bran Plus
TM
). These products contain 11 g
of dietary fiber per 40-g portion. Several baked goods
are enriched with wheat bran to increase the fiber
content. Enrichment should at least result in a doubling
of the dietary fiber content, and a good source of diet-
ary fiber should contain 2 g per portion. Although
coarse wheat bran increases the stool weight more
efficiently than fine wheat bran, the latter is often
used to enrich baked goods, as it is easier to process
and is preferred by the consumer.
0026 Pure oat bran flakes are also commercially avail-
able. One portion (25 g) of oat bran contains approxi-
mately 2.3 g of insoluble and 1.95 g of soluble dietary
fiber. Moreover, oat bran-enriched breakfast cereals
and oat flakes containing 80% oat bran are obtain-
able, and oat bran can be used as a fat replacement in
sausages like Frankfurters (6% oat bran, 30% water).
0027 Protex
TM
(Food Engineering International, Inc.) is a
specially processed rice bran derivative that may be
used in baked goods, breakfast cereals, macaroni and
noodle products, and milk-like beverages.
New Potential Sources
0028Psyllium is a new source of dietary fiber. It is made from
ground husks of psyllium seeds and has the ability to
hold water and form bulk. Psyllium contains up to
85% soluble fiber. Psyllium holds water better than
bran, so smaller amounts are needed to be effective.
Moreover, psyllium effectively lowers serum LDL chol-
esterol and blood glucose and insulin levels. In 1998,
the US Food and Drug Administration ruled that psy-
llium, like oat bran, may reduce the risk of heart dis-
ease. Psyllium has several advantages over oat bran. It
is more versatile, since it is a far more concentrated
form of fiber. The US Food and Drug Administration
allows the addition of psyllium to breakfast cereals,
and FDA officials expect more psyllium-containing
foods, from waffles to biscuits, to hit the market.
0029Soy bran is a dietary fiber concentrate that is
obtained during the production of soy protein, and
the insoluble portion is separated by centrifugation.
The concentrate is defatted and contains the soy
hulls. The dietary fiber content is approximately 67–
80% (Table 3). Soy bran is sold in competition
with other dietary fiber concentrates. It is effective
in increasing stool weight (Table 2), and 25 g of soy
fiber significantly reduces total serum cholesterol
levels, especially in patients with blood cholesterol
concentrations above 7.7 mmol l
1
(300 mg dl
1
).
0030Several other food residues with a high dietary fiber
content are obtained during food processing (Table 4).
They can be used by food technologists, e.g., for the
fiber enrichment of bread (beer draff) or for the pro-
cessing of fiber and b-carotene-rich fruit juices (carrot
husks).
0031Wheat fiber (fiber content 97%) is a concentrate of
cellulose and hemicellulose that is free of phytic acid
tbl0003Table 3 Effect of bran and other fiber-rich foods on stool weight
Efficiency
index
¼
increase in stool weight ðgÞ
fiber intake ðgÞ
Wheat bran, fine
a
3.1
Wheat bran, coarse
a
6.2
Bread, whole wheat
grain, fine
a
5.6
Bread, whole wheat
grain, coarse
a
8.2
Barley fiber
concentrate
a
4.2
Oat bran
a
4.54
Soy bran
b
1.5–2.3
Carrots, raw
a
3.6
a
Daily intake of 14 g of fiber;
b
daily intake of 30–65 g of fiber.
DIETARY FIBER/Bran 1849
and gluten. This insoluble wheat fiber can be used for
the production of common foods and also for the
preparation of dietetic foods (e.g., for patients with
celiac disease).
0032 The drying of apples results in a dietary fiber con-
centrate that is rich in soluble fiber (pectins). The
combination of wheat and apple fiber influences the
ratio of insoluble to soluble dietary fiber in processed
foods.
See also: Bioavailability of Nutrients; Cereals:
Contribution to the Diet; Dietary Fiber: Properties and
Sources; Effects of Fiber on Absorption; Phytic Acid:
Nutritional Impact; Nutritional Impact
Further Reading
Bartnik M and Jakubczyk T (1989) Chemical composition
and nutritive value of wheat bran. In: Bourne GH (ed.)
Nutritional Value of Cereal Products, Beans and
Starches. World Review of Nutrition and Dietetics 60:
92–131.
Batchelor AJ and Compston JE (1983) Reduced plasma
half-life of radio-labelled 25-hydroxyvitamin D
3
in
subjects receiving a high-fiber diet. British Journal of
Nutrition 49: 213–216.
Chen HL, Haack VS, Janecky CW, Vollendorf NW and
Marlett JA (1998) Mechanisms by which wheat bran
and oat bran increase stool weight in humans. American
Journal of Clinical Nutrition 68: 711–719.
Ferguson LR and Harris PF (1999) Protection against
cancer by wheat bran: role of dietary fibre and phyto-
chemicals. European Journal of Cancer Prevention 8:
17–25.
Lairon D (1996) Dietary fibres: effects on lipid metabolism
and mechanisms of action. European Journal of Clinical
Nutrition 50: 125–133.
Rose DP, Goldman M, Connolly JM and Strong LE (1991)
High-fiber diet reduces serum estrogen concentrations
in premenopausal women. American Journal of Clinical
Nutrition 54: 520–525.
Saunders RM (1990) The properties of rice bran as a food-
stuff. Cereal Foods World 35: 632–636.
Wood P (ed.) (1993) Oat Bran. St. Paul, MN: American
Association of Cereal Chemists.
Energy Value
S Brooks, 3W Banting Research Centre, Ottawa,
Canada
R Mongeau, Ottawa, Aylmer (Que
`
bec), Quebec,
Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Caloric Value in Human Nutrition
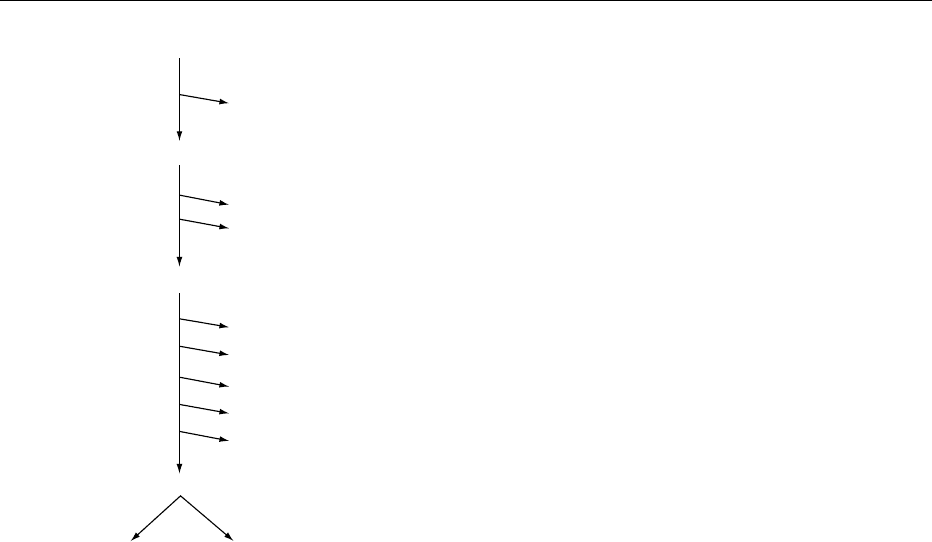
0001All food contains energy, which is needed by the body
to fuel metabolic processes. This energy is obtained
though the oxidation of macronutrients (carbo-
hydrate, fat, and protein) and is partly captured as
chemical energy in compounds such as adenosine
triphosphate (ATP). These ‘high-energy’ compounds
are used to drive many processes, including the bio-
synthesis of cellular components, the maintenance
of ion concentrations across membranes, the initial
phases of catabolic pathways, cell replication, mech-
anical function, etc. The large amount of energy
released by the hydrolysis of ATP (approximately
7 kcal mol
1
under cellular conditions) is captured
by coupling its hydrolysis to other chemical reactions
to drive them to completion.
0002The energy captured as ATP equivalents is one end
of a long chain of digestive and metabolic processes
that starts as the gross energy of the macronutrients in
ingested food (Figure 1). Significant losses in energy
occur during the digestive process. These can be
identified and quantified as losses due to incomplete
digestion and absorption (loss in fecal energy to
give apparent digestible energy; DE
app
), losses to fer-
mentation gases and urinary nitrogen energy (to
give apparent metabolizable energy), as well as
losses associated with the digestive process itself
and with the absorption and storage of food (to give
net energy). Calculating the metabolizable energy
from a food requires the collection of all excreta
(including methane and hydrogen gasses). For sub-
stances that are fermented, extra information is also
required as discussed later in this chapter. Net energy
values are more difficult to estimate and require some
assumptions about digestive processes (see section on
Factorial Models of Fermentation, eqn (19)).
0003Metabolizable energy values are the commonly
reported food energy values found on product labels.
These were initially determined in the 1900s by a
German scientist who was a pioneer in this area. To
honor his name, these factors are commonly referred to
as ‘Atwater factors.’ Both general and specific Atwater
factors exist. The specific values were measured in
individual foods. The general values (4 for carbohy-
drate, 9 for fat, 4 for protein, and 7 for alcohol) were
calculated from the specific Atwater factors using
tbl0004 Table 4 Different fiber concentrates of food residues
Concentrate Dietary fiber content
(gper100 g dry weight)
Beer draff 60–82
Maize bran 42–79
Soy bran 67–80
Pea seeds 83–90
Sugar-beet fiber 67–72
Citrus fiber, orange fiber 50–60
Cacao seeds 55
Carrot husks 60–68
1850 DIETARY FIBER/Energy Value
average intakes of mixed diets. The more modern
food energy values, as contained in tables such as
the US Department of Agriculture Handbook #8,
represent specific Atwater factors as modified by
Merrill and Watt. Although we refer to these factors
as metabolizable energy values for macronutrients,
the values for carbohydrate and fat are, in reality,
DE
app
values. This is because urinary and gaseous
energy losses were considered as insignificant for
these macronutrients. In the case of the protein
Atwater factors, urinary energy losses were taken into
account, but gaseous energy losses were, again, as-
sumed to be negligible. The Atwater factors for fat,
protein, and carbohydrate represent the total energy
available for metabolism, and this heat should be
equivalent to the energy measured with a whole-body
calorimeter.
0004 Digestible sugars, fats, and protein are readily
absorbed in the small intestine by physiological and
biochemical processes that capture the maximum
energy from these food components. These macronu-
trients are virtually completely absorbed by the small
intestine with little energy finding its way into the
large intestine. In fact, with diets high in digestible
sugars, fats, and protein, most of the energy entering
the large intestine comes from small intestine
secretions and from the sloughing off of dead intes-
tinal cells. It is the relative simplicity of the digestive
process coupled with the high degree of absorption
that allows the comparatively easy metabolic energy
calculation for these macronutrients.
0035Not all food components are readily digested
and absorbed in the small intestine. Nonstarch long-
chain polysaccharides such as those associated with
plant cell walls are not digested by human enzymatic
secretions. Many other carbohydrates are not well
absorbed by the small intestine. These pass into the
large intestine where they are anaerobically fer-
mented by the bacterial population that resides
there. For sake of clarity, the terms ‘undigested’ and
‘resistant’ will be used here to refer to the degradation
processes occurring in the upper gastrointestinal tract
(mostly by host digestive enzymes), and the term
‘fermentation’ and related words will be used to
refer to the bacterial degradation processes occurring
in the lower gastrointestinal tract. The energy losses
associated with fermentation are not as readily
measured, and so calculation of the metabolizable
energy for fermentable food components is not as
straightforward as it is for sugars, fats and protein.
The present chapter outlines different methods
for obtaining the metabolizable energy of food
components not digested and absorbed in the small
intestine.
0005A short note about the methods used to determine
macronutrient content of foods is in order. In some
countries like the USA and Canada, the carbohydrate
content is determined by subtracting the fat, protein,
water, and ash content of the food from the total
weight (difference method). This measurement in-
cludes both ‘available carbohydrate’ (i.e., carbohy-
drate that can be digested in the small intestine) as
well as dietary fiber. Other countries, such as Great
Britain and Australia, measure ‘available carbohy-
drate’ directly using chromatographic methods.
The carbohydrate values from this latter method do
not include dietary fiber. Specific Atwater factors
used in the USA and Canada take into account
some (but not all) of the energy losses associated
with dietary fiber because they rely on the apparent
digestibility of carbohydrate to calculate food
energy. This approach overestimates energyavailabil-
ity from mixed diets. In Europe, the general Atwater
factor for carbohydrate (4kcalg
1
, 16.7kJ) is used
to calculate carbohydrate energy after subtracting
the dietary fiber content. This method underestimates
the energy of mixed diets. When ‘available carbohy-
drate’ is measured directly, one must also measure
dietary fiber and use a dietary fiber-specific energy
value to calculate the energy contribution from diet-
ary fiber.
Ingested energy
Fecal energy
Apparent digestible energy
Gaseous energy
Urinary energy
Apparent metabolizable energy
Waste formation and excretion
Digestive processes
Heat of fermentation
Surface energy
Thermogenesis
(obligatory and nonobligatory)
Net energy
Retained energy
Milk production
Reproduction
Maintenance energy
Locomotory activity
Thermal regulation
fig0001 Figure 1 Diagrammatic representation of the energy losses
associated with various stages of digestion and the resulting
determined energy values.
DIETARY FIBER/Energy Value 1851

Digestible Energy and Metabolizable
Energy: Calculations
Digestible Energy
000 6 As noted above, the digestible energy of a substance is
the difference between the ingested energy and the
energy excreted in the feces. This is, in principle, a
simple definition but gives rise to two different digest-
ible energy values depending on how the measure-
ment is made: the apparent digestible energy and the
partial digestible energy. We begin by defining IM
s
as
the ingested mass of a measured substance FM
s
as the
amount of the substance of interest. This substance
can represent any dietary material but for the pur-
poses of this article, we will assume that it escapes
digestion in the small intestine and reaches the large
intestine where it may (or may not) be fermented. If
we further define recovered in the feces, the apparent
digestibility (D
app
) can be calculated as:
D
app
¼ðIM
s
FM
s
Þ=IM
s
:ð1Þ
The apparent digestible energy value (DE
app
)is
simply the apparent digestibility multiplied by the
heat of combustion ( DH
c
) of the material:
DE
app
¼ D
app
H
c
:ð2Þ
0007 The DE
app
takes into account only the energy loss
associated with incomplete digestion of a single food
component and not any potential interaction of the
component with macronutrients. If the food component
is fermented, part of its energy value will be captured
and utilized by the host, part of the remaining energy
will appear in the feces, and part will be lost to other
processes (discussed below). Thus, the DE
app
overesti-
mates the true digestible energy.
0008 Partial digestible energy values (DE
part
) describe
the influence of a dietary substance on the energy
digestibility of the whole diet. As such, they represent
a more complete picture of the energy intake and
excretion associated with ingestion of a particular
food component. Calculating DE
part
values requires
measurement of overall energy balance. The first step
is to define the energy digestibility (D
energy
) of the
overall diet as:
D
energy
¼ðIE
T
FE Þ=IE
T
, ð3Þ
where IE
T
is the total ingested energy, and FE is the
fecal energy. Partial digestible energy values are deter-
mined by adding a varying amount of a food com-
ponent (asa supplement) to an unchanging basal diet.
The energy digestibilities of the unsupplemented and
supplemented diets are then determined (eqn (3)) and
are plotted as a function of the fraction of ingested
energy that is derived from the supplement (IE
S
/IE
T
).
Ifthe supplement has a lower digestibility than that of
the overall diet, the graph should be a straight line
with a negative slope – the energy digestibility of the
overall diet will decrease with increasing amounts of
the supplement. The partial indigestibility of the sup-
plement (S
D
) is given by:
S
D
¼ D
energy
=ðIE
S
=IE
T
Þþð1 D
energy,0
Þ, ð4Þ
where D
energy,0
is the energy digestibility of the basal
(unsupplemented) diet. The DE
part
is obtained by
multiplying the partial digestibility (1 the partial
indigestibility) of the supplement by its heat of com-
bustion.
DE
part
¼ H
c
ð1 S
D
Þ: ð5Þ
0009Partial and apparent digestibilities can differ by
a considerable amount. In general, DE
part
<DE
app
because DE
part
includes energy losses that are
not accounted for by DE
app
measurements. This is
illustrated by an experiment that compared the
DE
app
and DE
part
values for the fiber component of
hard red wheat bran (bran is approximately 40%
fiber). Measurement of the diet and fecal fiber
content as well as the diet and fecal energy content
gaveaDE
app
¼1.82+ 0.17kcalg
1
(7.6kJg
1
)anda
DE
part
¼0.45+ 0.10kcalg
1
(1.9kJg
1
). The DE
app
value shows that, for every gram of wheat bran fiber
ingested, 4.151.82¼2.33kcal (9.7kJ) of energy
was lost to feces in the form of fiber. The DE
part
value shows that 1.820.45¼1.37kcal (5.8kJ) of
extra energy was lost to the feces in addition to that
lost from fecal fiber excretion. Thus, only 0.45/
1.82100%¼25% of the energy of the fermented
wheat fiber was potentially retained by the rats (see
below for other calculations on the metabolizable
energy value of wheat fiber). These calculations
show the importance of determining the total energy
losses in a digestion experiment involving ferment-
able substances.
Metabolizable Energy
0010Like digestible energy values, it is possible to define
two different metabolizable energy values. Livesey
(1993) calls the Atwater metabolizable energy values
‘apparent metabolizable energy’ values (ME
app
) be-
cause they do not take into account all the energy
losses normally associated with metabolizable energy
measurements:
ME ¼ IE ðFE þ UE þ GEÞð6Þ
where UE is the urinary energy and GE is the gaseous
energy. If a strict parallel between digestible energy
and metabolizable energy is to be made, one could
also define ME
app
as DE
app
(UE þ GE). In essence,
1852 DIETARY FIBER/Energy Value
this is the definition used by Atwater with some as-
sumptions. For fat and carbohydrate, it was assumed
that GE and UE were negligible. For protein, it was
assumed that GE was negligible but UE was not.
0012 Partial metabolizable energy values (ME
part
) can
also be calculated in a fashion analogous to DE
part
values. In this case, we define the total energy loss as:
P
E
losses
¼ FE þ GE þ UE: ð7Þ
Once again, we define the energy metabolizability of
the total diet as:
M
energy
¼ðIE
T
P
E
losses
Þ=IE
T
ð8Þ
and use this value to define a partial energy ‘non-
metabolizability’ (S
M
).
S
M
¼ M
energy
=ðIE
s
=IE
T
Þþð1 M
energy,0
Þ, ð9Þ
where M
energy,0
is the energy metabolizability of the
basal (unsupplemented) diet. The ME
part
is then given
by:
ME
part
¼ H
c
ð1 S
M
Þ: ð10Þ
Equations that minimize the error structure for ex-
perimentally determining DE
part
and ME
part
are also
available.
0013 LikeDE
part
values,ME
part
valuesdescribethe influ-
ence of a part of the diet on the energy metabolizabil-
ity of the whole diet. For protein and fat, it is thought
thatME
part
>ME
app
because fecal excretion of bacter-
ial nitrogen and fat will decrease the ME
app
value.
Note that energy losses to bacterial fat and nitrogen
are not simply due to the loss of macronutrient mass.
Energy is required for the de novo bacterial synthesis
of fat- and protein-containing macromolecular struc-
tures,whicharethenexcretedin the feces.Thisenergy
is also lost. For carbohydrates, it is thought that
ME
part
and ME
app
are equivalent since bacteria do
not contain appreciable amounts of carbohydrate.
For carbohydrates that escape digestion in the small
intestine and are partially or completely fermented,
like those in dietary fiber, ME
app
>ME
part
because of
theextraenergylossesthatoccurduringfermentation.
This energy loss is the subject of this chapter.
Metabolizable or Net Energy?
0014 The discrepancies between ME
app
and ME
part
energy
values mean that a more accurate system for measur-
ing food metabolizable energy must be employed for
diets containing appreciable amounts of undigested
and fermented carbohydrates as the greatest differ-
ences occur with these food components. Many
methods can be used to obtain the ME values of
food components that are not digested by the small
intestine. Whole-body calorimetric measurements (in
a chamber where an individual can be placed for
experimentation) can be used to determine the energy
value directly,estimates of the metabolizability can be
obtained from mixed diets, body energy measure-
ments can be made in laboratory animals and related
back to the metabolizable energy of the food, and
theoretical calculations can provide a general model
for estimating metabolizable energy.
0015Large differences exist between the energy losses
associated with the digestion of macronutrients (fat,
carbohydrate, and protein) and undigested and fer-
mented carbohydrates. Figure 2 shows an example of
thevariousprocesses that occurinthegastrointestinal
tract during the consumption of a partially digested
andpartially fermentedfood.Because the finalenergy
value is net of all major losses of energy, many re-
searchers have called it a net metabolizable energy
(NME). This should not be confused with the net
energy values used for livestock, which represent dif-
ferent energy measurements. Also, it is not the net
energy of Figure 1, which incorporates many more
energy losses. The NME is a more practical measure
of food energy for undigested and fermented sub-
strates because it takes into account energy losses
that do not occur for fat, carbohydrate, and protein.
Use of this value would allow comparisons between
the energy values of different food components.
Mathematical Models for Undigested and
Fermented Compounds: Sugar Alcohols
0016It is technically difficult and expensive to measure all
stages of the fermentation process and determine the
Oral ingestion
Urine
Small intestine
Absorption
Available
energy
Large intestine
Feces Fermentation
Increased
bacterial
mass
Heat
H
2
and CH
4
Absorption
Short-chain
fatty acids
Obligatory
heat loss
A
B
C
d
ab
c
fig0002Figure 2 Scheme showing various stages of digestion of a
macronutrient that is partially digested in the small intestine
and partially fermented in the large intestine. The letters repre-
sent fractions of the initial amount that proceed down various
pathways (see text).
DIETARY FIBER/Energy Value 1853

energy losses associated with each step. This is true of
human whole-body calorimetric measurements since
analysis requires a complete energy balance profile
for each subject (see below). A more simplistic ap-
proach involves the construction of a mathematical
model of the digestive events in the large intestine.
This approach has most commonly been applied to
the energy value of sugar alcohols but can also be
extended to other partially or completely fermentable
carbohydrates.
0017 Common dietary sugars (such as glucose, galact-
ose, and fructose) have a ketone or aldehyde group
that allows cyclization to a hexose or pentose ring
structure. Reduction of this carbonyl group to an
alcohol produces a sugar alcohol that cannot cyclize.
The resulting sugar alcohols are not well absorbed in
the small intestine so that a significant percentage
can pass intact into the large intestine. Isomalt (Pala-
tinit
1
), an equimolar mixture of two disaccharide
alcohols ( a-d-glucopyranosyl-1,1-d-mannitol and a-
d-glucopyranosyl-1,6-d-sorbitol), is an example of a
sugar alcohol that is virtually unabsorbed in the small
intestine. It passes intact into the large intestine,
where it is rapidly and completely fermented by the
bacterial population that resides there. As such, it
represents a model for a theoretical dietary fiber
that is completely fermented in the large intestine
and can be used as a basis for constructing a math-
ematical model.
0018 A good mathematical model seeks to define all
losses that occur during fermentation of ingested
material and assign reasonable factors to these losses.
Figure 2 presents the scheme for such a model.
Fermentable substances provide carbon units for
bacterial growth and reproduction and serve as
metabolizable substrates to meet the colonic bacterial
population’s energy requirements for maintenance
and growth. Four major products result from anaer-
obic colonic fermentation: increased bacterial mass
(factor a, Figure 2), heat energy loss due to fermenta-
tion (factor b, Figure 2), methane and hydrogen gas
(factor c, Figure 2), and short-chain fatty acids
(SCFAs). Humans can only use SCFAs as an energy
source. Thus, the metabolic energy yield of the sugar
alcohol has been reduced by the losses to bacterial
mass, as well as the heat and gas produced during
fermentation. It has also been reported that SCFAs
may produce significantly less ATP per gram of
absorbed SCFA than glucose, so an additional energy
loss to this difference in metabolic efficiency should
also be included (factor d, Figure 2).
0019 The fermentation model of Figure 2 can be
expressed as an equation that incorporates the four
major processes by which energy is lost during fer-
mentation. The equation describing the scheme of
Figure 2, as it applies to sugar alcohols, was first
developed for the Nutrition Council of Holland and
has been recognized by several authors as an alterna-
tive method for calculating the energy value of sugar
alcohols. For partly fermentable substances, it can be
written as:
NME ¼H
c
½ðA BÞþ a ð1 A CÞ b,
ð11Þ
where, DH
c
is the heat of combustion of the substance
in question, A represents the fraction of the ingested
substance absorbed in the small intestine, B repre-
sents the fraction of absorbed substance that is
metabolized, and C represents the fraction of the
ingested substance that is excreted intact in feces
(Figure 2). The factor a represents the proportion of
energy derived from fermentation of the substance. It
can be calculated from eqn (12):
a ¼ð1 a b c Þd :ð12Þ
The factor b was not present in the original equation
and represents the extra energy lost due to the pres-
ence of the fermentable carbohydrate. This can come
about through binding to macronutrients, changes in
osmotic balance, alterations in transit time, changes
in intestinal viscosity, inhibition of digestive enzymes,
inhibition of macronutrient uptake, or increased
sloughing of intestinal cells. As discussed above (see
section on DE
part
and ME
part
), measuring the appear-
ance of an undigested food component in the feces is
not sufficient to account for all of the energy losses
that can occur when a diet is supplemented with a
fermentable carbohydrate. In a practical sense, it is
impossible to differentiate between any ‘extra’ energy
lost to the feces (b) and the energy lost due to in-
creased bacterial mass that results from fermentation
(factor a). The solution is to set b ¼ 0 in eqn (11) and
measure total fecal output.
0020Using the mathematical model of eqns (11) and
(12) requires a knowledge of factors A, B,andC,
which are obtained from experimental evidence. For
sugaralcohols, valuesfor factorA have been obtained
from ileostomy patients or by direct sampling using
multiple lumen tubes. Factor B (the amount of sugar
alcohol metabolized) has been estimated indirectly by
measuring urinary sugar alcohol content (the amount
of sugar alcohol not metabolized).
0021In the original factorial model paper, the Nutrition
Council of Holland held a to be constant for all sugar
alcohols and estimated it at 0.5. This value included
fermentation energy losses resulting from increased
microbial mass excreted in the feces (a 0.2), loss
of H
2
and CH
4
(c ¼0.03–0.08), heat produced by
microbes (b ¼0.02–0.05), and higher heat loss during
utilization of short-chain fatty acids as compared
1854 DIETARY FIBER/Energy Value
with glucose (d ¼0.80–0.85). Summing these factors
gives a value of 0.40–0.53. The value of 0.5 was
adopted because the Nutrition Council of Holland
did not want to suggest that the value was known
with high precision (they could have used 0.46–0.47
as the average) and because they wanted to include
some energy lost to increased cell proliferation in the
large intestine caused by increased fermentation.
Thus, the slightly higher value of 0.5 was chosen as
an estimate of the total losses during fermentation. In
a later review of the value of a, it was determined that
the individual factors that contribute to a should give
avalue closerto 0.6. However, in the same review, the
author noted that the factorial procedure overesti-
mates energy values to the extent that it takes no
account of osmotic effects of the sugar alcohol.
Thus, the results of energy values calculated by the
Dutch method are probably fairly accurate. Many
researchers agree that the value of 0.5 represents a
reasonable estimate of the fermentation process.
0022 For completely fermentable substrates that are not
absorbed by the small intestine, like sugar alcohols,
the MNE value is 0.5DH
c
because A ¼0 and C ¼0.
As indicated above, the value of b is set to 0, and any
‘extra’ energy lost due to ingestion is incorporated
into the factor a, which has been estimated at 0.5.
Thus, for the sugar alcohol isomalt (which is com-
pletely fermented and not absorbed by the small in-
testine), the energy value is approximately 1.9kcal
g
1
(8kJ g
1
, approximately half the heat of combus-
tion). For completely fermentable dietary fibers, the
0.5 DH
c
rule of thumb represents an upper limit of
the NME since dietary fiber is known to bind fats and
cholesterol as well as inhibit carbohydrate absorp-
tion. Thus, dietary fiber may interact with other
macronutrients to decrease their metabolizable
energy values. This will give a lower NME value for
dietary fiber since there will be a higher excretion of
fecal energy.
0023When all the energy losses are included, the energy
value of dietary fiber can be small compared with the
ingested value. For example, measurements with hard
red spring wheat fiber give NME values of approxi-
mately 0.3kcalg
1
, even though the total ingested
energy is approximately 4kcalg
1
. In the case of
hard red spring wheat bran, much of the energy is
lost to excretion; wheat bran dietary fiber is incom-
pletely fermented so that approximately 60% is lost
directly to the feces. In addition, a significant loss of
energy comes from dietary fiber interactions with
macronutrients plus other losses due to the presence
of wheat bran fiber (b). Finally, energy is lost during
the fermentation process itself. The energy value of
hard red spring wheat fiber is not typical of other
dietary fibers, as shall be shown in the following
sections (Table 1).
Whole-body Calorimetric Measurements:
Lactitol as a Model
0024Indirect calorimetry (a type of whole body calorim-
etry where all gas consumption and gas exhalation
are continuously monitored) canbe used todetermine
energy expenditure through the use of equations re-
lating total O
2
consumption, CO
2
expiration, and
urinary nitrogen excretion to energy utilization. One
such equation is:
Heat production ¼ 16:175O
2
þ 5:021 CO
2
5:987 urea N 4:5H
2
: ð13Þ
In addition to total energy production, indirect calor-
imetry provides information on macronutrient util-
ization through the nonprotein respiratory quotient
tbl0001 Table 1 Potential error associated with oxidation of 50 g of a carbohydrate versus fermentation and oxidation of the released short-
chain fatty acids
a
Substrate RQ Oxidation substrates (mol)
b
O
2
consumed (mol) H
eqc
kcalper 50 g
50 g of sucrose 1.00 0.146 CHO 1.753 5.012 250.6
50 g of lactitol 0.943 0.409 (CHO) þ 0.099 (fat) 1.113 (CHO) þ 0.269 (fat) 4.981 (CHO) þ4.682 (fat) 153.1
50 g of lactitol 0.943 0.508 SCFA 1.382 4.663 144.5
a
The table illustrates the potential error involved in measuring the apparent metabolic energy value of a completely fermentable supplement (lactitol) by
indirect calorimetry. This was done by calculating the apparent metabolic energy value using two different methods. It was assumed that lactitol was
fermented to a mixture of SCFAs followed by complete oxidation to CO
2
and water plus fermentation gases. In an indirect calorimeter, oxidation of SCFAs
can be mistaken as oxidation of fat and carbohydrate in a 0.195:0.808 ratio. Oxidation of these substrates would give 153.1 kcal per 50 g. Oxidation of the
SCFA directly would give 144.5 kcal per 50 g of original lactitol. Thus, an error of approximately 6% can occur when indirect calorimetry is used to
measure the metabolic energy value of lactitol. The yield of SCFAs from lactitol and the proportion of acetate, propionate, and butyrate (115.1:35.38:24.5
per 100 mol of glucose) represent weighted averages of literature values for ruminants. As such, these calculations approximate the actual error
associated with indirect calorimetry, which varies from individual to individual.
b
Equivalent mol of sucrose or SCFAs obtained from 50 g of substrate.
c
H
eq
values from Livesey G and Elia M (1988) Estimation of energy expenditure, net carbohydrate utilization, and net oxidation and synthesis by indirect
calorimetry: evaluation of errors with special reference to the detailed composition of fuels. American Journal of Clinical Nutrition 47: 608–628. The H
eq
value for glucose was used for carbohydrate and the H
eq
value for the fat mixture of an omnivore diet were used for fat. The H
eq
value for SCFAs
represents a weighted average.
DIETARY FIBER/Energy Value 1855
