Brennen Ch.E. Fundamentals of Multiphase Flow
Подождите немного. Документ загружается.

quently collapse as they are convected into regions of higher pressure within
the blade passages of the pump. The displacement of liquid by this volume
growth and collapse introduces an additional flow area restriction into the
flow, an additional inlet nozzle caused by the cavitation. Stripling and Acosta
(1962) and others have suggested that the head degradation due to cavita-
tion could be due to a lack of pressure recovery in this effective additional
nozzle.
219
9
HOMOGENEOUS FLOWS
9.1 INTRODUCTION
In this chapter we shall be concerned with the dynamics of multiphase flows
in which the relative motion between the phases can be neglected. It is clear
that two different streams can readily travel at different velocities, and in-
deed such relative motion is an implicit part of the study of separated flows.
On the other hand, it is clear from the results of section 2.4.2 that any two
phases could, in theory, be sufficiently well mixed and therefore the disperse
particle size sufficiently small so as to eliminate any significant relative mo-
tion. Thus the asymptotic limit of truly homogeneous flow precludes relative
motion. Indeed, the term homogeneous flow is sometimes used to denote a
flow with negligible relative motion. Many bubbly or mist flows come close
to this limit and can, to a first approximation, be considered to be homoge-
neous. In the present chapter some of the properties of homogeneous flows
will be considered.
9.2 EQUATIONS OF HOMOGENEOUS FLOW
In the absence of relative motion the governing mass and momentum conser-
vation equations for inviscid, homogeneous flow reduce to the single-phase
form,
∂ρ
∂t
+
∂
∂x
j
(ρu
j
) = 0 (9.1)
ρ
∂u
i
∂t
+ u
j
∂u
i
∂x
j
= −
∂p
∂x
i
+ ρg
i
(9.2)
220

where, as before, ρ is the mixture density given by equation 1.8. As in single-
phase flows the existence of a barotropic relation, p = f(ρ), would complete
the system of equations. In some multiphase flows it is possible to establish
such a barotropic relation, and this allows one to anticipate (with, perhaps,
some minor modification) that the entire spectrum of phenomena observed in
single-phase gas dynamics can be expected in such a two-phase flow. In this
chapter we shall not dwell on this established body of literature. Rather,
attention will be confined to the identification of a barotropic relation (if
any) and focused on some flows in which there are major departures from
the conventional gas dynamic behavior.
From a thermodynamic point of view the existence of a barotropic relation,
p = f(ρ), and its associated sonic speed,
c =
dp
dρ
1
2
(9.3)
implies that some thermodynamic property is considered to be held constant.
In single-phase gas dynamics this quantity is usually the entropy or, occa-
sionally, the temperature. In multiphase flows the alternatives are neither
simple nor obvious. In single-phase gas dynamics it is commonly assumed
that the gas is in thermodynamic equilibrium at all times. In multiphase
flows it is usually the case that the two phases are not in thermodynamic
equilibrium with each other. These are some of the questions that must be
addressed in considering an appropriate homogeneous flow model for a mul-
tiphase flow. We begin in the next section by considering the sonic speed of
a two-phase or two-component mixture.
9.3 SONIC SPEED
9.3.1 Basic analysis
Consider an infinitesimal volume of a mixture consisting of a disperse phase
denoted by the subscript A and a continuous phase denoted by the subscript
B. For convenience assume the initial volume to be unity. Denote the initial
densities by ρ
A
and ρ
B
and the initial pressure in the continuous phase
by p
B
. Surface tension, S, can be included by denoting the radius of the
disperse phase particles by R. Then the initial pressure in the disperse phase
is p
A
= p
B
+2S/R.
Now consider that the pressure, p
A
, is changed to p
A
+ δp
A
where the
difference δp
A
is infinitesimal. Any dynamics associated with the resulting
fluid motions will be ignored for the moment. It is assumed that a new equi-
221
librium state is achieved and that, in the process, a mass, δm, is transferred
from the continuous to the disperse phase. It follows that the new disperse
and continuous phase masses are ρ
A
α
A
+ δm and ρ
B
α
B
− δm respectively
where, of course, α
B
=1− α
A
. Hence the new disperse and continuous phase
volumes are respectively
(ρ
A
α
A
+ δm)/
ρ
A
+
∂ρ
A
∂p
A
QA
δp
A
(9.4)
and
(ρ
B
α
B
− δm)/
ρ
B
+
∂ρ
B
∂p
B
QB
δp
B
(9.5)
where the thermodynamic constraints QA and QB are, as yet, unspecified.
Adding these together and subtracting unity, one obtains the change in total
volume, δV , and hence the sonic velocity, c,as
c
−2
= −ρ
δV
δp
B
δp
B
→0
(9.6)
c
−2
= ρ
α
A
ρ
A
∂ρ
A
∂p
A
QA
δp
A
δp
B
+
α
B
ρ
B
∂ρ
B
∂p
B
QB
−
(ρ
B
− ρ
A
)
ρ
A
ρ
B
δm
δp
B
(9.7)
If it is assumed that no disperse particles are created or destroyed, then the
ratio δp
A
/δp
B
may be determined by evaluating the new disperse particle
size R + δR commensurate with the new disperse phase volume and using
the relation δp
A
= δp
B
−
2S
R
2
δR:
δp
A
δp
B
=
1 −
2S
3α
A
ρ
A
R
δm
δp
B
/
1 −
2S
3ρ
A
R
∂ρ
A
∂p
A
QA
(9.8)
Substituting this into equation 9.7 and using, for convenience, the notation
1
c
2
A
=
∂ρ
A
∂p
A
QA
;
1
c
2
B
=
∂ρ
B
∂p
B
QB
(9.9)
the result can be written as
1
ρc
2
=
α
B
ρ
B
c
2
B
+
α
A
ρ
A
c
2
A
−
δm
δp
B
1
ρ
A
−
1
ρ
B
+
2S
3ρ
A
ρ
B
c
2
A
R
1 −
2S
3ρ
A
c
2
A
R
(9.10)
This expression for the sonic speed, c, is incomplete in several respects.
First, appropriate thermodynamic constraints QA and QB must be identi-
fied. Second, some additional constraint is necessary to establish the rela-
tion δm/δp
B
. But before entering into a discussion of appropriate practical
222

choices for these constraints (see section 9.3.3) several simpler versions of
equation 9.10 should be identified.
First, in the absence of any exchange of mass between the components
the result 9.10 reduces to
1
ρc
2
=
α
B
ρ
B
c
2
B
+
α
A
ρ
A
c
2
A
1 −
2S
3ρ
A
c
2
A
R
(9.11)
In most practical circumstances the surface tension effect can be neglected
since S ρ
A
c
2
A
R;thenequation9.11becomes
1
c
2
= {ρ
A
α
A
+ ρ
B
α
B
}
α
B
ρ
B
c
2
B
+
α
A
ρ
A
c
2
A
(9.12)
In other words, the acoustic impedance for the mixture, namely 1/ρc
2
,is
simply given by the average of the acoustic impedance of the components
weighted according to their volume fractions. Another popular way of ex-
pressing equation 9.12 is to recognize that ρc
2
is the effective bulk modulus
of the mixture and that the inverse of this effective bulk modulus is equal
to an average of the inverse bulk moduli of the components (1/ρ
A
c
2
A
and
1/ρ
B
c
2
B
) weighted according to their volume fractions.
Some typical experimental and theoretical data obtained by Hampton
(1967), Urick (1948) and Atkinson and Kyt¨omaa (1992) is presented in figure
9.1. Each set is for a different ratio of the particle size (radius, R)tothe
wavelength of the sound (given by the inverse of the wavenumber, κ). Clearly
the theory described above assumes a continuum and is therefore relevant
to the limit κR → 0. The data in the figure shows good agreement with
the theory in this low frequency limit. The changes that occur at higher
frequency (larger κR) will be discussed in the next section.
Perhaps the most dramatic effects occur when one of the components is
a gas (subscript G), that is much more compressible than the other com-
ponent (a liquid or solid, subscript L). In the absence of surface tension
(p = p
G
= p
L
), according to equation 9.12, it matters not whether the gas is
the continuous or the disperse phase. Denoting α
G
by α for convenience and
assuming the gas is perfect and behaves polytropically according to ρ
k
G
∝ p,
equation 9.12 may be written as
1
c
2
=[ρ
L
(1 − α)+ρ
G
α]
α
kp
+
(1 − α)
ρ
L
c
2
L
(9.13)
This is the familiar form for the sonic speed in a two-component gas/liquid or
gas/solid flow. In many applications p/ρ
L
c
2
L
1 and hence this expression
223
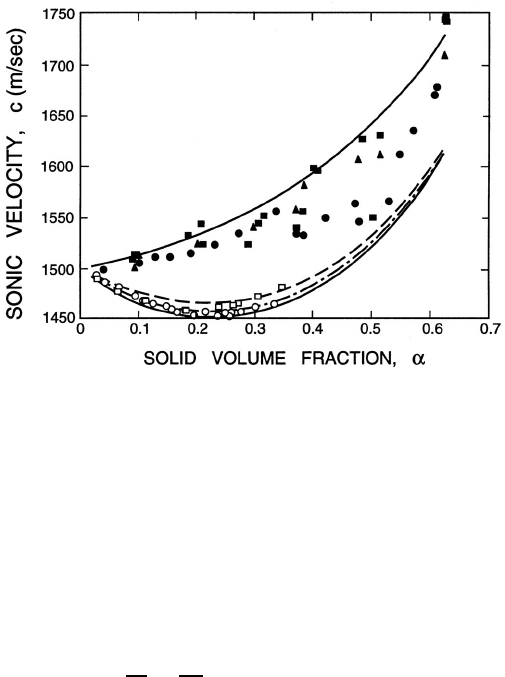
Figure 9.1. The sonic velocities for various suspensions of particles in
water: , frequency of 100kHz in a suspension of 1µm Kaolin particles
(Hampton 1967) (2κR =6.6 × 10
−5
); , frequency of 1MHz in a suspen-
sion of 0.5µm Kaolin particles (Urick 1948) (2κR =3.4 × 10
−4
); solid sym-
bols, frequencies of 100kHz − 1MHz in a suspension of 0.5mm silica parti-
cles (Atkinson and Kyt¨omaa 1992) (2κR =0.2 − 0.6). Lines are theoretical
predictions for 2κR =0, 6.6 × 10
−5
,3.4 × 10
−4
,and2κR =0.2 − 0.6in
ascending order (from Atkinson and Kyt¨omaa 1992).
may be further simplified to
1
c
2
=
α
kp
[ρ
L
(1 − α)+ρ
G
α] (9.14)
Note however, that this approximation will not hold for small values of the
gas volume fraction α.
Equation 9.13 and its special properties were first identified by Minnaert
(1933). It clearly exhibits one of the most remarkable features of the sonic
velocity of gas/liquid or gas/solid mixtures. The sonic velocity of the mix-
ture can be very much smaller than that of either of its constituents. This is
illustrated in figure 9.2 where the speed of sound, c, in an air/water bubbly
mixture is plotted against the air volume fraction, α. Results are shown for
both isothermal (k = 1) and adiabatic (k =1.4) bubble behavior using equa-
tion 9.13 or 9.14, the curves for these two equations being indistinguishable
on the scale of the figure. Note that sonic velocities as low as 20 m/s occur.
Also shown in figure 9.2 is experimental data of Karplus (1958) and Gouse
and Brown (1964). Data for frequencies of 1.0 kHz and 0.5 kHz are shown
in figure 9.2, as well as data extrapolated to zero frequency. The last should
224
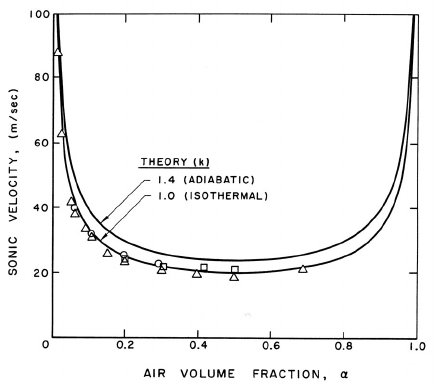
Figure 9.2. The sonic velocity in a bubbly air/water mixture at atmo-
spheric pressure for k =1.0and1.4. Experimental data presented is from
Karplus (1958) and Gouse and Brown (1964) for frequencies of 1 kHz (),
0.5 kHz (), and extrapolated to zero frequency().
be compared with the low frequency analytical results presented here. Note
that the data corresponds to the isothermal theory, indicating that the heat
transfer between the bubbles and the liquid is sufficient to maintain the air
in the bubbles at roughly constant temperature.
Further discussion of the acoustic characteristics of dusty gases is pre-
sented later in section 11.4 where the effects of relative motion between the
particles and the gas are included. Also, the acoustic characteristics of di-
lute bubbly mixtures are further discussed in section 10.3 where the dynamic
response of the bubbles are included in the analysis.
9.3.2 Sonic speeds at higher frequencies
Several phenomena can lead to dispersion, that is to say to an acoustic
velocity that is a function of frequency. Among these are the effects of bubble
dynamics discussed in the next chapter. Another is the change that occurs at
higher frequencies as the wavelength is no longer effectively infinite relative
to the size of the particles. Some experimental data on the effect of the
ratio of particle size to wavelength (or κR) was presented in figure 9.1.
Note that the minimum in the acoustic velocity at intermediate volume
fractions disappears at higher frequencies. Atkinson and Kyt¨omaa (1992)
225
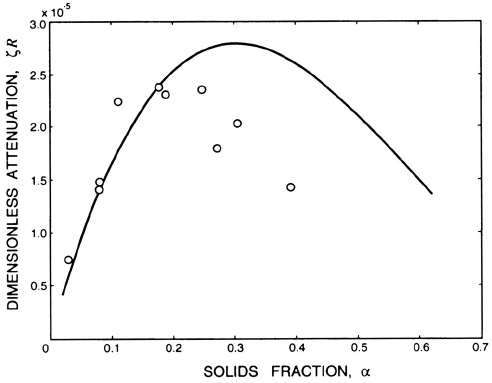
Figure 9.3. An example of the dimensionless attenuation, ζR,atlowfre-
quencies as a function of solids fraction, α. The experimental data (◦)is
for a suspension of Kaolin particles in water with 2κR =3.4 × 10
−4
(Urick
1948); the theoretical line is from Atkinson and Kyt¨omaa (1992).
modeled the dynamics at non-zero values of κR using the following set of
governing equations: (a) continuity equations 1.21 for both the disperse and
continuous phases with no mass exchange (I
N
= 0) (b) momentum equations
1.45 for both phases with no gravity terms and no deviatoric stresses σ
D
Cki
=
0 and (c) a particle force, F
k
(see equation 1.55) that includes the forces
on each particle due to the pressure gradient in the continuous phase, the
added mass, the Stokes drag and the Basset memory terms (see section 2.3.4,
equation 2.67). They included a solids fraction dependence in the added
mass. The resulting dispersion relation yields sound speeds that depend on
frequency, ω, and Reynolds number, ρ
C
ωR
2
/µ
C
, but asymptote to constant
values at both high and low Reynolds numbers. Typical results are plotted
in figure 9.1 for various κR and exhibit fair agreement with the experimental
measurements.
Atkinson and Kyt¨omaa (1992) also compare measured and calculated
acoustic attenuation rates given non-dimensionally by ζR where the am-
plitude decays with distance, s, according to e
−ζs
. The attenuation results
from viscous effects on the relative motion between the particles and the
continuous fluid phase. At low frequencies the relative motion and therefore
the attenuation is dominated by contribution from the Stokes drag term in
equation 2.67; this term is proportional to ω
2
. Though the measured data on
attenuation is quite scattered, the theory yields values of the dimensionless
226

attenuation, ζR, that are roughly of the correct magnitude as shown by the
example in figure 9.3. On the other hand at high frequencies (large κR)the
theoretical attenuation is dominated by the Basset term and is proportional
to (µ
C
ω)
1
2
; it also increases nearly linearly with the solids fraction. However
the measured attenuation rates in this frequency range appear to be about
an order of magnitude larger than those calculated.
Weir (2001), following on the work of Gregor and Rumpf (1975), uses
a similar perturbation analysis with somewhat different basic equations to
generate dispersion relations as a function of frequency and volume fraction.
Acknowledging that solutions of this dispersion relation yield a number of
propagation velocities including both kinematic and dynamic wave speeds
(see section 15.7.3), Weir chooses to focus on the dynamic or acoustic waves.
He demonstrates that, in general, there are two types of dynamic wave. These
have the same kinds of high and low frequency asymptotes described above.
The two low frequency wave speeds converge to yield a single dynamic wave
speed that has a functional dependence on frequency and α that is qualita-
tively similar to that of Atkinson and Kyt¨omaa (1992). It also agrees well
with the measured sound speeds in Musmarra et al.(1995) for suspensions
of various types of particles in liquid. Weir also analyzes the wave speeds in
fluidized beds and compares them with those in unfluidized or static beds.
He also examines the data on wave attenuation; as with the other attenu-
ation data the experimental measurements are quite scattered and do not
agree well with the theoretical predictions, particularly at high frequencies.
9.3.3 Sonic speed with change of phase
Turning now to the behavior of a two-phase rather than two-component
mixture, it is necessary not only to consider the additional thermodynamic
constraint required to establish the mass exchange, δm, but also to recon-
sider the two thermodynamic constraints, QA and QB, that were implicit in
the two-component analysis of section 9.3.1, in the choice of the polytropic
index, k, for the gas and the choice of the sonic speed, c
L
, for the liquid. Note
that a nonisentropic choice for k (for example, k =1)impliesthatheatis
exchanged between the components, and yet this heat transfer process was
not explicitly considered, nor was an overall thermodynamic constraint such
as might be placed on the global change in entropy.
We shall see that the two-phase case requires more intimate knowledge of
these factors because the results are more sensitive to the thermodynamic
constraints. In an ideal, infinitely homogenized mixture of vapor and liq-
uid the phases would everywhere be in such close proximity to each other
227
that heat transfer between the phases would occur instantaneously. The en-
tire mixture of vapor and liquid would then always be in thermodynamic
equilibrium. Indeed, one model of the response of the mixture, called the
homogeneous equilibrium model, assumes this to be the case. In practice,
however, there is a need for results for bubbly flows and mist flows in which
heat transfer between the phases does not occur so readily. A second com-
mon model assumes zero heat transfer between the phases and is known as
the homogeneous frozen model. In many circumstances the actual response
lies somewhere between these extremes. A limited amount of heat transfer
occurs between those portions of each phase that are close to the interface.
In order to incorporate this in the analysis, we adopt an approach that in-
cludes the homogeneous equilibrium and homogeneous frozen responses as
special cases but that requires a minor adjustment to the analysis of section
9.3.1 in order to reflect the degree of thermal exchange between the phases.
As in section 9.3.1 the total mass of the phases A and B after application of
the incremental pressure, δp,areρ
A
α
A
+ δm and ρ
B
α
B
− δm, respectively.
We now define the fractions of each phase,
A
and
B
that, because of their
proximity to the interface, exchange heat and therefore approach thermody-
namic equilibrium with each other. The other fractions (1 −
A
)and(1−
B
)
are assumed to be effectively insulated so that they behave isentropically.
This is, of course, a crude simplification of the actual circumstances, but it
permits qualitative assessment of practical flows.
It follows that the volumes of the four fractions following the incremental
change in pressure, δp,are
(1 −
A
)(ρ
A
α
A
+ δm)
[ρ
A
+ δp(∂ρ
A
/∂p)
s
]
;
A
(ρ
A
α
A
+ δm)
[ρ
A
+ δp(∂ρ
A
/∂p)
e
]
(1 −
B
)(ρ
B
α
B
− δm)
[ρ
B
+ δp(∂ρ
B
/∂p)
s
]
;
B
(ρ
B
α
B
− δm)
[ρ
B
+ δp(∂ρ
B
/∂p)
e
]
(9.15)
where the subscripts s and e refer to isentropic and phase equilibrium deriva-
tives, respectively. Then the change in total volume leads to the following
modified form for equation 9.10 in the absence of surface tension:
1
ρc
2
=(1−
A
)
α
A
ρ
A
∂ρ
A
∂p
s
+
A
α
A
ρ
A
∂ρ
A
∂p
e
+(1−
B
)
α
B
ρ
B
∂ρ
B
∂p
s
+
B
α
B
ρ
B
∂ρ
B
∂p
e
−
δm
δp
1
ρ
A
−
1
ρ
B
(9.16)
The exchange of mass, δm, is now determined by imposing the constraint
that the entropy of the whole be unchanged by the perturbation. The entropy
228
