Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.


1. LIESST and Related Phenomena
The abbreviation LIESST refers to the phenomenon
of light-induced excited spin state trapping, which
was discovered in 1984 on [Fe(ptz)
6
](BF
4
)
2
(ptz ¼1-
propyl-tetrazole), an iron(II) coordination compound
known to exhibit thermal spin transition between
high-spin (HS,
5
T
2
) and low-spin (LS,
1
A
1
) states (see
Spin Transition Compounds (Decurtins et al. 1984).
The material, as a single-crystal, polycrystalline, or
embedded in a polymer foil, converts from the LS to
the HS state by irradiation with green light into the
1
A
1
-
1
T
1,2
absorption band, followed by two suc-
cessive intersystem crossing (ISC) processes
1
T
1,2
-
3
T
1,2
-
5
T
2
to populate the HS state
5
T
2
. Ra-
diative decay
5
T
2
-
1
A
1
is spin and parity forbidden,
and due to the considerably larger M–L bond dis-
tance in the HS state as compared to the LS state,
which builds up an energy barrier between the po-
tential wells of the two states (see Fig. 1), the lifetime
of the metastable LIESST state can be very long (e.g.,
weeks at 20 K in the case of [Fe(ptz)
6
](BF
4
)
2
) (Gu
¨
tlich
et al. 1994).
Thermal relaxation of the metastable LIESST state
does not occur in a classical manner by passing over
the barrier, but by nonadiabatic tunneling through
the barrier, whereby the observed temperature de-
pendence of the relaxation rate is due to thermal
population of the vibronic levels of the
5
T
2
manifold
(Hauser 1995). Optical switching back from the
5
T
2
LIESST state to the
1
A
1
ground state is also pos-
sible by irradiation with red light for the initial
excitation
5
T
2
-
5
E followed by two ISC processes
5
E-
3
T
1,2
-
1
A
1
(Hauser 1991). It has been found
that LIESST and reverse LIESST may be observed
with all iron(II) compounds exhibiting thermal SC, a
major difference, however, being the lifetime of the
light-induced LIESST state at a given temperature,
which depends on the ligand field strength, viz., the
weaker the ligand field strength, the smaller the dif-
ference DE
0
HL
between the lowest vibronic levels of
the HS and LS states, the longer the lifetime of the
LIESST state. LIESST has also been observed in
Langmuir–Blodgett thin films (Letard et al. 1999).
More recently, photophysical phenomena in iro-
n(II) SC systems, which are related to LIESST have
been observed. If a sample is continuously irradiated
with green light while the temperature is raised and
then lowered again, a thermal hysteresis may develop;
this phenomenon has been termed light-induced ther-
mal hysteresis (Desaix et al. 1998). In another case
existing thermal hysteresis of an iron(II) SC com-
pound was observed to shift to lower temperatures
under continuous irradiation with green light, and to
higher temperatures with red light (light-perturbed
thermal hysteresis) (Renz et al. 2000). Finally there is
the SOXIESST (soft x-ray induced excited spin state
trapping) which uses a source of soft x rays to initiate
the
1
A
1
-
5
T
2
transition (Collison et al. 1997), but
the relaxation mechanism should be the same as for
LIESST.
2. NIESST Effect
The acronym NIESST stands for nuclear decay-in-
duced excited spin state trapping. This phenomenon
is closely related to LIESST, as it makes use of the
nuclear decay and its energy release as an intrinsic
molecular excitation source, whereas LIESST is the
result of irradiation with an external visible light
source. Thus the initial step of electronic excitation is
different, but the final step of ligand field state relax-
ations has been found to be the same for both phe-
nomena.
57
Fe Mo
¨
ssbauer emission (ME) spectroscopy has
been a most elegant tool for the observation of me-
tastable ligand field states at ca. 100 ns after electron
capture decay of
57
Co(EC)
57
Fe in transition metals
compounds (Sano et al. 1984). A
57
Co labeled com-
pound is used as the Mo
¨
ssbauer source at variable
temperatures vs. K
4
[Fe(CN)
6
] as a single-line absorb-
er. If
57
Co is embedded in strong field surroundings,
which causes LS behavior in the corresponding
iron(II) compound like the tris-phen or tris-bpy
complexes (phen ¼1,10-phenanthroline; bpy ¼2,2
0
-
bipyridine), one observes the nucleogenic
57
Fe
II
ions
in LS (
1
A
1
) state at room temperature, which is the
ground state in the corresponding synthesized iro-
n(II) compound. Below ca. 200 K, however, one ob-
serves typical
57
Fe
II
-HS resonances in the ME spectra
with increasing intensity towards lower temperatures
at the expense of the
57
Fe
II
-LS resonances. In iron(II)
SC systems with weaker ligand field strength one ob-
serves no more
57
Fe
II
-LS resonances in the ME spec-
tra, even at very low temperatures where the
corresponding synthesized iron(II) complex has al-
ready turned to the LS (
1
A
1
) ground state. The
trapped HS state of the nucleogenic
57
Fe
II
ion was
found to have very similar lifetimes as the corre-
sponding LIESST state under comparable conditions
(Deisenroth et al. 1998). It was, therefore, concluded
that NIESST and LIESST are closely related phe-
nomena. Whereas the LIESST effect bears basically
the potential for practical applications, NIESST is
evidently of more academic interest and has contrib-
uted much to a deeper knowledge of mechanistic
aspects in hot atom chemistry in the solid state.
3. Stilbenoid Complexes
Another elegant way to change spin states of
some transition metal molecular compounds can be
achieved using the well-known cis–trans photo-
isomerization of stilbenoid units incorporated as
ligand molecules. This effect, called ligand driven
light-induced spin change (LD-LISC), was first ob-
served on the iron(II) complex [Fe(stpy)
4
(NCBPh
3
)
2
]
1060
Photomagnetism of Molecular Systems

(stpy ¼4-styrylpyridine) (Boillot et al. 1996). This
compound undergoes sharp thermal spin transition
around ca. 190 K under its trans form whereas the cis
form remains permanently HS. This is the conse-
quence of the different ligand field strength for these
two forms due to the different conformations of the
coordinated ligand. Irradiation of a thin film of trans-
[Fe(stpy)
4
(NCBPh
3
)
2
] with UV light around 140 K,
that is in the LS area, resulted in a LS-HS conver-
sion which was followed by absorption spectroscopy.
This transformation was found to be incomplete,
probably due to the rigidity of the matrix at this
temperature. Thus light irradiation affords a cis–trans
isomerization of the ligand with subsequent spin
transition at the iron(II) center as a consequence of a
change in ligand field strength.
It has recently become possible to observe the LD-
LISC effect at room temperature for [Fe(4-methyl-4
0
-
trans-styryl-bpy)
2
(NCS)
2
] (Boillot et al. 1999) and also
for some iron(III) complexes but only in solution. The
limiting point of this phenomenon is that it has for now
only been observed in thin films or in solution, the
reason being the enormous space required for the
photoisomerization to proceed. This weak point
regarding practical applications could be overcome
by implementation of these photosensitive materials in
organized media on the mesoscopic scale, such as
Langmuir–Blodgett films, which is actually in progress.
4. Nitrosyl Complexes
The discovery of light-induced long lived metastable
states (41 10
9
s) in Na
2
[Fe(CN)
5
(NO)].2H
2
O (Ha-
user et al. 1977) represents an important step in view
of possible applications for optical information stor-
age and data recording. Irradiating a single crystal of
sodium nitroprusside (SNP) below typically 100 K
with green light results in the subsequent population
of two metastable states (MS
1
and MS
2
) accompa-
nied by a color change. It was also proved that it is
possible to go from MS
1
to MS
2
with red light and
more generally, that all possible photoswitching path-
ways between the ground state, MS
1
and MS
2
, are
accessible using an appropriate wavelength. Upon
heating, radiationless relaxation is observed around
ca. 200 K (MS
1
) and 150 K (MS
2
). The photoswitch-
ing mechanism involves a two-step process: a charge
transfer transition from the metal d orbital to an an-
tibonding p* NO ligand orbital followed by a relax-
ation into the metastable potential minima. It may
also involve a reorientation of the nitrosyl group at-
oms upon photoexcitation. This possibility as well as
the origin of the longevity of the metastable states is
still debated. It should be mentioned that the photo
switching of SNP differs from the LIESST phenom-
enon as the ground state and the metastable states are
diamagnetic and as no change of the lattice constants
is observed during the photoexcitation.
The existence of photo-induced metastable states is
not restricted to SNP. It has also been found for in-
stance for related complexes such as XY[Fe(CN)
5
(NO)]
2
.10H
2
O(X¼Rb or Cs), K
2
[Ru(CN)
5
(NO)].
2H
2
O and for K
2
[RuCl
5
(NO)] (Woike et al. 1990);
this latter compound points out the crucial role of the
nitrosyl group for the photoswitching process.
Thus, SNP and its derivatives represent an appeal-
ing class of photochromic compounds, which are
suitable for optical memory devices with a very high
storage capacity and for holographic information
storage (Haussu
¨
hl et al. 1995). It is noteworthy that
the effect is not limited to solids but also occurs
in glassy matrices. Research efforts are currently
underway towards materials with a faster popula-
tion velocity and high decay temperatures. Chemical
modification of the SNP system in a controlled
manner might be the appropriate way to reach this
exciting goal.
5. Prussian Blue Analogues
The design of molecular magnets with high Curie
temperature (T
c
) which can be switched by light rep-
resents a challenging topic in materials science. Par-
ticular attention has been devoted to the Prussian
blue family, which can show spontaneous magneti-
zation above room temperature (Ferlay et al. 1995).
Soon after, the first photo-switchable, cobalt–iron
cyanide was reported (Sato et al. 1996). K
0.2
Co
1.4
[Fe(CN)
6
].6.9H
2
O possesses an f.c.c. structure in
which cobalt(II) ions are bridged to iron(III) ions
via cyano groups with a few cobalt(III)–iron(II) di-
amagnetic pairs also being present. Irradiation of a
powdered sample with red light at 5 K results in an
increase in the magnetization together with a rise of
T
c
from 16 K to 19 K. This light-induced magnetiza-
tion remains trapped at 5 K for several days in the
dark. Switching back to the ground state was
achieved by raising the temperature to 150 K or by
irradiation with blue light.
Before the excitation, the cobalt(III) and iron(II)
ions are in their diamagnetic LS states (S ¼0 for
both). Excitation with red light induces an electron
transfer from the iron to the cobalt leading to co-
balt(II) ions in the HS state (S ¼3/2) and iron(III)
ions in the LS state (S ¼1/2), which couple anti-
ferromagnetically. More interesting is that the reverse
electron transfer is accessible with blue light. The lo-
cal electron transfer switches increases the number of
magnetic neighbors and as a result the ordering tem-
perature (Verdaguer 1996). The key role of this phe-
nomenon is not only the presence of Co
III
–Fe
II
diamagnetic pairs but also crystal defects which
render the network flexible and allow the extension
of the light-induced electron transfer.
From an application viewpoint, the slow switch-
ing process (a few nanometers), as well as the low
1061
Photomagnetism of Molecular Systems

temperature range of the observation of the phenom-
enon, are the weak points of this system. Anyhow, it
represents the first example of fine-tuning of long-
range magnetic ordering by light. Photomagnetic
studies on related materials using different alkali cat-
ions are in progress to further explore this fascinating
phenomenon.
6. Concluding Remarks
The photo-control of the magnetic and optical prop-
erties remains a challenging topic in material science
in view of the possible implementation in optical
switching and memory devices. Various examples be-
longing to this appealing class of materials have been
covered in this article. There are no doubts that a
sizeable number of new compounds, as well as new
photophysical phenomena, may emerge in the near
future from this rapidly evolving field. We can, for
instance, mention a novel composite material com-
prising Prussian blue intercalated into photorespon-
sive vesicles based on azobenzene moieties, whose
magnetic properties can be controlled by photoillu-
mination (Einaga et al. 1999) or a nitrosyl derivative,
trans-[Ru(en)
2
(H
2
O)(NO)]Cl
3
(en ¼ethylenediamine)
which show a remarkably long lived metastable state
up to 267 K (Kawano et al. 2000).
Bibliography
Boillot M-L, Chantraine S, Zarembowitch J, Lallemand J-Y,
Prunet J 1999 First ligand-driven, light-induced spin change
at room temperature in a transition-metal molecular com-
pound. New J. Chem., 179–83
Boillot M-L, Roux C, Audie
´
re J-P, Dausse A, Zarembowitch J
1996 Ligand-driven light-induced spin change in transition-
metal complexes: selection of an appropriate system and first
evidence of the effect, in Fe
II
(4-styrylpyridine)
4
(NCBPh
3
)
2
.
Inorg. Chem. 35, 3975–80
Collison D, Garner C D, McGrath C M, Mosselmans J F,
Roper M D, Seddon J M W, Sinn E, Young N A 1997 Soft
X-ray induced excited spin state trapping and soft X-ray
photochemistry at the iron L-2, L-3 edge in [Fe(phen)
2
(NCS)
2
] and [Fe(phen)
2
(NCSe)
2
] (phen ¼1,10-phenanthro-
line). J. Chem. Soc. Dalton Trans. 4371–6
Decurtins S, Gu
¨
tlich P, Ko
¨
hler C P, Spiering H, Hauser A 1984
Light-induced excited spin state trapping in a transition metal
complex. Chem. Phys. Lett. 105, 1–4
Deisenroth S, Spiering H, Nagy D L, Gu
¨
tlich P 1998 Lamb–
Mo
¨
ssbauer factor of electronically excited molecular states
measured by time-differential Mo
¨
ssbauer emission spectro-
scopy. Hyperfine Interact. 113, 351–5
Desaix A, Roubeau O, Jeftic J, Haasnoot J G, Boukheddaden
K, Codjovi E, Linares M, Nogues M, Varret F 1998 Light-
induced bistability in spin transition solids leading to thermal
and optical hysteresis. Eur. Phys. B 6, 183–93
Einaga Y, Sato O, Iyoda T, Fujishima A, Hashimoto K 1999
Photofunctional vesicles containing Prussian blue and azo-
benzene. J. Am. Chem. Soc. 121, 3745–50
Ferlay S, Mallah T, Ouahes R, Veillet P, Verdaguer M 1995 A
room-temperature organometallic magnet based on Prussian
blue. Nature 378, 701–3
Gu
¨
tlich P, Hauser A, Spiering H 1994 Thermal and optical
switching of iron(II) complexes. Angew. Chem. Int. Ed. Engl.
33, 2024–54
Hauser A 1995 Intersystem crossing in iron(II) coordination
compounds: a model process between classical and quantum
mechanical behaviour. Comments Inorg. Chem. 17, 17–40
Hauser U, Oestereich V, Rohrweck H D 1977 On optical
dispersion in transparent molecular systems. Z. Phys. A 280,
17–25
Haussu
¨
hl S, Schetter G, Woike Th 1995 Nitroprussides, a new
group of materials for holographic information storage on
the basis of metastable electronic states. Opt. Comm. 114,
219–22
Kawano M, Ishikawa A, Morioka Y, Tomizawa H, Miki E -i,
Ohashi Y 2000 X-ray diffraction and spectroscopic studies
of the light-induced metastable state of a ethylenediamine
nitrosyl ruthenium complex. J. Chem. Soc. Dalton Trans.
2425–31
Letard J-F, Nguyen O, Soyer H, Mingotaud C, Delhae
´
sP,
Kahn O 1999 First evidence of the LIESST effect in a
Langmuir–Blodgett film. Inorg. Chem. 38, 3020–1
Renz F, Spiering H, Goodwin H A, Gu
¨
tlich P 2000 Light per-
turbed hysteresis in an iron(II) crossover compound observed
by the Mo
¨
ssbauer effect. Hyperfine Interact. 126, 155–8
Sano H, Gu
¨
tlich P 1984 Hot atom chemistry in relation to
Mo
¨
ssbauer emission spectroscopy. In: Matsuura T (ed.) Hot
Atom Chemistry. Kodanski, Tokyo, pp. 265–302
Sato O, Iyoda T, Fujishima A, Hashimoto K 1996 Photoin-
duced magnetization of a cobalt–iron cyanide. Science 272,
704–5
Verdaguer M 1996 Molecular electronics emerge from molec-
ular magnetism. Science 272, 698–9
Woike Th, Zo
¨
llner H, Krasser W 1990 Raman-spectroscopic
and differential scanning calorimetric studies of the light
induced metastable states in K
2
[RuCl
5
NO]. Solid State
Commun. 73, 149–52
P. Gu
¨
tlich and Y. Garcia
Institut fu
¨
r Anorganische Chemie und Analytische
Chemie, Johannes-Gutenberg Universita
¨
t, Mainz
Germany
Pnictides and Chalcogenides: Transition
Metal Compounds
Research on the magnetic properties of 3d transition
metal pnictides and chalcogenides has advanced
enormously. Realistic energy band calculations as
well as experimental techniques such as photoemis-
sion spectroscopy are available and play an impor-
tant role in clarifying the variety of magnetic and
related electronic characteristics. Chalcogens (M)
(pnicogens (M
0
)), elements from the 6a (5a) group
of the periodic table, have a valence electron config-
uration ns
2
np
4
(ns
2
np
3
). The former group includes
oxygen, sulfur, selenium, tellurium, and polonium
and the latter nitrogen, phosphorus, arsenic, anti-
mony, and bismuth. Chalcogens and pnicogens form
many compounds with 3 d elements. The total number
1062
Pnictides and Chalcogenides: Transition Metal Compounds
of transition metal (T) pnictides T
n
M
0
m
and chalco-
genides T
n
M
m
exceeds 150. This article does not
include oxides (see Transition Metal Oxides: Mag-
netism), nitrides, or polonides.
The generally stronger electronegativity of chal-
cogens compared to pnicogens implies two principal
differences between chalcogenides and pnictides.
First, in chalcogenides like Fe
7
Se
8
and Fe
3
Se
4
order-
ing of vacancies in the crystals often occurs, whereas
in pnictides vacancies are generally absent. A second
important difference is observed in the magnetic and
electronic characteristics. For example, NiS is anti-
ferromagnetic with a nickel magnetic moment of
1.7m
B
and undergoes a nonmetal to metal transition
at T ¼263K, whereas NiAs is a weakly paramagnetic
metal. The most interesting chalcogenides and pnic-
tides are of the types TM, TM
0
,TM
2
, and T
2
M
0
. This
article briefly reviews the magnetic and electronic
properties mainly of the typical compounds men-
tioned above. Further details can be found in the
review by Beckman and Lundgren (1991).
1. Chalcogenides
1.1 Pyrite-type Compounds of TM
2
A pyrite-type structure is adopted by the TM
2
com-
pounds with manganese, iron, cobalt, nickel, and
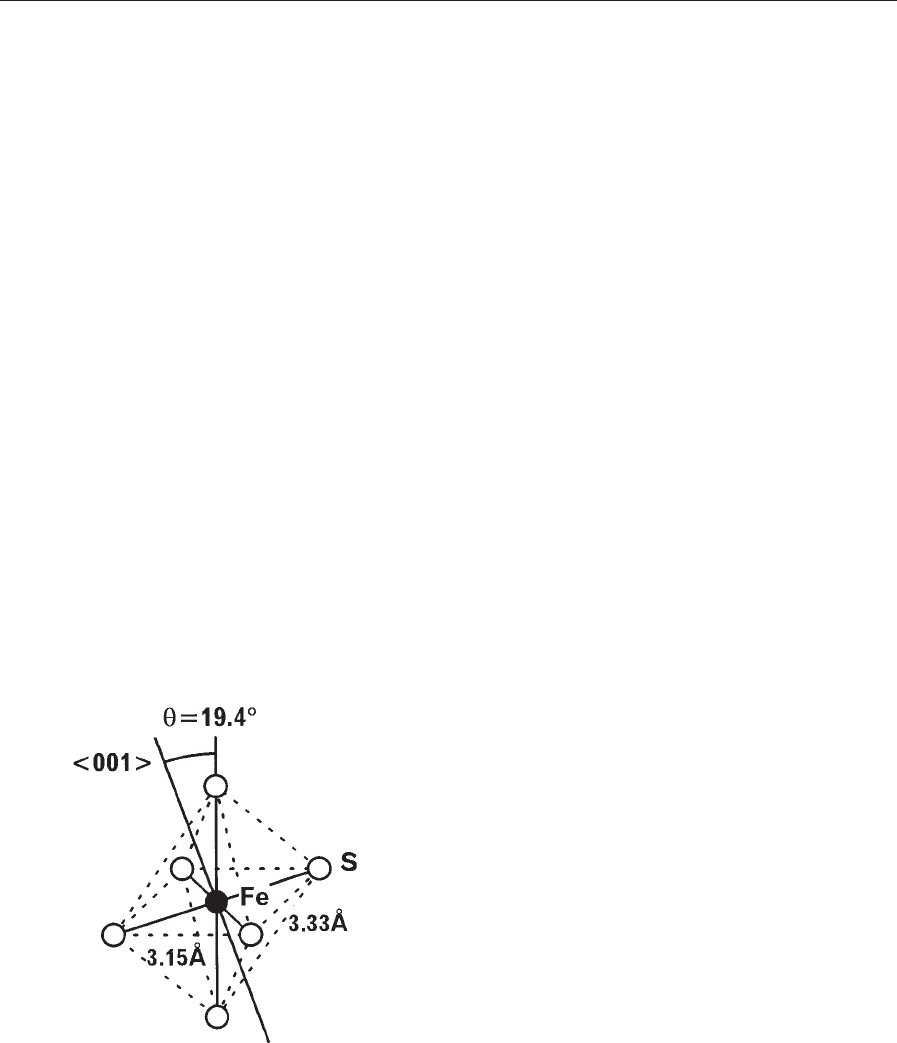
copper. The nearest neighbor sulfur atoms to iron in
FeS
2
are shown in Fig. 1. The 3d orbitals in the oc-
tahedral crystal field are split into the three de (or t
2g
)
and two dg (or e
g
) orbitals. The distorted octahedron
and the influence from more distant neighbors cause
further splitting of the de and dg orbitals. The related
energy levels are broadened into energy bands owing
to the mixing with s and p orbitals of the neighboring
atoms. The MnM
2
compounds have lattice para-
meters several percent larger than the other TM
2
compounds. Owing to strong electron correlation,
they do not form energy bands and become antif-
erromagnetic with a local manganese magnetic mo-
ment of 5m
B
in the high-spin state of the de
3
and dg
2
configuration.
The Ne
´
el temperature, T
N
, is 48K, 47K, and 87K
for MnS
2
, MnSe
2
, and MnTe
2
, respectively (Ishikawa
and Miura 1991). Energy band calculations per-
formed by Bullet (1982) for TS
2
with T ¼Fe, Co, Ni,
Cu, and Zn are successful in explaining the magnetic
and electric properties such as Pauli paramagnetic
and semiconductive FeS
2
and ferromagnetic and me-
tallic CoS
2
, etc. However, the band calculations can-
not explain semiconductive NiS
2
(antiferromagnetic
with a nickel moment of 1.17m
B
). Note that the band
description is not relevant for this strongly correlated
electron system.
The band calculations predict NiS
2
to be a Mott–
Hubbard insulator because of the existence of a
half-filled 3d band at the Fermi level. Also, NiS
2
is
regarded as an insulator of the charge-transfer type
(Fujimori et al. 1996, 1997). Since the nickel atom
appears in the octahedral surrounding as in Fig. 1,
p–d charge transfer is likely to occur. The photo-
emission spectrum of NiS
2
has been explained by a
cluster model with a charge transfer energy of 1.8eV,
a d–d electron correlation energy, U, of 3.3eV, and a
p–d transfer integral, pds,of1.5eV.
Both CoS
2
and CoSe
2
are metallic. The former is
ferromagnetic with T
C
¼125K and a cobalt magnetic
moment of 0.85m
B
, and the latter is apparently a
Curie–Weiss-type paramagnet. The solid solutions
Co(S
x
Se
1x
)
2
in the composition region 0.12 pxp0.4
show a very interesting metamagnetic transition at
T ¼4.2K (Adachi et al. 1970, 1979). The metamag-
netism was successfully explained based on the energy
band structure (Takahashi and Tano 1982). The
magnetic characteristics of Co(S
x
Se
1x
)
2
at finite
temperature are typical examples for which the self-
consistent renormalization (SCR) theory is applied
(Moriya 1979).
1.2 TM Compounds and Related Types
The TM compounds, which crystallize mainly as a
hexagonal NiAs-type structure (Fig. 2(a)), often con-
tain vacancies at the T site, and the crystal becomes
distorted owing to vacancy ordering. Therefore, the
magnetic and electrical properties of TM compounds
are generally complex. For example, within the Fe–S
system, FeS, Fe
1x
S with xo0.075, and Fe
7
S
8
com-
pounds have been found. The magnetic and electrical
properties of such materials depend on the type of
Figure 1
The iron atom in FeS
2
with the cubic pyrite-type crystal
structure (space group Pa3) is surrounded octahedrally
by six nearest neighbor sulfur atoms with the same Fe–S
distance of 2.29A
˚
. Two sides of the rectangle are not
equal. If the position parameter, u, is just 0.5, the
octahedron becomes perfect with y ¼0.
1063
Pnictides and Chalcogenides: Transition Metal Compounds
vacancy order, which varies with temperature as well
as with the composition. Other known compounds
with vacancies are Fe
7
Se
8
,Fe
3
Se
4
,Cr
7
Te
8
,Cr
3
Te
4
,
and Cr
2
Te
3
. Generally, the TM systems with T ¼V,
Cr, and M ¼S, Se show crystallographic similarities
with the Cr–Te system.
The antiferromagnetic compound FeS with
T
N
¼598K undergoes an interesting transition at
T
a
¼420K, which is called the a transition. At T
a
the
lattice parameters, magnetic susceptibility, and elec-
trical resistivity measured along the c-axis (r
8
D
0.2Ocm for TpT
a
) change very sharply, while the
resistivity perpendicular to the c-axis (r
8
D10
3
Ocm)
does not change significantly, and r
8
Dr
>
for TXT
a
(Wijn 1990). The coefficient of the electronic specific
heat, g, has been found to be zero within experimental
error (Kobayashi et al. 1999), which suggests that
FeS is an insulator at low temperatures. T
a
of the
Fe
1x
S compounds is strongly concentration depend-
ent. The mechanism responsible for the a transition is
still not clear.
NiS shows an insulator to metal transition at
T
t
¼263K with increasing temperature. The low-
temperature phase is antiferromagnetic with a nickel
magnetic moment of 1.7m
B
and the high-temperature
phase is paramagnetic. The insulator phase is known
to be of a charge transfer type and it is considered
to be at the metal–insulator boundary. An energy
gap of 0.1eV has been obtained for NiS (Fujimori
et al. 1996). The paramagnetic state of NiS is not
considered to be of a usual Pauli type (Takahashi and
Kanamori 1991).
2. Pnictides
All the 3d pnictides are metallic with resistivities of
the order of 10
3
–10
4
O cm at 300 K, and their mag-
netic properties are described by the itinerant electron
model. TM
0
and T
2
M
0
compounds are reviewed here.
The TM
0
compounds mainly crystallize either as
NiAs-or MnP-type structures (Fig. 2), while the T
2
M
0
compounds crystallize as Cu
2
Sb- or Fe
2
P-type struc-
tures (Fruchart et al. 1969). The most interesting are
the TM
0
compounds. The magnetic moment per
transition metal atom is displayed as a function of the
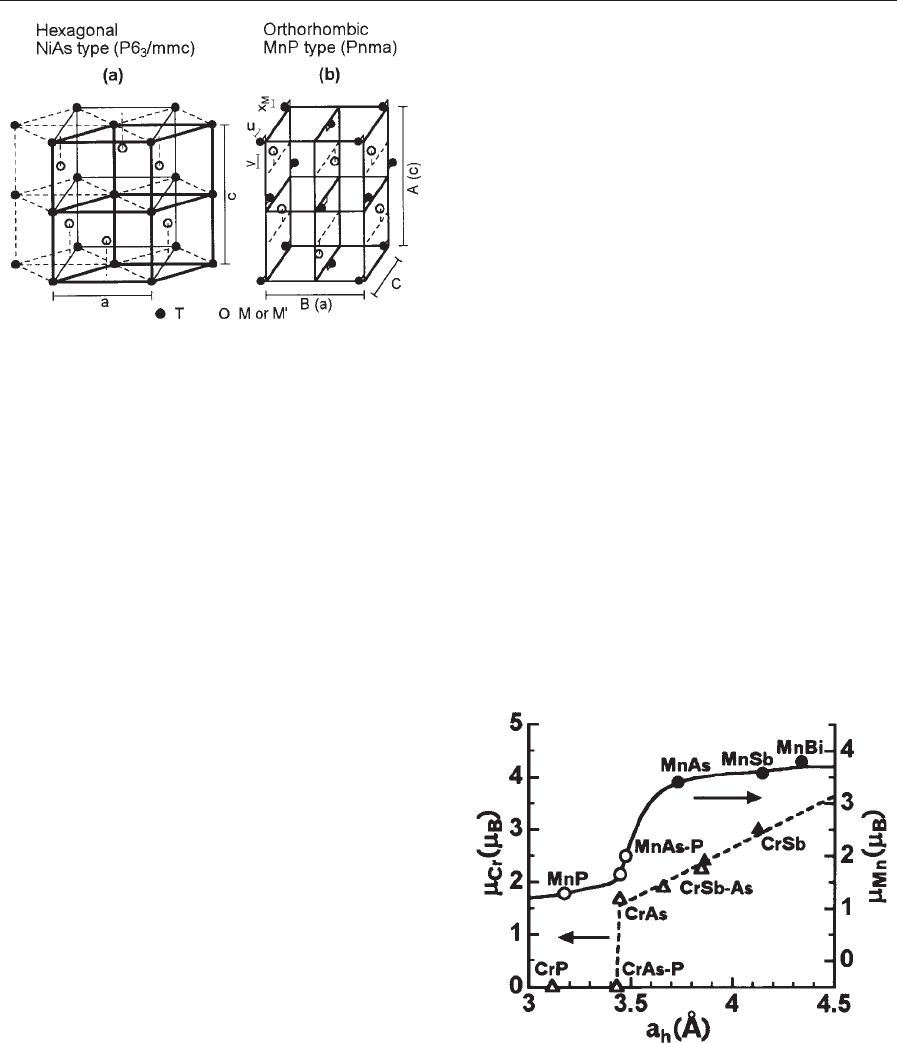
lattice parameter for T ¼Mn and Cr in Fig. 3. Man-
ganese pnictides are generally ferromagnetic, while
chromium ones are antiferromagnetic except for
Pauli paramagnetic CrP. The B-axis of CrP (the
B-axis corresponds to the a-axis of the hexagonal
NiAs-type structure; see also Fig. 2 and its caption.)
is about 25% smaller than the a-axis of CrSb, while
the c-axis (or the A-axis) of the chromium pnictides
shown in Fig. 3 remains within about 2% variation.
Therefore, m
Cr
seems to be a reasonable function of a
(or B).
Somewhat analogous features are observed for
manganese pnictides (Fig. 3). These findings imply
somewhat the existence of a critical separation where
the spontaneous atomic magnetic moment changes
drastically. The crossover is located around MnAs
Figure 2
Crystal structures of (a) the hexagonal NiAs-type
structure and (b) the orthorhombic MnP-type structure.
The MnP- becomes the NiAs-type structure if C ¼
ffiffiffi
3
p
B=2 and U ¼V ¼X
M
¼0. The parameter d, which
expresses a degree of the distortion from the hexagonal
to the orthorhombic structure, is defined as
d ¼ðC
ffiffiffi
3
p
BÞ=
ffiffiffi
3
p
B. The parameters U, V, and d are
generally less than 0.05, and X
M
is negligibly small in
general. The lattice parameters A, B, and C in (b)
correspond to c, a, and b ( ¼
ffiffiffi
3
p
a=2) in (a), respectively.
Figure 3
Relationship between the magnetic moment per T atom
and the lattice parameter a
h
, where a
h
means either the
a-axis in (a) or the B-axis in (b) of Fig. 2. The filled and
the open circles and triangles are the values for the
NiAs- and the MnP-type structures, respectively. The
data are taken from Wijn (1990) and Kallel et al . (1974).
1064
Pnictides and Chalcogenides: Transition Metal Compounds
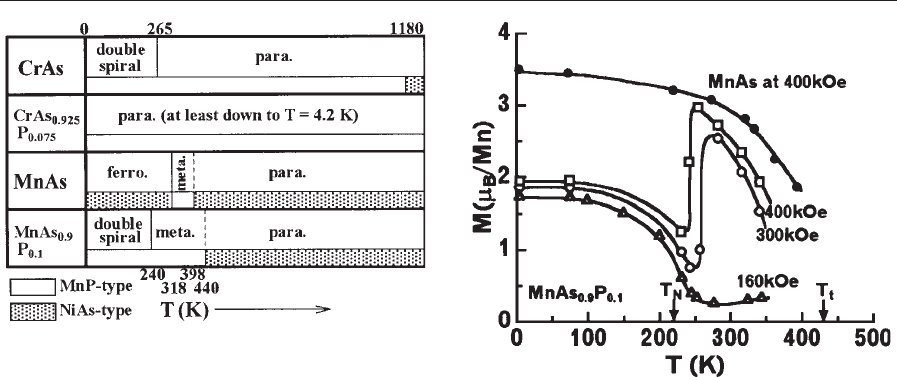
and CrAs. This conclusion is corroborated by the fact
that both MnAs and CrAs undergo a first-order
transition at T ¼318 K and 265 K, respectively, where
the magnetic order disappears, accompanied by an
abrupt shrinkage of the a-orB-axis. Besides, the
magnetic properties of MnAs and CrAs are also very
sensitive to substitutions of other elements. Typical
examples are summarized in Fig. 4. A substitution of
only 7.5% of phosphorus for arsenic in CrAs yields a
collapse of the double spiral magnetic order (Selte
et al. 1975). The B-axis of CrAs extrapolated to
T ¼0 K is about 6.1% (or 0.21A
˚
) larger than that of
nonmagnetic CrAs
0.9
P
0.1
(Suzuki and Ido 1993).
The substitution of phosphorus for arsenic in
MnAs also has a strong effect on magnetic proper-
ties. Magnetic properties and the various phase tran-
sitions in these materials have been explained in the
framework of energy band calculations together with
SCR theory (Motizuki and Katoh 1984). A large field
effect on the magnetic properties is shown in Fig. 5.
At temperatures in the paramagnetic region of
240 KoToT
t
, a metamagnetic (field-induced) tran-
sition accompanied by a crystallographic transition
from the MnP- to the NiAs-type structure is ob-
served. The mechanism responsible for the various
phase transitions mentioned above has not been fully
clarified.
Mn
2
Sb with the tetragonal Cu
2
Sb-type structure is
ferrimagnetic with T
C
¼550 K. This compound is in-
teresting because of the strong effect of substitutions
of other elements such as chromium for manganese.
For example, Mn
1.9
Cr
0.1
Sb undergoes a first-order
transition from antiferromagnetic to ferrimagnetic
order at T ¼300 K with increasing temperature. The
magnetic structures in the Cu
2
Sb-type compounds
have been explained on the basis of energy band cal-
culations (Shirai and Motizuki 1992).
The ground state magnetic and electrical proper-
ties of 3d metal chalcogenides and pnictides can be
explained on the basis of the electronic structures.
However, numerous phase transitions induced at fi-
nite temperatures by the external field and/or by the
temperature change remain unexplained theoreti-
cally. Detailed band calculations should be extended
to compounds with complex magnetic structures such
as CrAs, etc. SCR theory has been exploited to ex-
plain the magnetic properties at finite temperatures.
Some magnetic and electrical properties often de-
pend strongly on the crystallographic states, especially
for the chalcogenides. In addition to the application
of new experimental techniques, preparation of single
crystals and polycrystals with well-controlled compo-
sition and the related better experimental data may
enhance progress in this field. High-pressure studies in
the area up to about 10GPa may throw new light on
the physics of these materials.
See also: Magnetic Refrigeration at Room Tempera-
ture; Magnetism in Solids: General Introduction;
Transition Metal Oxides: Magnetism
Bibliography
Adachi K, Matuura M, Ohashi M, Kawai M 1979 Further
investigations of magnetic properties of Co(S
x
Se
1x
)
2
(0pxp1). J. Phys. Soc. Jpn. 46, 1474–82
Adachi K, Sato K, Matsui M 1970 New type metamagnetism
in Co(S
x
Se
1x
)
2
: exchange-compensated paramagnetism.
J. Phys. Soc. Jpn. 29, 323–32
Beckman O, Lundgren L 1991 Compounds of transition ele-
ments with nonmetals. In: Buschow K H J (ed.) Handbook of
Figure 4
Some examples of the temperature dependences of
magnetic and crystallographic properties of the pnictides
(Wijn 1990, Selte et al. 1975).
Figure 5
Temperature dependences of magnetizations at constant
fields for MnAs and MnAs
0.9
P
0.1
. See also main text.
1065
Pnictides and Chalcogenides: Transition Metal Compounds

Magnetic Materials. North-Holland, Amsterdam, Vol. 6,
Chap. 3
Bullett D W 1982 Electronic structure of 3d pyrite-and mar-
casite-type sulphides. J. Phys. C15, 6163–74
Fruchart R, Roger A, Senateur J P 1969 Crystallographic and
magnetic properties of solid solutions of the phosphides. J.
Appl. Phys. 40, 1250–7
Fujimori A, Mamiya K, Mizokawa T 1997 Photoemission
spectroscopy of correlated transition-metal compounds in the
charge transfer regime. Physica B237–238, 137–41
Fujimori A, Mamiya K, Mizokawa T, Miyadai T, Sekiguchi T,
Takahashi H, Mori N 1996 Resonant photoemission study of
pyrite-type NiS
2
, CoS
2
and FeS
2
. Phys. Rev. B 54, 16329–32
Ishikawa Y, Miura N 1991 Physics and Engineering Application
of Magnetism, Solid State Science 92. Springer, Berlin
Kallel A, Boller H, Bertaut E F 1974 Helimagnetism in MnP-
type compounds: MnP, FeP, and CrAs
1x
Sb
x
mixed crystals.
J. Phys. Chem. Solids 35, 1139–52
Katoh K, Motizuki K 1984 Role of spin fluctuation in spon-
taneous magneto-volume effect of intermetallic compounds
MnAs and MnAs
1x
P
x
. J. Phys. Soc. Jpn. 53, 3166–71
Kobayashi H, Nozue T, Matsumura T, Suzuki T, Kamimura T
1999 The low temperature specific heat of FeS and M
0.875
X
(M ¼Fe, Co; X ¼S, Se) with a NiAs-like structure. J. Phys.
Condens. Matter 11, 8673–9
Moriya T 1979 Recent progress in the theory of spin fluctua-
tions in itinerant electron magnets. J. Magn. Magn. Mater.
14, 1–46
Motizuki K, Katoh K 1984 Spin fluctuation theory of
intermetallic compound MnAs. J. Phys. Soc. Jpn. 53,
735–46
Selte K, Hjersing H, Kjekshus A, Andresen A F, Fischer P 1975
Magnetic structures and properties of CrAs
1x
P
x
. Acta
Chem. Scand. A29, 695–8
Shirai M, Motizuki K 1992 In: Kotani A, Suzuki N (eds.) Re-
cent Advances in Magnetism of Transition Metal Compounds.
World Scientific, Singapore, pp. 67–77
Suzuki T, Ido H 1993 Magnetic–nonmagnetic transition in
CrAs and related compounds. J. Appl. Phys. 73, 5686–8
Takahashi M, Kanamori J 1991 Electron correlation in Ni
compounds. J. Phys. Soc. Jpn. 60, 3154–61
Takahashi Y, Tano M 1982 The metamagnetism and temper-
ature dependence of the magnetic susceptibility of
Co(S
x
Se
1x
)
2
. J. Phys. Soc. Jpn. 51, 1792–8
Wijn H P J (ed.) 1990 Landolt-Bo
¨
rnstein New Series. Springer,
Berlin, Group III, Vol. 27a
H. Ido
Tohoku Gakuin University, Tagajo City, Japan
1066
Pnictides and Chalcogenides: Transition Metal Compounds
Radiation and Particle Detectors
Superconducting radiation and particle detectors
measure the energy deposited by photons or parti-
cles that interact with the detector. Superconducting
detectors measure this energy with orders of magni-
tude more accuracy than semiconductor detectors.
Most superconducting photon and particle detectors
operate at temperatures below 1 K. At this low tem-
perature, the energy deposited by the photons and
particles of interest is not obscured by the thermal
energy of the detector. It is the low temperature of
operation that allows superconducting detectors to
measure the energy of each incident photon or par-
ticle with high precision.
Superconducting radiation and particle detectors
are energy dispersive, that is, they provide spectral
information by measuring the energy of particles or
photons absorbed in the active volume of the detec-
tor. Superconducting detectors can often be con-
structed so that almost every incident particle or
photon interacts with the detector. Thus, these de-
tectors can provide a combination of high resolution
and high quantum efficiency which is not available in
more conventional detectors.
The quest for new detectors with high resolution
and high efficiency has been strongly motivated by
x-ray astrophysics. However, superconducting detec-
tors are now being developed to study astronomical
objects at energies from the infrared to gamma-rays.
Superconducting x-ray detectors are also being de-
veloped for laboratory plasma experiments and the
measurement of nuclear materials. These detectors
are being used to improve x-ray fluorescence analysis
of many types of materials ranging from integrated
circuits to proteins. Superconducting detectors are
also used to search for proposed weakly interacting
massive particles (WIMPs), which may compose the
‘‘dark matter’’ of the universe. They may also be
useful for studying ions and very large molecules.
Various detector designs have been developed
which take advantage of the unique thermal and elec-
trical properties of low-temperature superconductors.
1. Microcalorimeters
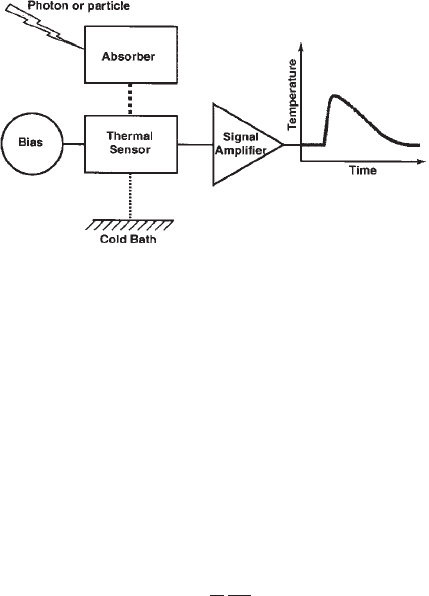
A microcalorimeter is a small detector used to meas-
ure the total energy of incident photons or particles.
As illustrated in Fig. 1, the device consists of an ab-
sorber, which is heated by individual photons or
particles, and a thermal sensor, which measures the
resulting change in temperature. The absorber and
thermal sensor are in good thermal contact, while the
assembly is isolated from the cold bath by a weak
thermal link. The temperature rise due to an incident
particle is DT ¼E/C where E is its energy and C is the
heat capacity of the device. A typical microcalori-
meter operates at 0.1 K where its heat capacity can be
so small that even optical photons will raise the tem-
perature by a significant amount.
In addition to having a low heat capacity absorber,
the microcalorimeter must include a sensitive thermal
sensor. Thermistors have provided the best results
to date. The sensitivity of the thermistor is typically
parameterized by a
a
T
R
dR
dT
ð1Þ
If a low-noise amplifier is used such that the dom-
inant noise contributions are from the thermal fluc-
tuations in the detector and the Johnson noise in the
thermistor, then the limit to the accuracy one can
measure the energy is
DE
FWHM
¼ x2:35
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
k
B
T
2
C
p
ð2Þ
where k
B
is the Boltzmann constant and x is a pre-
factor that depends on a and the power dissipated in
the detector by the bias circuit.
For semiconducting thermistors, aB5 and xB3.
Values of xo1, and therefore better energy resolu-
tion, can be achieved with a superconducting transi-
tion-edge sensor (TES). A TES is a superconducting
film operated at the transition between the supercon-
ducting and normal state where the change in resist-
ance versus temperature is very steep and a4100 can
be obtained. The transition temperature of a TES can
be tuned to almost any value with the proper com-
bination of superconducting and normal metals.
When the photon or particle deposits energy in the
microcalorimeter, the temperature quickly increases,
and then returns to the operating temperature. The
Figure 1
Schematic diagram of a microcalorimeter photon or
particle detector.
R
1067

time constant for a device operating without bias
power is C/G where G is the thermal conductivity of
the weak link. When bias power is applied to the
thermistor, electrothermal feedback can significantly
lengthen or shorten the pulse.
For the TES, a constant voltage bias is usually
used. With a constant voltage bias, V, the detector is
heated above the base temperature by the bias power
P ¼V
2
/R. When the detector is heated by a photon or
particle, its temperature increases, thereby increasing
the resistance, R, of the TES. This increase in R
decreases the bias power providing negative electro-
thermal feedback. This feedback reduces the pulse
length. For a TES device with a large a, electro-
thermal feedback can shorten the pulse time by more
than an order of magnitude. In this case, the prefactor
in Eqn. (2) is given by xE2:5=
ffiffiffi
a
p
, which can be much
less than one. A high-bandwidth (41 MHz) super-
conducting quantum interference device (SQUID)
current amplifier is typically used to operate the
low-resistance voltage-biased TES detectors.
While the resolution achieved with microcalorim-
eters has been remarkable, the results are not gener-
ally limited by Eqn. (2). For instance, Eqn. (2)
assumes that 100% of the photon or particle energy is
converted to heat in the absorber. In real devices,
however, some of the particle’s energy can either ex-
cite long-lived states in the absorber material, or leave
the detector before being measured by the thermal
sensor. In both cases, the energy resolution is de-
graded. For optimal results, one must therefore find
absorber materials with good photon or particle ab-
sorption properties, low specific heat, and good ther-
malization properties. Insulators and semiconductors
can have very low specific heat, but statistical fluc-
tuations in the generation of long-lived electronic
states typically degrades the energy resolution to a
much worse value than Eqn. (2). Good results, how-
ever, have been obtained with high-purity germanium
and silicon when all the trapping centers are filled by
infrared photon excitation prior to the measurement.
Normal metals have excellent thermalization, but
they also contain much more specific heat. Semimet-
als, bismuth in particular, provide an excellent com-
bination of good thermalization with low specific
heat. Superconductors cooled far below their transi-
tion temperature have low specific heat, and their
small energy gap can minimize statistical fluctuations
from trapped states. Tin appears to work exception-
ally well.
X-ray microcalorimeters are typically o1mm
2
in
area, and several microns thick. When cooled to
TB0.1 K, resolutions of about 5 eV full width at half
maximum (FWHM) have been obtained at 6 keV,
and 2 eV at 1.4 keV. The response time of these de-
vices generally limits their count rate to a few 100
counts per second. These results have been achieved
with TES devices and with germanium neutron-
transmutation-doped thermistors attached to tin-foil
absorbers by epoxy. For comparison, a good energy-
dispersive semiconductor detector has a resolution 20
times worse, about 110 eV FWHM at 6 keV. Optical
TES devices have also been developed, where 400 mm
2
pixels operating at TB80 mK provide single photon
counting with a resolving power of about 15, and
count rates as high as 3.0 10
4
counts per second.
Another thermal sensor under development uses the
change in magnetization of a paramagnetic material.
A resolution of 13 eV FWHM at 6 keV has been
obtained using an erbium-doped gold sensor and a
0.01 mm
2
gold absorber.
Larger microcalorimeters are being developed to
improve our understanding of the nonbaryonic mat-
ter of the universe. When a proposed WIMP particle
scatters off a nucleus in a semiconductor crystal, the
energy transferred to the detector is divided between
electron–hole pairs and thermal energy. By measur-
ing both the electron–hole pairs, and the prompt
phonons, dark-matter detectors can differentiate nu-
clear-recoil events from electron-recoil events which
tend to produce more electron–holes for a given en-
ergy input. This allows electron-recoil events due to
background radiation to be excluded from the meas-
urement, increasing the sensitivity to WIMPs by two
orders of magnitude. A similar differentiation has
been achieved by comparing the scintillation light
and thermal energy collected when photons and par-
ticles scatter in calcium tungstate crystals. Despite
their modest size (o1 kg), superconducting WIMP
detectors have achieved more sensitivity than much
more massive detectors operating at higher temper-
atures where it is not possible to reject background
interactions to such a high degree.
2. Superconducting Tunnel Junctions
The superconducting tunnel junction (STJ) detector
typically consists of a superconductor-insulator-
superconductor (SIS) thin-film structure where the
insulator is a very thin (B2 nm) native-oxide layer,
usually Al
2
O
3
. The STJ is cooled below 1/10 of the
superconducting transition temperature to condense
almost all its electrons into Cooper pairs. An incident
photon or particle breaks some of these Cooper pairs
and produces electronic excitations called quasipar-
ticles. When the Josephson current is suppressed by a
magnetic field and the junction is biased at a nonzero
voltage, these excess quasiparticles tunnel through
the insulating barrier, producing a current pulse (see
Josephson Junctions: Low-T
c
). The number of quasi-
particles produced is N
qp
¼E/e, where e is the average
energy required to produce a quasiparticle. For most
superconductors, eE1.7 D where D is the supercon-
ducting energy gap (see Electrodynamics of Super-
conductors: Flux Properties; Electrodynamics of
Superconductors: Weakly Coupled). Quasiparticle
can tunnel back and forth through the barrier of an
1068
Radiation and Particle Detectors
STJ detector and this multiple tunneling can increase
the measured signal.
If a low-noise amplifier is used such that the total
noise in the measurement is dominated by the statis-
tical fluctuations in the generation and tunneling of
quasiparticles, then the resolution of the STJ detector
would be limited by
DE
FWHM
¼ 2:35
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
EeðF þ F
0
Þ
p
ð3Þ
Where F is the so-called Fano factor that parame-
terizes the statistical fluctuations in the generation of
quasiparticles, and F
0
is a term which describes the
noise associated with the variations in how many
times each quasiparticle tunnels through the barrier.
Monte Carlo simulations find that FE0.2 for most
superconductors. The total integrated current meas-
ured with the device is Q ¼nN
qp
where n is the
average number of times a quasiparticle tunnels
through the barrier. Typically n ¼G
tun
.t
life
where
G
tun
is the tunneling rate, and .t
life
is the quasiparticle
lifetime. For a symmetric STJ detector with multiple
tunneling, F
0
¼ 1 þ 1=n. Thus the multiple tunneling
of quasiparticles can produce a bigger signal, but
usually increases the statistical noise.
In general, a high tunnel rate, G
tun
, will produce
larger signals and better results. The tunnel rate is
inversely proportional to the barrier thickness and
the thickness of the superconductor. The barrier can
be only so thin before it becomes leaky, allowing re-
sistive current flow and excess noise. A thin super-
conductor will have a high tunnel rate, but poor x-ray
absorption efficiency. It is possible, however, to use
two or more superconductors to concentrate the qua-
siparticles from a larger absorbing layer into a thin-
ner layer next to the tunnel barrier. If the
superconducting energy gap of the absorbing layer
(typically niobium or tantalum) is larger than the en-
ergy gap of the layer next to the tunnel barrier (typ-
ically aluminum), then quasiparticles created in the
higher-gap material can emit a phonon while in the
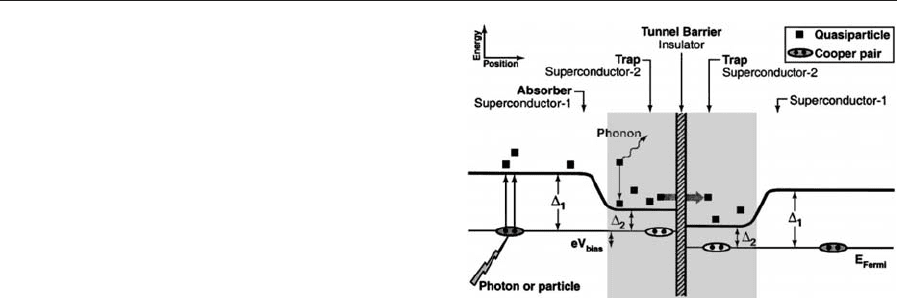
lower-gap material. As illustrated in Fig. 2, the qua-
siparticle is then trapped in the lower gap material
close to the barrier. Quasiparticle trapping can be
used to improve the performance of an STJ x-ray
detector.
In general, STJ photon detectors have not achieved
the resolution described in Eqn. (3), but the discrep-
ancy is mostly understood. STJ detectors are gener-
ally operated with room-temperature current or
charge amplifiers. The noise contribution from these
amplifiers depends on the resistance of the STJ. This
noise contribution is typically measured during in-
tervals when no photons strike the detector. When
this quiescent noise estimate is subtracted in quad-
rature from the measured resolution, the estimated
intrinsic resolution can approach the limit in Eqn. (3).
Better agreement is obtained with measurements of
the electronic noise contribution when a photon
strikes the detector. The quasiparticles created by the
photon causes a reduction in the STJ’s resistance and
an increase in electronic noise. This increase tends to
bring the measurements into closer agreement with
the limit described in Eqn. (3).
Five-layer STJ detectors of Nb/Al/Al
2
O
3
/Al/Nb
and Ta/Al/Al
2
O
3
/Al/Ta have successfully been imple-
mented as both x-ray and optical detectors. For x-rays
below 1 keV, these detectors have good absorption
efficiency and resolution between 2 eV FWHM and
10 eV FWHM, and can operate at temperatures as
warm as 0.5 K. At 6 keV, the resolution achieved is
about 25 eV FWHM, and the efficiency of these de-
tectors is not as good. A notable exception is a Al/
Al
2
O
3
/Al STJ coupled to a lead film through a thick
insulating barrier. In this case, the photon energy is
converted to phonons in the lead, which pass through
the insulator into the aluminum where the phonons
break Cooper pairs. A resolution of 12 eV FWHM at
6vkeV was obtained with this device, and the lead film
provides very high absorption efficiency even at 6 keV.
Compared to the calorimeters developed so far, the
STJ detectors can operate at higher count rates. STJ
x-ray detectors have been operated at count rates up
to 2.0 10
4
counts per second with little degradation
in energy resolution. STJ detectors have also been
successfully implemented to measure individual opti-
cal photons with a resolving power of about 15.
Quasiparticle trapping is a widely used technique to
collect quasiparticles at various locations on a super-
conducting film or crystal. When a photon or particle
interacts with the superconductor, the resulting qua-
siparticles will diffuse throughout the material, until
recombining or becoming trapped. If most of the
quasiparticles end up in the trapping centers adjacent
to STJs, then both the energy and the position of the
absorption event can be determined. Position-sensi-
tive detectors have been successfully fabricated in
both one and two-dimensional geometries. An energy
Figure 2
Energy levels in a symmetric superconducting tunnel
junction photon or particle detector.
1069
Radiation and Particle Detectors
