Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


internet by ProMED-mail, the Program for Moni-
toring Emerging Diseases. ProMED-mail is a pro-
gram of the International Society for Infectious
Diseases (ISID) and acts as a global electronic
reporting system for outbreaks of emerging infec-
tious diseases. These outbreaks are all unconfirmed
and the only source for these outbreaks is media
reports.
0016 Two large unconfirmed botulism outbreaks have
been reported in the north African countries Algeria
and Morocco. Algerian state-run radio was cited as
reporting that an outbreak of botulism killed 17
people in July 1988 in eastern Algeria. One of the
foods suspected in this outbreak was a processed
meat known as kashir. Another unconfirmed out-
break was reported by Associated Press, stating that
mortadella sausage was responsible for a suspected
botulism outbreak in Morocco during September
1999 with at least 10 deaths.
0017 Reuters news agency had reported an unconfirmed
botulism outbreak involving 90 cases with four
deaths from consumption of fish at a wedding recep-
tion in the former Soviet republic of Azerbaijan. The
Russian news agency Itar-Tass has reported an out-
break of botulism in southern Siberian Buryat, with
48 cases and four deaths after consumption of fish
from Lake Baikal. More than 70 cases of botulism,
including eight deaths, had been reported in Buryatia
in 1999.
Conclusions Regarding Epidemiology
0018 Most botulism outbreaks have occurred in the north-
ern hemisphere, particularly in countries north of the
Tropic of Cancer. Argentina is the only country in the
southern hemisphere which has reported several out-
breaks. Seasonal trends in outbreak frequencies are
apparent in some areas. In Canada, Alaska, and
Poland most outbreaks occur from May until Octo-
ber, whereas in China most outbreaks occur in the
late winter and spring. There is a strong association
between the prevalent outbreak type and the
prevalent environmental type, as well as the impli-
cated food. In colder regions, Canada, Alaska,
Greenland, Scandinavia, parts of Russia, Iran, and
northern Japan, type E causes most botulism out-
breaks and is the prevalent environmental type.
The implicated foods are usually fish or marine
mammals. In central Europe, type B causes most out-
breaks and is the prevalent environmental type.
Meats, particularly home-cured smoked ham, are
the major cause of botulism. In the western USA,
Argentina, and China, type A causes the majority of
outbreaks and is the prevalent environmental isolate.
In these areas, the most frequently implicated foods
are vegetables.
Clinical Aspects
Symptoms
0019The disease may vary from a mild illness which may
be overlooked or misdiagnosed to a serious disease
which may be fatal within 24 h. The onset of symp-
toms typically occurs 12–36 h after ingestion of toxin,
with a range from a few hours to 14 days. Usually,
the earlier symptoms appear, the more serious the
disease. The first symptoms are generally nausea
and vomiting. Mainly neurological signs and symp-
toms appear next, including visual impairments
(blurred or double vision, ptosis, fixed and dilated
pupils), loss of normal mouth and throat functions
(difficulty in speaking and swallowing, dry mouth,
throat and tongue, a sore throat), general fatigue
and lack of muscle coordination, and respiratory im-
pairment. Other gastrointestinal symptoms include
abdominal pain, diarrhea, and constipation. Diarrhea
occurs relatively early in the course of the disease,
whereas constipation persists in the advanced stages.
Respiratory failure and airway obstruction are the
main causes of death. Fatality rates in the first half
of the century were about 60%, but with the avail-
ability today of antisera and modern respiratory sup-
port systems, they have decreased to about 10%.
Treatment
0020Initially, treatment of botulism is directed toward
removing or inactivating the toxin by (1) neutralizing
circulating toxin with antiserum; (2) enema or treat-
ment with cathartics to remove residual toxin from
the bowel; and (3) in the absence of vomiting, gastric
lavage or treatment with emetics. Induced emesis
or gastric lavage is recommended if exposure has
occurred within several hours. Treatment with anti-
serum is most effective in the early stages of the
illness. The impact of antiserum is obvious from the
Chinese data; before the availability of antisera in
1960, the death rate in China was approximately
50%, but it was only 8% in the nearly 4000 patients
who received antitoxin. Subsequent treatment is
mainly to counteract the paralysis of the respiratory
muscles by artificial ventilation.
Diagnosis
0021The initial diagnosis of foodborne botulism is based
on the patient’s signs and symptoms, and perhaps
food history. It must be confirmed by detecting
toxin or viable C. botulinum in a suspect food or
clinical sample, or by epidemiological association
with a laboratory-confirmed case. Serum, feces,
enema fluid, stomach contents, and autopsy sections
of the small and large intestines, and of the liver, are
1416
CLOSTRIDIUM
/Botulism

suitable specimens for neurotoxin detection. Except
for serum, these specimens are also suitable for
detecting viable C. botulinum. The methods for
detecting the neurotoxin and C. botulinum are given
in the previous article Clostridium: Occurrence of
Clostridium botulinum. Other than neutralizing any
acidic samples, little special treatment is required.
Occasionally, extracts prepared from feces cannot be
filter-sterilized. In that case, tetracycline should be
added to 200 p.p.m. to control infection of the mouse.
Case History
0022 One of the most severe, nonfatal cases of botulism
was documented in a patient in the UK. Within ap-
proximately 10 h of eating the suspect food, he had
blurred and double vision followed by nausea and
vomiting, difficulty in swallowing and talking, dry-
ness of the mouth, and arm weakness. He suffered a
respiratory arrest on arrival at the hospital and was
intubated. A progressive flaccid paralysis ensued. He
was given polyvalent antitoxin, a tracheostomy was
done and total parenteral nutrition (TPN) was
started. There was little change in his condition for
100 days. His ptosis did not resolve until 46 days after
onset of symptoms. TPN was stopped on day 158,
and respiratory support was withdrawn after 173
days. He was not discharged until after 237 days.
Prevention
0023 Usually, the preservation of high-moisture foods is
geared toward control of C. botulinum, which usually
involves inhibition rather than destruction. Such con-
trol generally also insures control of other foodborne
pathogens and of many spoilage microorganisms. The
effects of different factors on the growth of C. botuli-
num in foods are described in the previous entry Clos-
tridium: Occurrence of Clostridium botulinum.
Control of C. botulinum in commercial foods is gener-
ally achieved by one of the following methods:
1.
0024 Low-acid shelf-stable canned foods are preserved
by a full thermal process.
2.
0025 Shelf-stable canned cured meats are preserved by a
combination of thermal processing and addition
of salt and nitrite.
3.
0026 Canned acid foods are preserved by a pasteurizing
thermal process and acidity.
4.
0027 Products such as dry fermented sausages are pre-
served by reduced water activity (a
w
) and pH, and
added nitrite.
5.
0028 Packaged raw or cooked meats or fish and shell-
fish are preserved by refrigeration alone < 3
C.
6.
0029 Many meat and fish products are preserved by a
combination of added salt and refrigeration.
7.
0030Many cured meat products are preserved by a
combination of added salt and nitrite, and refriger-
ation.
8.
0031Vacuum-packaged smoked fish are preserved by a
combination of thermal processing, added salt,
smoking, and refrigeration.
0032Foodborne botulism, caused by home-prepared
foods, can be prevented by strict adherence to proper
home-canning instructions. The type of container,
levels of acidity, and salt and heat treatments are
especially important for home-canning. All home-
canned foods should be brought to a boil before
they are eaten. An effective botulinal toxoid is avail-
able from the US Centers for Disease Control for
immunization. Immunization of high-risk popula-
tions, such as Alaskan and Canadian Inuits, has
been repeatedly proposed, but never implemented.
At present, only laboratory workers at risk are
immunized.
Neurotoxin
0033As previously stated, seven serologically different
neurotoxins are produced by various strains of C.
botulinum. Some strains of C. butryricum produce
type E neurotoxin and some strains of C. barati can
produce type F neurotoxin. In general, the designa-
tion of the strain is that of the toxin it produces.
Strains of subtypes. AB, AF, BA, and BF are rare and
produce, in addition to the first toxin type, lesser
amounts of toxin of the second type. Type C and D
strains often produce small amounts of D and C
toxin, respectively. They may also produce a toxin,
C2, which is distinctly different from the neurotoxin
and will not be discussed here.
Structure
0034The neurotoxins are all very similar proteins with a
molecular weight of approximately 150 kDa. They
are synthesized as a single-chain protein with rela-
tively low toxicity, which is activated when it is
‘nicked’ by a number of proteases, including prote-
ases of group I C. botulinum, into a double-chain
molecule which is held together by a disulfide bond.
The two components of the nicked toxin, a light
(L) and heavy (H) chain, have molecular weights
of approximately 50 and 100 kDa, respectively.
Individually, the two components are not toxic, but
toxicity can be restored by reestablishing the disulfide
bond.
0035The neurotoxins exist as complexes of four
molecular sizes: 7S, 12S, 16S, and 19S, designated S
(small), M (medium), L (large), and LL (extra large)
toxins, respectively. The molecular weights range
CLOSTRIDIUM
/Botulism 1417

from 150 to 900 kDa. The M form is the most
common natural form, found in foods and cultures,
along with the L form. It is a complex of the S form
with an atoxic component. In the L form, a hemag-
glutinin is also part of the complex. The LL form is
only known for type A, and was the first form of the
botulinum toxin to be purified and crystallized. It has
not been found in cultures and is probably an artifi-
cial aggregate. The M and L forms are referred to as
progenitor toxins. They dissociate under mild alka-
line conditions into the S, or derivative toxin, which is
the single-chain protein.
Mode of Action
0036 Botulinum neurotoxins cause paralysis by blocking
acetylcholine release at neuromuscular junctions.
Cholinergic systems are affected the most, but adre-
nergic systems may also be affected by high concen-
trations of toxin. Paralysis appears to be caused by a
four-step process. First, the toxin binds to a type-
specific receptor on the presynaptic membrane. This
step is mediated by the carboxy-terminus of the H
chain. Second, the toxin is internalized into the nerve
cell by an initial receptor-mediated endocytosis. Acid-
ity in the endosome is believed to induce the amino-
terminus of the H chain to form channels in the
endosome membrane which permit the L chain to
enter the cytoplasm. Finally, the light chain acts as a
zinc endopeptidase and hydrolyzes the SNARE pro-
teins responsible for synaptic vesicle fusion: synapto-
brevin, syntaxin, or SNAP-25. Cleavage of the
SNARE proteins results in inhibition of acetylcholine
release, ultimately resulting in flaccid paralysis.
Inactivation
0037 The most effective means of inactivating botulinum
toxins in foods is by heat. Heat inactivation curves
are biphasic, with an initial steep decline that levels
off with time. Foods, especially those high in protein,
colloidal components, or ionic strength, have a pro-
tective effect. The toxin is most stable between pH 4
and 5. For the safe thermal inactivation of toxin at
concentrations up to 10
5
LD
50
per gram, time/tem-
perature combinations of 20 min at 79
C or 5 min at
85
C have been recommended. Other means of toxin
inactivation include treatment with chlorine or
ozone.
See also: Canning: Principles; Chilled Storage:
Microbiological Considerations; Packaging Under
Vacuum; Clostridium: Occurrence of Clostridium
botulinum; Fish: Spoilage of Seafood; Heat Treatment:
Chemical and Microbiological Changes; Lactic Acid
Bacteria; Marine Foods: Marine Mammals as Meat
Sources; Meat: Preservation; Microbiology: Detection of
Foodborne Pathogens and their Toxins; Nisin; Nitrates
and Nitrites; Nitrosamines; Smoked Foods: Principles;
Water Activity: Principles and Measurement
Further Reading
Centers for Disease Control and Prevention (1998) Botu-
lism in the United States, 1899–1966. Handbook for
Epidemiologists, Clinicians, and Laboratory Workers.
Atlanta, GA: Centers for Disease Control and Preven-
tion.
Dodds KL and Austin JW (1997) Clostridium botulinum.
In: Doyle MP, Beuchat LR, and Montville TJ (eds)
Food Microbiology. Fundamentals and Frontiers,
pp. 288–304. Washington: ASM Press.
Galazka A and Przybylska A (1999) Surveillance of food-
borne botulism in Poland: 1960–1998. Eurosurveillance
4: 69–72.
Gao QY, Huang YF, Wu JG, Liu HD and Xia HQ (1990) A
review of botulism in China. Biomedical and Environ-
mental Sciences 3: 326–336.
Hauschild AHW and Dodds KL (1993) Clostridium botuli-
num: Ecology and Control in Foods. New York: Marcel
Dekker.
Pellizzari R, Rossetto O, Schiavo G and Montecucco C
(1999) Tetanus and botulinum neurotoxins: mechanism
of action and therapeutic uses. Philosophical Transac-
tions of the Royal Society of London Series B – Bio-
logical Sciences 354(1381): 259–268.
Proulx JF, Milot-Roy V and Austin J (1997) Four outbreaks
of botulism in Ungava Bay, Nunavik, Quebec. Canada
Communicable Disease Report 23: 30–32.
Shapiro RL, Hatheway CL and Swerdlow DL (1998) Botu-
lism in the United States: a clinical and epidemiologic
review. Annals of Internal Medicine 129: 221–228.
Smith LDS and Sugiyama H (1988) Botulism: The Organ-
ism, Toxins, the Disease, 2nd edn. Toronto: Academic
Press.
St Louis ME, Peck SH, Bowering D et al. (1988) Botulism
from chopped garlic: delayed recognition of a major
outbreak. Annals of Internal Medicine 108: 363–368.
Therre H (1999) Botulism in the European Union. Euro-
surveillance 4: 2–7.
Villar RG, Shapiro RL, Busto S et al. (1999) Outbreak of
type A botulism and development of a botulism surveil-
lance and antitoxin release system in Argentina. Journal
of the American Medical Association 281: 1334–1338,
1340.
Weber JT, Hibbs RJ Jr, Darwish A et al. (1993) A massive
outbreak of type E botulism associated with traditional
salted fish in Cairo. Journal of Infectious Diseases 167:
451–454.
1418
CLOSTRIDIUM
/Botulism

COBALAMINS
Contents
Properties and Determination
Physiology
Properties and Determination
MJa
¨
gerstad and K Arkba
˚
ge, Swedish University of
Agricultural Sciences, Uppsala, Sweden
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Discovery
0001 The history of cobalamin (vitamin B
12
) started almost
two centuries ago, when a fatal anemia, resulting
from some disorder of the digestive and assimilative
organs, was described. The symptoms of this anemia,
named pernicious anemia, was further outlined during
this century, but a successful treatment of this fatal
disease was reported first in 1926, when raw liver
was introduced as therapy. In 1929, it was shown
that the intestinal absorption of the antipernicious
anemia principle in liver (extrinsic factor) required
prior binding to an ‘intrinsic factor’ secreted by the
stomach. The search for the active principle in liver
culminated with the isolation of vitamin B
12
in 1948
by a team of Merck scientists in the USA, which
was confirmed by a British team 3 weeks later. Injec-
tions of the isolated vitamin B
12
into muscular tissues
induced a dramatic beneficial response in patients
with pernicious anemia. Hodgkin and coworkers elu-
cidated the chemical structure of cyanocobalamin
(vitamin B
12
) by X-ray crystallography in 1964,
for which they were rewarded the Nobel Prize in
chemistry (the second Nobel Prize designated for
this vitamin).
Structure, Nomenclature, and Physical
Properties
0002 Cobalamins have a complex chemical structure based
on a corrin ring system. Four reduced pyrrole rings
are linked together to form a macrocyclic ring desig-
nated corrin, which is chelated by the four pyrrole
nitrogens. Corrin resembles the heme of hemoglobin,
but has one less a-methene bridge and has cobalt (Co)
instead of iron at the center. All the compounds con-
taining this ring are designated corrinoids. The fifth
co-ordinate covalent bound to cobalt is a nitrogen
of a dimethylbenzimidazole moiety. Thus, as with
nucleic acids, cobalamins contain a nucleotide, in
this case 5,6-dimethylbenzimidazole, rather than
various purine or pyrimidine bases. And the sugar,
ribose, has an a-glycosidic linkage, unlike the b-link-
age in the nucleic acids. The d-1-amino-2-propanol
moiety of the molecule is esterified to the nucleotide
and joined in amide linkage to the porphyrin-like
nucleus. The sixth position of cobalt may be
occupied by different anionic ligands, (R) according
to Figure 1 and Table 1. This ligand can be one of the
following types, e.g., – CN(cyanocobalamin), – OH
(hydroxocobalamin), – H
2
O (aquacobalamin), –NO
2
(nitritocobalamin), – SO
3
(sulfitocobalamin), – CH
3
(methylcobalamin), or – 5
0
-deoxy-5
0
-adenosyl (adeno-
sylcobalamin). Approximately 20 naturally occurring
analogs of cobalamins have been identified. Some of
these have no biological activity in mammals,
whereas others exhibit at least partial vitamin activity
but are often poorly absorbed. Human serum con-
tains not only the active cobalamins (mainly the
hydroxo-, methyl-, and adenosyl-cobalamins) but
also a variable number of analogs that are biologic-
ally inactive noncobalamin corronoids. These vita-
min B
12
analogs have their base of nucleotide,
5,6-dimethylbenzimidazole replaced by other nucle-
otides, e.g., adenine, guanine, 5-methyl-adenine, or
5-hydroxybenzimidazole.
0003According to the nomenclature of the
corrinoids, a cobalamin is a cobamide in which 5,6-
dimethylbenzimidazole is the aglycon attached by a
glycosyl link. Consequently, the systemic name of
cobalamin given by IUNS (International Union of
Nutritional Sciences) and IUPAC (International
Union of Pure and Applied Chemistry) is: Coa-
[a-(5,6-dimethylbenzimidazolyl)]-Cob-‘R’ cobamide,
where ‘R’ denotes ‘cyano,’‘aqua,’‘hydroxo,’
‘nitrito,’‘adenosyl,’‘methyl,’ etc) IUNS and IUPAC
state that the term ‘vitamin B
12
’ should be used as the
generic description for all corrinoids exhibiting quali-
tatively the biological activity of cyanocobalamin,
whereas the term ‘corrinoid(s)’ should be used as the
generic descriptor for all compounds containing the
corrin nucleus and thus chemically related to cyano-
cobalamin. The term ‘vitamin B
12
’ has two meanings.
COBALAMINS/Properties and Determination 1419
To the chemist, it means only cyanocobalamin,
whereas in the nutrition and pharmacology literature,
vitamin B
12
is a generic term for all the cobamides
exerting biological activity in humans. So far, all the
cobamides found to play a role in human metabolism
have been cobalamins. Table 1 summarizes the
generic or trivial names for different cobalamins,
together with their abbreviations, CAS numbers,
and physical properties.
Chemical Properties, and Stability
Cyanocobalamin
0004 Cyanocobalamin, a synthetic form of vitamin B
12
,is
commercially available for medical use, food fortifi-
cation, and nutrient supplements. The original iden-
tification of vitamin B
12
as cyanocobalamin involved
its formation as an artefact of the isolation procedure.
Little or no naturally occurring cyanocobalamin
exists in foods. Thus, cyanocobalamin is normally
not present in humans except in very small amounts
in smokers. It is a tasteless, odorless, red crystalline
substance with good water solubility. The color of the
compound may pose a limitation in the possible add-
ition of cyanocobalamin to certain foods, especially
light-colored products (e.g., white bread).
0005 The cyanide group of cyanocobalamin can be
replaced by other ions to form hydroxycobalamin,
chlorocobalamin, nitritocobalamin, thiocyanatoco-
balamin, and others; for example, bisulfite ions cause
conversion of aquacobalamin to sulfitcobalamin, and
similar reactions can occur to form cobalamins sub-
stituted with ammonia and nitrite. These reactions
have little influence on the net vitamin B
12
activity
of foods, since all the cobalamines are readily recon-
verted to cyanocobalamin after treatment with
cyanide, except methylcobalamin.
0006Cyanocobalamin appears to be the most stable of
the various vitamin B
12
analogs, being stable in air
and in dry form, and is even relatively stable at 100
C
for a few hours. Aqueous solutions at pH 4–7 can be
autoclaved at 120
C. Cyanocobalamin is slowly de-
composed by ultraviolet or strong visible light. The
cyano group is split off, yielding hydroxocobalamin,
which is also a biological active form of vitamin B
12
(and is available for medical use and used in fortifica-
tion by the food industry). Prolonged exposure to
light, however, causes irreversible decomposition
and inactivation.
0007Mild acid hydrolysis of cyanocobalamin induces
the removal of the nucleotide, and additional frag-
mentation occurs as the severity of the acidic condi-
tions increases. Exposure to alkaline conditions
causes hydrolysis of amides, yielding biologically in-
active carboxylic acid derivatives of vitamin B
12
.In
solution, thiamin and nicotinamide, or nicotinic acid,
destroy cyanocobalamin slowly, whereas the addition
of small amounts of iron or thiocyanate appear to
protect it. However, the literature is not definitive.
N
N
H
O
O
O
O
HO
OH
O
O
−
P
HN
H
N
N
N
CN
H
2
NOC
CONH
2
CONH
2
CONH
2
H
2
NOC
H
2
NOC
H
3
C
H
3
C
H
3
C
H
3
C
Co
+
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
N
N
N
H
O
O
O
O
HO
OH
O
O
−
P
HN
H
N
N
N
R
H
2
NOC
CONH
2
CONH
2
CONH
2
H
2
NOC
H
2
NOC
H
3
C
H
3
C
H
3
C
H
3
C
Co
+
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
N
fig0001 Figure 1 Left: Chemical structure of cyanocobalamin; right: Coenzyme B
12
/adenosylcobalamin (R ¼5
0
-deoxy-5
0
-adenosyl); methyl-
cobalamin (R ¼methyl); aquacobalamin (R ¼H
2
O
þ
); hydroxocobalamin (R ¼OH), with kind permission of Kra
¨
utler.
1420 COBALAMINS/Properties and Determination

tbl0001 Table 1 Nomenclature and physical properties of cobalamins (vitamin B
12
)
Generic name Ligand (R) Abbreviation Molar mass Formula Solubility Crystal form l
max
(nm) Absorption
(E
1cm
)
e10
3
Solvent
Cyanocobalamin
(vitamin B
12
)
– CN CNCbl 1355.38 C
63
H
88
CoN
14
O
14
P 1 g in 80 ml of water Dark red
hygroscopic
278 115 [15.6] Water
361 204 [27.6] Water
CAS No. 68-19-9
a
551 64 [8.7] Water
Aquacobalamin
(vitamin B
12a
)
–H
2
OH
2
OCbl
þ
1347.0 C
62
H
90
CoN
13
O
15
P 274 [153] 20.6 Water
351 [197] 26.5 Water
CAS No. 13422-52-1 499 [60] 8.1 Water
Hydroxocobalamin
(vitamin B
12b
)
– OH HOCbl 1346.37 C
62
H
89
CoN
13
O
15
POH Moderately soluble
in water; insoluble
in acetone,
ether, petroleum
ether, benzene
Dark red
orthorombic
279 [141] 19.0 Water
325 [85] 11.4 Water
CAS No. 13422-51-0 359 [153] 20.6 Water
516 [66] 8.9 Water
537 [71] 9.5 Water
Nitritocobalamin
(vitamin B
12c
)
–NO
2
1374.6 C
62
H
88
CoN
14
O
16
P Red crystalline
solids
352 153 [21.0] Water
528 60 [8.4] Water
CAS No. 20623-13-6
Sulfitocobalamin – SO
3
1409.5 C
62
H
89
CoN
13
O
17
PS 275 328 [46.2] Water, pH 7
CAS No. 15671-27-9 365 130 [18.3] Water, pH 7
516 61 [8.6] Water, pH 7
Adenosylcobalamin (cobamide
or coenzyme B
12
)
5
0
-deoxy-
5
0
-adenosyl
AdoCbl 1579.6 C
72
H
100
CoN
18
O
17
P Soluble in
ethanol, phenol;
insoluble in
acetone, ether,
dioxane
Yellow–orange
six-faced crystal
288 [115] 18.1 Water, pH 7
340 [78] 12.3 Water, pH 7
375 [60] 10.9 Water, pH 7
CAS No. 13870-90-1 522 [51] 8.0 Water, pH 7
Methylcobalamin
(methyl B
12
)
–CH
3
MeCbl 1344.4 C
63
H
91
CoN
13
O
14
P Bright red 266 [148] 19.9 Water
342 [107] 14.4 Water
CAS No. 13422-55-4 522 [70] 9.4 Water
a
CAS No., Chemical Abstract Service Number.
Values in brackets are calculated from the corresponding e value or E
1cm
value.
Modified from Eitenmiller.

Coenzyme B
12
0008 The coenzyme forms of vitamin B
12
are methyl-
cobalamin and adenosylcobalamin. Methylcobala-
min functions coenzymatically in the transfer of a
methyl group (from 5-methyltetrahydrofolate to
homocysteine) in methionine biosynthesis, whereas
5
0
-deoxyadenosylcobalamin serves as the coenzyme
in an enzymatic rearrangement reaction catalyzed by
methylmalonyl-CoA mutase. Both forms of vitamin
B
12
coenzymes are converted to the biological active
aquacobalamin upon light exposure. In fact, vitamin
B
12
coenzymes exhibit extreme photosensitivity,
which necessitates the use of subdued lighting and
low actinic glassware during vitamin B
12
analyses.
The photochemical degradation of vitamin B
12
coen-
zymes interferes with experimental studies of vitamin
B
12
metabolism and function, but this conversion has
no influence on the total vitamin B
12
activity of foods,
because aquacobalamin retains vitamin B
12
activity.
Surprisingly, methylcobalamin and adenosylcobala-
min are relatively stable in neutral aqueous solution
in the dark and can be heated for 20 min at 100
C.
0009 Adenosylcobalamin is unstable under acidic and
alkaline conditions, whereas methylcobalamin is
stable in the presence of dilute acid or alkali. In
general, the natural forms of cobalamins (hydroxo-,
aqua-, methyl-, or adenosylcobalamin) are inacti-
vated by severe alkaline conditions, heavy metals,
strong oxidizing agents, or strong reducing agents
(like ascorbate). A possible interaction with ascorbic
acid has been reported to be detrimental to the vita-
min, especially under heating conditions. The signifi-
cance of these observations for the processing of food
remains unclear (see further discussion below). The
mechanism of vitamin B
12
degradation has not been
fully determined, in part because of the complexity of
the molecule and the very low concentration in foods.
Cobalamins in Food
0010 Only microorganisms are able to synthesize cobala-
min, and therefore, higher plants are devoid of
vitamin B
12
unless processed microbiologically or
contaminated by fecal material, e.g., from fertilizer.
Certain legumes have been reported to absorb small
amounts of vitamin B
12
produced by bacteria associ-
ated with root nodules, but little enters the seeds.
Food from animal origin, especially organs like liver
and kidney, contain > 30 mg vitamin B
12
per 100 g.
Other rich sources are meat muscles, fish, dairy prod-
ucts, and eggs. Bovine milk contains about 0.4 mgof
vitamin B
12
per 100 g (range 0.24–0.74 mg per 100 g).
Human milk contains only about 15% as much vita-
min B
12
as bovine milk. The predominant source of
vitamin B
12
in bovine milk is biosynthesized by
microorganisms in the rumen. Hydroxocobalamin is
the predominant form of vitamin B
12
in bovine milk.
There may also be some of the light-sensitive forms of
vitamin B
12
, adenosylcobalamin and methylcobala-
min, present. In milk, most of the vitamin B
12
is
protein-bound. The significance of this is still un-
known, although a protective effect against microbial
breakdown has been suggested. The vitamin B
12
in
most animal tissues consists mainly of the coenzyme
forms, methylcobalamin and adenosylcobalamin, in
addition to aquacobalamin. Some foods or microor-
ganisms contain other corrinoids, with varying bio-
logical activity, some of which may have antivitamin
activity. Foods are classified according to their
vitamin B
12
content, as shown in Table 2.
Process and Storage Losses
0011Under most conditions of food processing and preser-
vation, there is little nutritionally significant loss of
vitamin B
12
. Cyanocobalamin added to breakfast
cereal products has been reported to undergo an aver-
age loss of 17% during processing, with an additional
17% loss during storage for 12 months at ambient
temperature. When milk is pasteurized for 2–3s, it
loses 7% of its available vitamin B
12
, and boiling
for 2–5 min destroys 30%. Sterilization in a bottle
for 13 min at 119–120
C causes a loss of 77%, and
rapid sterilization (3–4 s) with superheated steam at
143
C, i.e., ultrahigh temperature (UHT) destroys
about 10% of the vitamin.
0012There are also processes like fermentation used for
manufacture of certain dairy products, e.g., yogurt
tbl0002 Table 2 Grouping of food according to vitamin B
12
content
Food Vitamin B
12
(mg per 100 g edible weight)
Very rich sources: organ meats (liver, kidney, heart), bivalves (clams and oysters) > 10
Moderately rich sources: some fish, crabs, egg yolk 3–10
Moderate sources: muscle meats (beef), some fish, cheese, egg, milk powder 1–3
Good sources: milk, yogurt, muscle meats (pig) <1
Poor sources: vegetables, cereals, fruits and berries n.d.
n.d., not detectable.
1422 COBALAMINS/Properties and Determination

and cheeses, which might result in either increased or
decreased vitamin B
12
concentrations depending on
the vitamin B
12
-producing or -consuming micro-
organisms.
0013 Studies indicate that the storage temperature and
time have a significant influence on the rate of break-
down. Thus, if UHT milk is stored at a low tempera-
ture (þ7
C), as for pasteurized milk, the losses of
vitamin B
12
are low for up to at least half a year.
However, if storage is at ambient temperature,
which is a normal condition for an aseptic product
such as UHT milk, breakdown of the vitamin may be
considerable even within a few weeks. Fermented
dairy products might lose or gain vitamin B
12
during
storage for the reason given above.
0014 Like all water-soluble vitamins, vitamin B
12
is sub-
ject to losses through leaching into the cooking water.
This indicates the potential for substantial losses of
vitamin B
12
during prolonged heating of foods at or
near neutral pH. Typical oven heating of commer-
cially prepared convenience dinners has been shown
to yield 79–100% retention of vitamin B
12
.
0015 Ascorbic acid has long been known to accelerate
the degradation of vitamin B
12
, especially during
high-temperature heating, although this may be of
little practical significance, because foods containing
vitamin B
12
usually do not contain significant
amounts of ascorbic acid. In curing solutions for
ham, neither ascorbate nor erythorbate has been
found to have any influence on vitamin B
12
retention,
probably since this process does not include high-
temperature heating.
Determination of Cobalamins
General
0016 For detection purposes, cobalamins are all UV ab-
sorbers. Unfortunately, their absorption spectra vary
between different congeners, and detection is compli-
cated by the low concentration at which they occur
normally in foods. Available methods for assay of
cobalamins developed over the years include both
general methods, e.g., polarographic, spectrophoto-
metric, various chromatographic procedures (paper,
thin-layer, and open-column chromatography), and
more specific and/or sensitive methods, e.g., micro-
biological, protein-binding, and high-performance
liquid chromatography (HPLC) procedures.
0017 Almost all available data on vitamin B
12
in food
have been obtained by microbiological assay (MA)
using Lactobacillus delbrueckii as the test organism.
Still, the microbiological assay is the reference
method for assaying cobalamins in foods. For clinical
tissues and serum samples, rapid radioprotein-
binding assays with
57
Co-labeled cyanocobalamin
are routine. However, radioassay kits for clinical
assay are not reliable for assay of food unless care-
fully validated for matrix effects. A more recent de-
velopment of this protein-binding assay is the use of a
biosensor technique to quantify cobalamins in food.
Although the various forms of vitamin B
12
can be
separated chromatographically, HPLC methods are
not readily suitable for food analysis, even if precon-
centration steps are used, because of the very low
concentrations typically found. Below, only methods
currently in use for the assay of naturally occurring
cobalamins in foods and/or fortified foods are pre-
sented and discussed.
Extraction
0018All methods for cobalamin analyses of food samples
need a preparative step prior to analysis. Since animal
products are the sole food sources of vitamin B
12
, the
vitamin must be extracted from protein-rich matrices
under conditions that will not lead to its destruction.
As a result, extraction procedures often involve diges-
tion with protease followed by extraction in hot
(80
C) ethanol. For quantitation as total vitamin
B
12
, heating in a cyanide- or sulfate-containing buffer
has been used to extract the endogenous vitamin B
12
forms, thereby converting them to the more stable
cyano- or sulfitocobalamin forms. The AOAC official
method (952.20) recommends mixing approximately
1 g or 1 ml of the sample in 25 ml of a freshly
prepared solution containing sodium phosphate,
citric acid, and metabisulfite. The extraction is com-
pleted by homogenizing the sample in the extraction
solution, autoclaving the mixture at 121–123
C for
10 min, adjusting the cooled mixture to pH 6.0,
filtering and/or centrifugation, and diluting with
water to a vitamin B
12
concentration of approxi-
mately 0.2 ng ml
1
. However, most extraction pro-
cedures described in the literature add either sodium
cyanide (NaCN) or potassium cyanide (KCN) to
0.1 M acetate buffer, pH 4.5–4.7, during homogen-
ization and extraction. The heating time varies be-
tween 3 and 30 min, and a heating temperature of
either 100
C (boiling water) or 121
C (autoclava-
tion) is used. The heat-extraction procedure including
centrifugation and/or filtration are enough of sample
preparation for microbiological assays and protein-
binding assays (immunoassays). In the use of
HPLC, further clean-up work might be necessary,
e.g., ultracentrifugation, ultrafiltering, affinity chro-
matography, or adsorption on to charcoal or chroma-
tography on anion exchange resin and/or solid-phase
extraction on C
18
cartridges.
0019The extraction conditions have been shown to affect
significantly the efficiency of the overall extraction
COBALAMINS/Properties and Determination 1423

process. It has therefore been suggested that the ex-
traction conditions should be optimized for each type
of food sample in order to achieve reproducible and
good recoveries of the complete analysis.
Microbiological Assay
0020 Over the years, the growth response of various micro-
organisms has been used to analyze cobalamins in
foods and other biological material. The micro-
organisms that have been used are Lactobacillus
delbrueckii (previously, leichmanni), Escherichia
coli, Euglena gracilis,andOchromonas malhamen-
sis. Below, only the AOAC (Association of Official
Analytical Chemists) Official method (reference
method) based on Lactobacillus delbrueckii is pre-
sented. The other microorganisms have different dis-
advantages compared with Lactobacillus delbrueckii,
i.e., less sensitive and/or specific also towards less
active biological analogs. In addition, Euglena graci-
lis and Ochromonas malhamensis grow more slowly
and are thus more painstaking.
0021 The AOAC Official Method 952.20: Cobalamin
(Vitamin B
12
-activity) in Vitamin preparations use a
microbiological assay with Lactobacillus delbrueckii
(ATCC 7830) (45.2.02). This method was originally
collaborated for use on vitamin preparations, but the
AOAC Task Force on Methods for Nutrition Label-
ing recommended the procedure for use on all food
matrices. The Lactobacillus delbrueckii growth re-
sponse is sufficiently sensitive to quantify cyanoco-
balamin at concentrations approaching 1.0 pg per
milliliter of assay growth medium (corresponding to
less than 0.5 mg per 100 g). It has a variable response
to various cobalamins. A similar growth response has
been reported for cyanocobalamin, hydroxocobala-
min, sulfitocobalamin, nitritocobalamin, and dicya-
nocobalamin. However, adenosylcobalamin produces
a greater response, and methylcobalamin a lower
growth response. This will generally not cause a prob-
lem, since excess of cyanide or sulfite is used during
the extraction procedure prior to analysis, thereby
converting all different native forms of cobalamins
except methylcobalamin into one stable form.
0022 An important aspect in all methods of analysis is
their specificity. Lactobacillus delbrueckii can utilize
vitamin B
12
analogs, e.g., deoxyribonucleotides and
deoxyribonucleosides, in addition to biologically
active cobalamins. Older literature suggests that dilu-
tion of deoxyriboside levels (e.g., thymidine) to less
than 1 mg per milliliter of the assay medium will elim-
inate the effect.
Radioprotein-Binding Assays
0023 Radioprotein-binding assays have been used rou-
tinely for blood and tissue analysis since early work
by Rothenberg and several others during the 1960s.
The principle of this assay is based on the competition
of added isotope-labeled cyanocobalamin (
57
Co)
versus cyanocobalamin from the unknown sample
for a limited number of binding sites on cobalamin-
binding proteins (often intrinsic factor from hog). The
amount of labeled cobalamin bound to the binding
protein is quantified in a gamma counter, and the
concentration of cobalamin in the unknown sample
is inversely proportional to the concentration of
labeled cobalamin, and calculated from a calibration
curve.
0024Early types of radioprotein-binding assays for de-
termination of vitamin B
12
in clinical specimens and
foods were often inaccurate because the binding pro-
tein could bind to active forms of vitamin B
12
as well
as biologically inactive analogs. The specificity of
such assays has been greatly improved through
the use of a vitamin B
12
-binding protein (generally
porcine intrinsic factor, IF) that is specific for the
biologically active forms of the vitamin. Radioligand
binding methods based on monospecific antisera that
eliminate any cross-reactions with other cobalamins
are now available and should be used routinely in all
clinical laboratories.
0025The relative binding affinities of porcine IF for
various cobalamins have also been studied. An
equivalent affinity has been found for cyano-,
dicyano, nitrito-, and methylcobalamins, whereas
significantly different binding affinities have been
found for hydroxo-, sulfito, and adenosylcobala-
mins. Therefore, with cyanocobalamin as the calibra-
tion standard, it is necessary to add cyanide to the
samples prior to quantification during the extraction
procedure to convert all native forms to cyanocobal-
amins.
0026Studies by several research groups indicate that
radioprotein-binding assays with IF can be used for
food analysis. When optimized extraction procedures
have been used, radioprotein binding assays and
microbiological procedures show agreement for
most foods; however, differences have been noted
often enough to conclude that the two methods
are not universally interchangeable for the assay of
all foods. Thus, recent work has shown that the
radioprotein binding assay could be reliable for food
analysis, providing careful method validation includ-
ing control of selectivity. One should bear in mind
that not only the food matrix itself but also sub-
stances used during the extraction procedure, e.g.,
buffer strength, pH, cyanide concentration, and
added enzymes needed to release vitamin B
12
bound
to the food matrix, might cause nonspecific binding
and therefore must be accounted for in order to
achieve reliable results.
1424 COBALAMINS/Properties and Determination
0027 An alternative to the protein-binding assay is an
enzyme-linked protein-binding assay that appears to
be useful for fortified foods in which cyanocobalamin
is the predominant cobalamin. The procedure uses
an R-protein–enzyme conjugate and microtiter plate
techniques for quantitation of cyanocobalamin. Since
R-proteins bind cobalamins and their analogs, the
assay has not been applied to nonfortified foods.
Another drawback is that the R-protein enzyme con-
jugate is not commercially available. Applications to
(a)
(
c
)
(b)
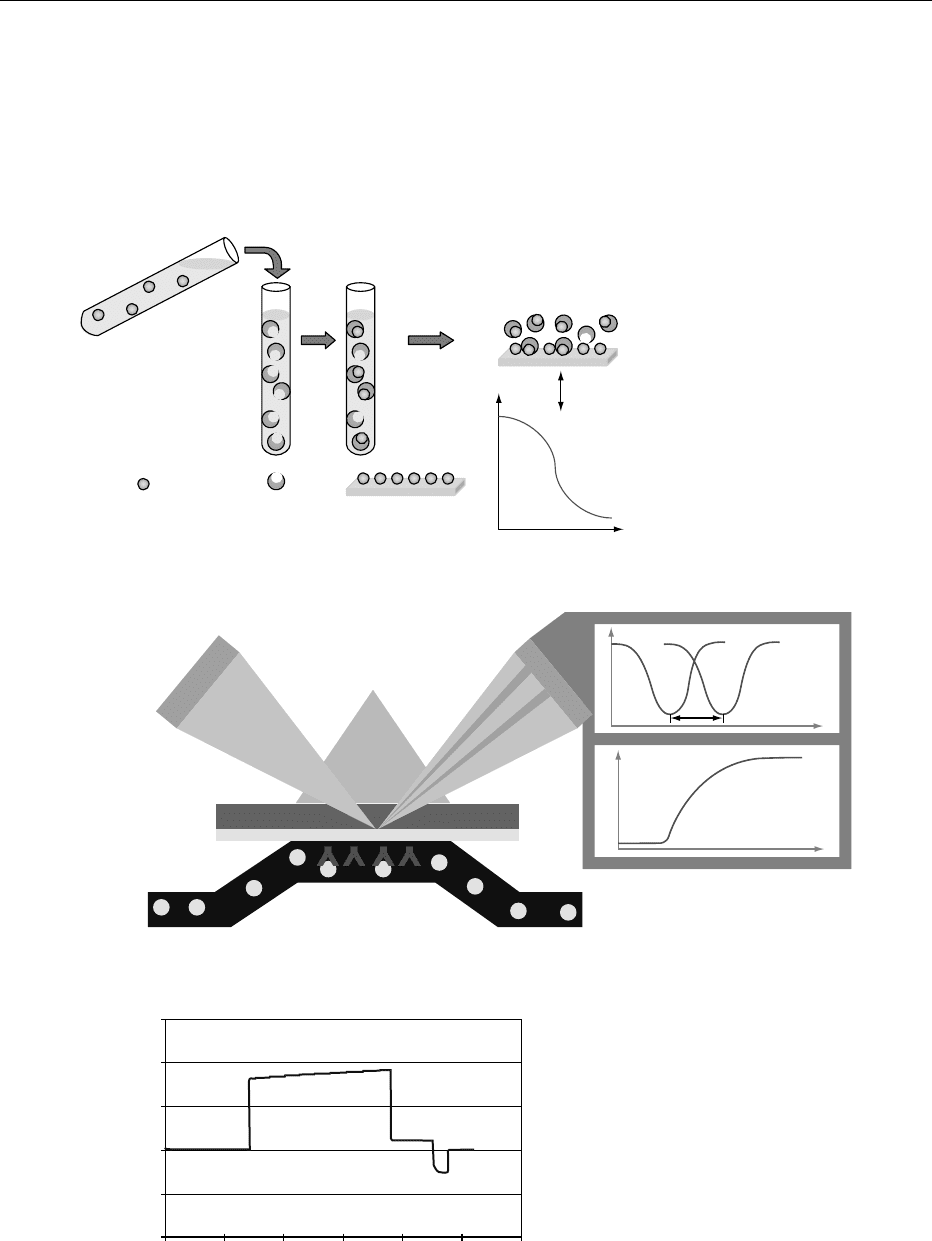
Vitamin-specific molecules are added to sample extract
Vitamin-specific
molecule
Vitamin Vitamin sensor
chip
Measurement of unbound
vitamin-specific molecules
Vitamin concentration
Response (RU)
+
Light-
source
Sensor chip with
gold film
Prism
Optical
detection
unit
Intensity
Angle
Time
Resonance
signal
Sensorgram
Flow channel
III
II
I
Polarized
light
Reflected
light
II
I
0
10 000
12 000
14 000
16 000
18 000
20 000
200 400 600
Time (s)
Sensorgram
Cobalamin, 0.6 ng/ml
Response (RU)
800 1000 1200
fig0002 Figure 2 Assay principle for determination of vitamin B
12
in food using optical biosensor technology (BIACORE).
COBALAMINS/Properties and Determination 1425
