Brealey, Myers. Principles of Corporate Finance. 7th edition
Подождите немного. Документ загружается.

Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
only 1 or 2 may ever get to market. The 1 or 2 that are marketed have to
generate enough cash flow to make up for the 9,999 or 9,998 that fail.)
2. Market success. FDA approval does not guarantee that a drug will sell. A
competitor may be there first with a similar (or better) drug. The company
may or may not be able to sell the drug worldwide. Selling prices and
marketing costs are unknown.
Imagine that you are standing at the top left of Figure 10.5. A proposed research
program will investigate a promising class of compounds. Could you write down
the expected cash inflows and outflows of the program up to 25 or 30 years in the
future? We suggest that no mortal could do so without a model to help; simulation
may provide the answer.
13
Simulation may sound like a panacea for the world’s ills, but, as usual, you pay
for what you get. Sometimes you pay for more than you get. It is not just a matter
of the time and money spent in building the model. It is extremely difficult to esti-
mate interrelationships between variables and the underlying probability distri-
butions, even when you are trying to be honest.
14
But in capital budgeting, fore-
casters are seldom completely impartial and the probability distributions on which
simulations are based can be highly biased.
In practice, a simulation that attempts to be realistic will also be complex. There-
fore the decision maker may delegate the task of constructing the model to man-
agement scientists or consultants. The danger here is that, even if the builders un-
derstand their creation, the decision maker cannot and therefore does not rely on
it. This is a common but ironic experience: The model that was intended to open
up black boxes ends up creating another one.
268 PART III
Practical Problems in Capital Budgeting
13
N. A. Nichols, “Scientific Management at Merck: An Interview with CFO Judy Lewent,” Harvard Busi-
ness Review 72 (January–February 1994), p. 91.
14
These difficulties are less severe for the pharmaceutical industry than for most other industries.
Pharmaceutical companies have accumulated a great deal of information on the probabilities of scien-
tific and clinical success and on the time and money required for clinical testing and FDA approval.
15
Some simulation models do recognize the possibility of changing policy. For example, when a phar-
maceutical company uses simulation to analyze its R&D decisions, it allows for the possibility that the
company can abandon the development at each phase.
10.3 REAL OPTIONS AND DECISION TREES
If financial managers treat projects as black boxes, they may be tempted to think only
of the first accept–reject decision and to ignore the subsequent investment decisions
that may be tied to it. But if subsequent investment decisions depend on those made
today, then today’s decision may depend on what you plan to do tomorrow.
When you use discounted cash flow (DCF) to value a project, you implicitly as-
sume that the firm will hold the assets passively. But managers are not paid to be
dummies. After they have invested in a new project, they do not simply sit back and
watch the future unfold. If things go well, the project may be expanded; if they go
badly, the project may be cut back or abandoned altogether. Projects that can easily
be modified in these ways are more valuable than those that don’t provide such flex-
ibility. The more uncertain the outlook, the more valuable this flexibility becomes.
That sounds obvious, but notice that sensitivity analysis and Monte Carlo
simulation do not recognize the opportunity to modify projects.
15
For example,
Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
think back to the Otobai electric scooter project. In real life, if things go wrong
with the project, Otobai would abandon to cut its losses. If so, the worst out-
comes would not be as devastating as our sensitivity analysis and simulation
suggested.
Options to modify projects are known as real options. Managers may not al-
ways use the term real option to describe these opportunities; for example, they
may refer to “intangible advantages” of easy-to-modify projects. But when they
review major investment proposals, these option intangibles are often the key to
their decisions.
The Option to Expand
In 2000 FedEx placed an order for 10 Airbus A380 superjumbo transport planes for
delivery in the years 2008–2011. Each flight of an A380 freighter will be capable of
making a 200,000 pound dent in the massive volume of goods that FedEx carries
each day, so the decision could have a huge impact on FedEx’s worldwide busi-
ness. If FedEx’s long-haul airfreight business continues to expand and the super-
jumbo is efficient and reliable, the company will need more superjumbos. But it
cannot be sure they will be needed.
Rather than placing further firm orders in 2000, FedEx has secured a place in the
Airbus production line by acquiring options to buy a “substantial number” of ad-
ditional aircraft at a predetermined price. These options do not commit the com-
pany to expand but give it the flexibility to do so.
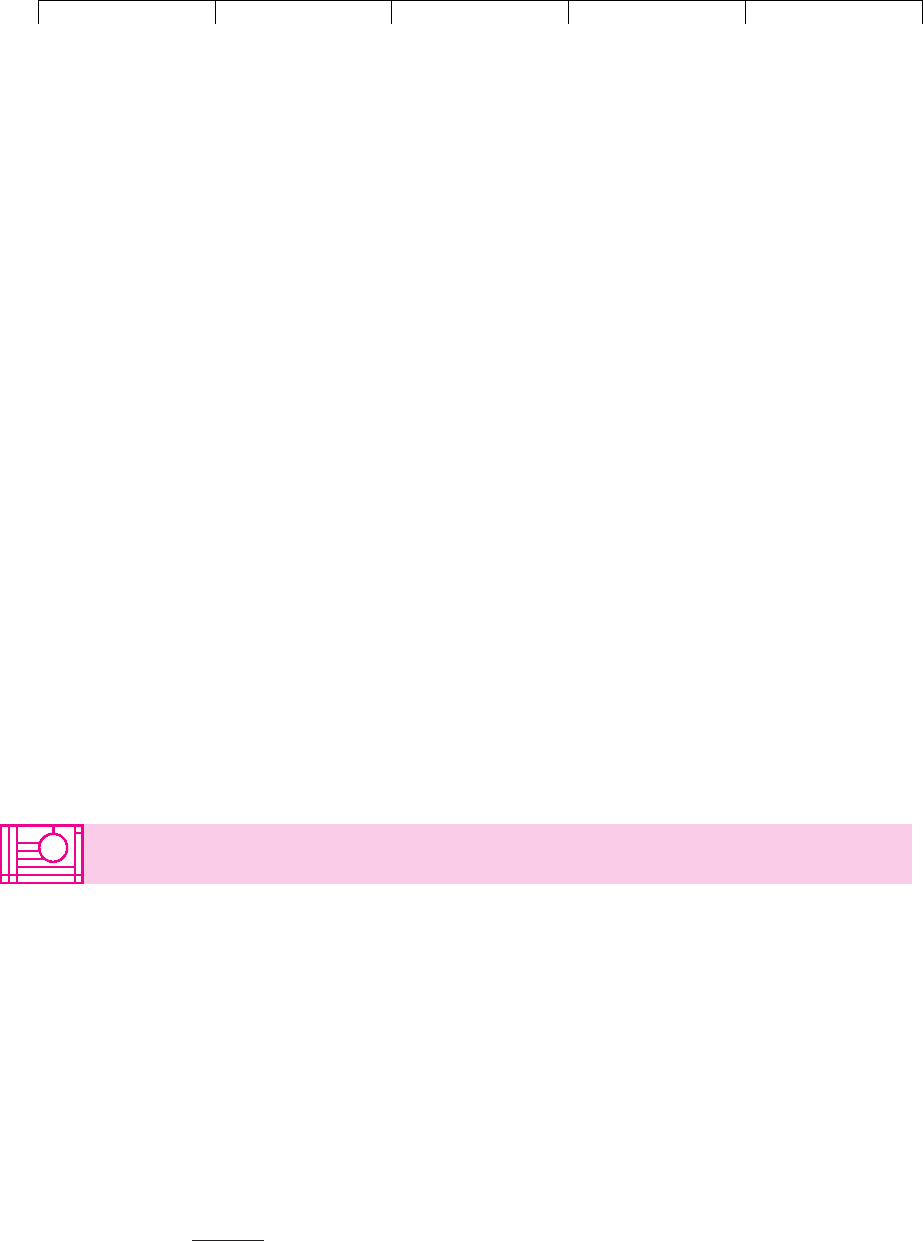
Figure 10.6 displays FedEx’s expansion option as a simple decision tree. You
can think of it as a game between FedEx and fate. Each square represents an action
or decision by the company. Each circle represents an outcome revealed by fate. In
this case there is only one outcome in 2007,
16
when fate reveals the airfreight de-
mand and FedEx’s capacity needs. FedEx then decides whether to exercise its op-
tions and buy additional A380s. Here the future decision is easy: Buy the airplanes
only if demand is high and the company can operate them profitably. If demand is
low, FedEx walks away and leaves Airbus with the problem of selling the planes
that were reserved for FedEx to some other customer.
CHAPTER 10
A Project Is Not a Black Box 269
High
demand
2007: Observe
demand for
airfreight
2000: Acquire
delivery option
in 2008–2011
Exercise
delivery
option
Don't take
delivery
Low
demand
FIGURE 10.6
FedEx’s expansion option
expressed as a simple
decision tree.
16
We assume that FedEx can wait until 2007 to decide whether to acquire the additional planes.

Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
You can probably think of many other investments that take on added value be-
cause of the further options they provide. For example
• When launching a new product, companies often start with a pilot program to
iron out possible design problems and to test the market. The company can
evaluate the pilot and then decide whether to expand to full-scale production.
• When designing a factory, it can make sense to provide extra land or floor
space to reduce the future cost of a second production line.
• When building a four-lane highway, it may pay to build six-lane bridges so
that the road can be converted later to six lanes if traffic volumes turn out to
be higher than expected.
Such options to expand do not show up in the assets that the company lists in its
balance sheet, but investors are very aware of their existence. If a company has
valuable real options that can allow it to invest in new profitable projects, its mar-
ket value will be higher than the value of its physical assets now in place.
In Chapter 4 we showed how the present value of growth opportunities (PVGO)
contributes to the value of a company’s common stock. PVGO equals the fore-
casted total NPV of future investments. But it’s better to think of PVGO as the
value of the firm’s options to invest and expand. The firm is not obliged to grow. It
can invest more if the number of positive-NPV projects turns out high or slow
down if that number turns out low. The flexibility to adapt investment to future op-
portunities is one of the factors that makes PVGO so valuable.
The Option to Abandon
If the option to expand has value, what about the decision to bail out? Projects
don’t just go on until assets expire of old age. The decision to terminate a project
is usually taken by management, not by nature. Once the project is no longer
profitable, the company will cut its losses and exercise its option to abandon the
project.
17
Some assets are easier to bail out of than others. Tangible assets are usually eas-
ier to sell than intangible ones. It helps to have active secondhand markets, which
really exist only for standardized items. Real estate, airplanes, trucks, and certain
machine tools are likely to be relatively easy to sell. On the other hand, the knowl-
edge accumulated by a software company’s research and development program is
a specialized intangible asset and probably would not have significant abandon-
ment value. (Some assets, such as old mattresses, even have negative abandonment
value; you have to pay to get rid of them. It is costly to decommission nuclear
power plants or to reclaim land that has been strip-mined.)
Example. Managers should recognize the option to abandon when they make
the initial investment in a new project or venture. For example, suppose you
must choose between two technologies for production of a Wankel-engine out-
board motor.
1. Technology A uses computer-controlled machinery custom-designed to
produce the complex shapes required for Wankel engines in high volumes
and at low cost. But if the Wankel outboard doesn’t sell, this equipment will
be worthless.
270 PART III
Practical Problems in Capital Budgeting
17
The abandonment option was first analyzed by A. A. Robichek and J. C. Van Horne, “Abandonment
Value in Capital Budgeting,” Journal of Finance 22 (December 1967), pp. 577–590.
Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
2. Technology B uses standard machine tools. Labour costs are much higher,
but the machinery can be sold for $10 million if the engine doesn’t sell.
Technology A looks better in a DCF analysis of the new product because it was de-
signed to have the lowest possible cost at the planned production volume. Yet you
can sense the advantage of technology B’s flexibility if you are unsure about
whether the new outboard will sink or swim in the marketplace.
We can make the value of this flexibility concrete by expressing it as a real op-
tion. Just for simplicity, assume that the initial capital outlays for technologies A
and B are the same. Technology A, with its low-cost customized machinery, will
provide a payoff of $18.5 million if the outboard is popular with boat owners
and $8.5 million if it is not. Think of these payoffs as the project’s cash flow in
its first year of production plus the present value of all subsequent cash flows.
The corresponding payoffs to technology B are $18 million and $8 million.
CHAPTER 10
A Project Is Not a Black Box 271
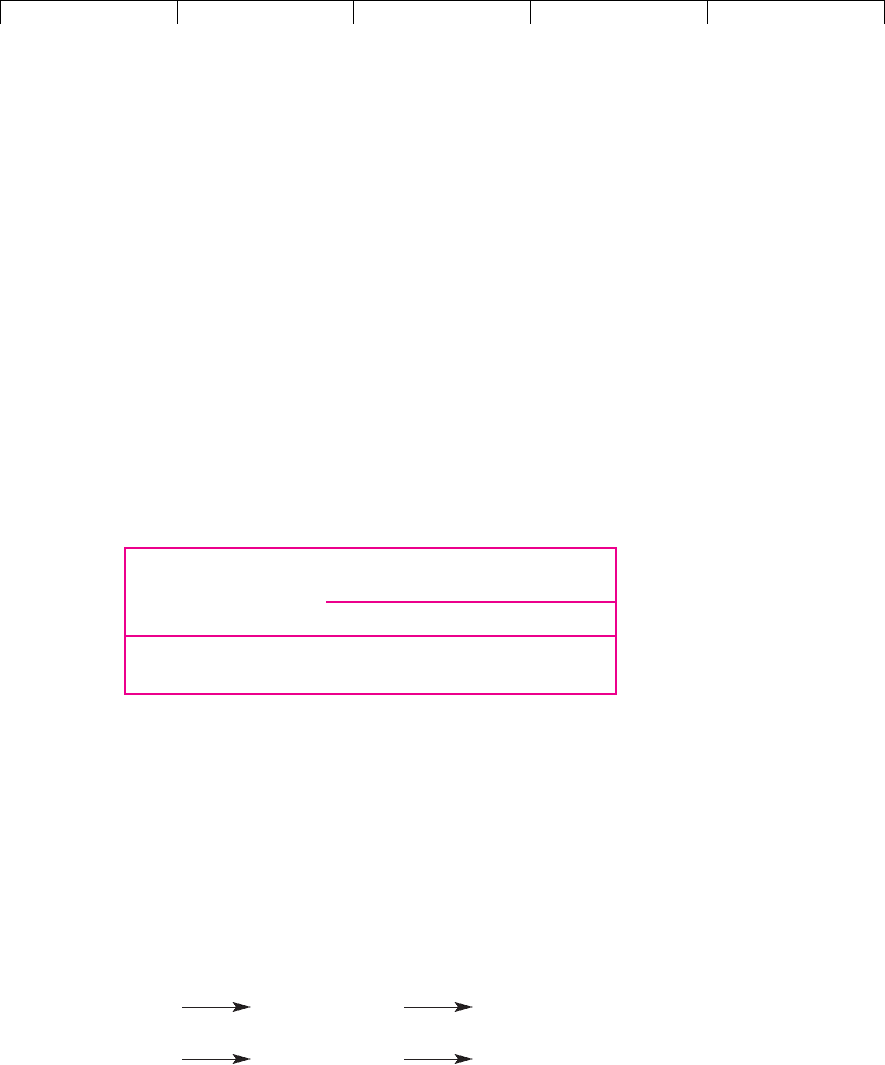
Payoffs from Producing
Outboard ($ millions)
Technology A Technology B
Buoyant demand $18.5 $18
Sluggish demand 8.5 8
If you are obliged to continue in production regardless of how unprofitable the project
turns out to be, then technology A is clearly the superior choice. But remember
that at year-end you can bail out of technology B for $10 million. If the outboard
is not a success in the market, you are better off selling the plant and equipment
for $10 million than continuing with a project that has a present value of only
$8 million.
Figure 10.7 summarizes this example as a decision tree. The abandonment op-
tion occurs at the right-hand boxes for Technology B. The decisions are obvious:
continue if demand is buoyant, abandon otherwise. Thus the payoffs to Technol-
ogy B are:
Buoyant continue own business
demand production worth $18 million
Sluggish exercise option receive
demand to sell assets $10 million
Technology B provides an insurance policy: If the outboard’s sales are disap-
pointing, you can abandon the project and recover $10 million. You can think of
this abandonment option as an option to sell the assets for $10 million. The total
value of the project using technology B is its DCF value, assuming that the com-
pany does not abandon, plus the value of the abandonment option. When you
value this option, you are placing a value on flexibility.
Two Other Real Options
These are not the only real options. For example, companies with positive-NPV
projects are not obliged to undertake them right away. If the outlook is uncertain,
you may be able to avoid a costly mistake by waiting a bit. Such options to post-
pone investment are called timing options.
Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
When companies undertake new investments, they generally think about the
possibility that at a later stage they may wish to modify the project. After all, to-
day everybody may be demanding round pegs, but, who knows, tomorrow
square ones could be all the rage. In that case you need a plant that provides the
flexibility to produce a variety of peg shapes. In just the same way, it may be
worth paying up front for the flexibility to vary the inputs. For example, in Chap-
ter 22 we will describe how electric utilities often build in the option to switch be-
272 PART III
Practical Problems in Capital Budgeting
Technology A
Demand
revealed
Demand
revealed
Technology B
Buoyant
Sluggish
$18.5 million
$8.5 million
$18 million
$10 million
$8 million
$10 million
Abandon
Continue
Continue
Abandon
Buoyant
Sluggish
FIGURE 10.7
Decision tree for the
Wankel outboard motor
project. Technology B
allows the firm to
abandon the project and
recover $10 million if
demand is sluggish.

Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
tween burning oil to burning natural gas. We refer to these opportunities as pro-
duction options.
More on Decision Trees
We will return to all these real options in Chapter 22, after we have covered the the-
ory of option valuation in Chapters 20 and 21. But we will close this chapter with
a closer look at decision trees.
Decision trees are commonly used to describe the real options imbedded in cap-
ital investment projects. But decision trees were used in the analysis of projects
years before real options were first explicitly identified.
18
Decision trees can help
to understand project risk and how future decisions will affect project cash flows.
Even if you never learn or use option valuation theory, decision trees belong in
your financial toolkit.
The best way to appreciate how decision trees can be used in project analysis is
to work through a detailed example.
An Example: Magna Charter
Magna Charter is a new corporation formed by Agnes Magna to provide an executive
flying service for the southeastern United States. The founder thinks there will be a
ready demand from businesses that cannot justify a full-time company plane but nev-
ertheless need one from time to time. However, the venture is not a sure thing. There
is a 40 percent chance that demand in the first year will be low. If it is low, there is a 60
percent chance that it will remain low in subsequent years. On the other hand, if the
initial demand is high, there is an 80 percent chance that it will stay high.
The immediate problem is to decide what kind of plane to buy. A turboprop
costs $550,000. A piston-engine plane costs only $250,000 but has less capacity and
customer appeal. Moreover, the piston-engine plane is an old design and likely to
depreciate rapidly. Ms. Magna thinks that next year secondhand piston aircraft
will be available for only $150,000.
That gives Ms. Magna an idea: Why not start out with one piston plane and buy
another if demand is still high? It will cost only $150,000 to expand. If demand is
low, Magna Charter can sit tight with one small, relatively inexpensive aircraft.
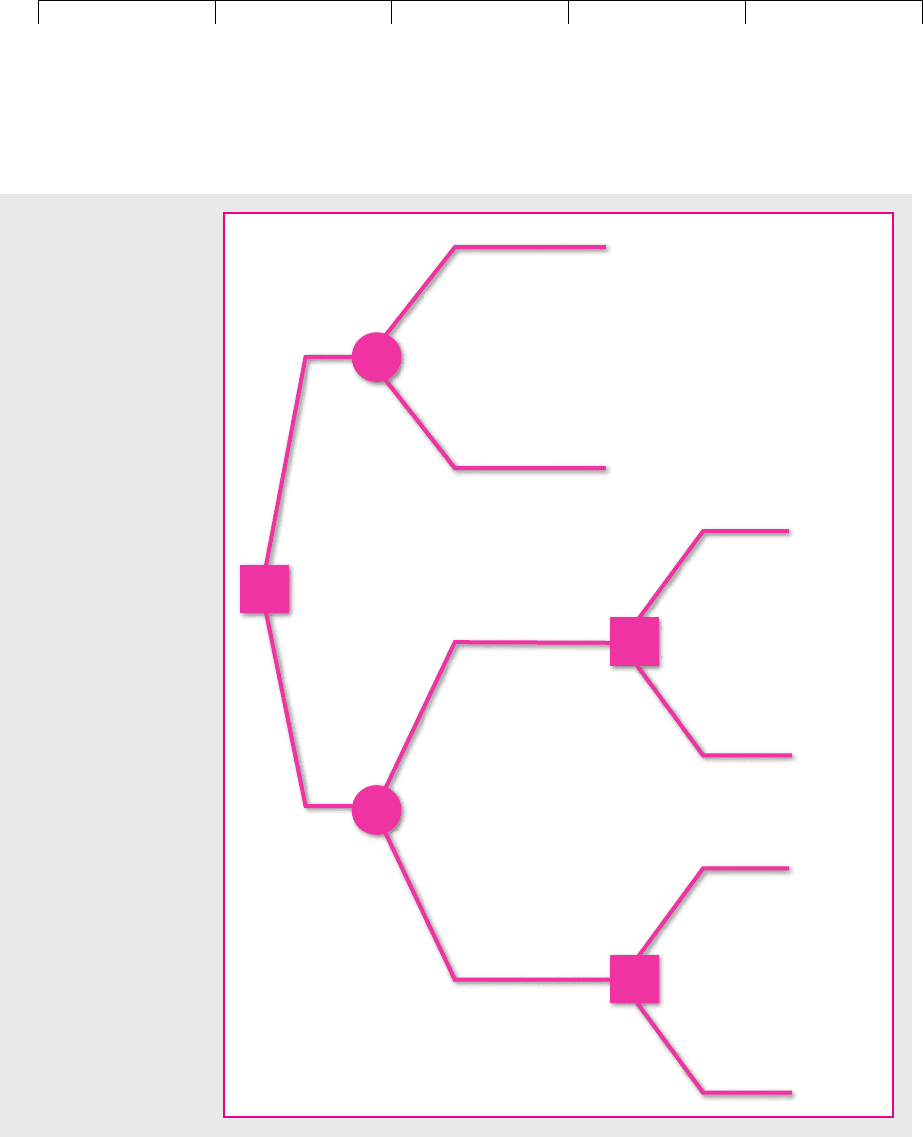
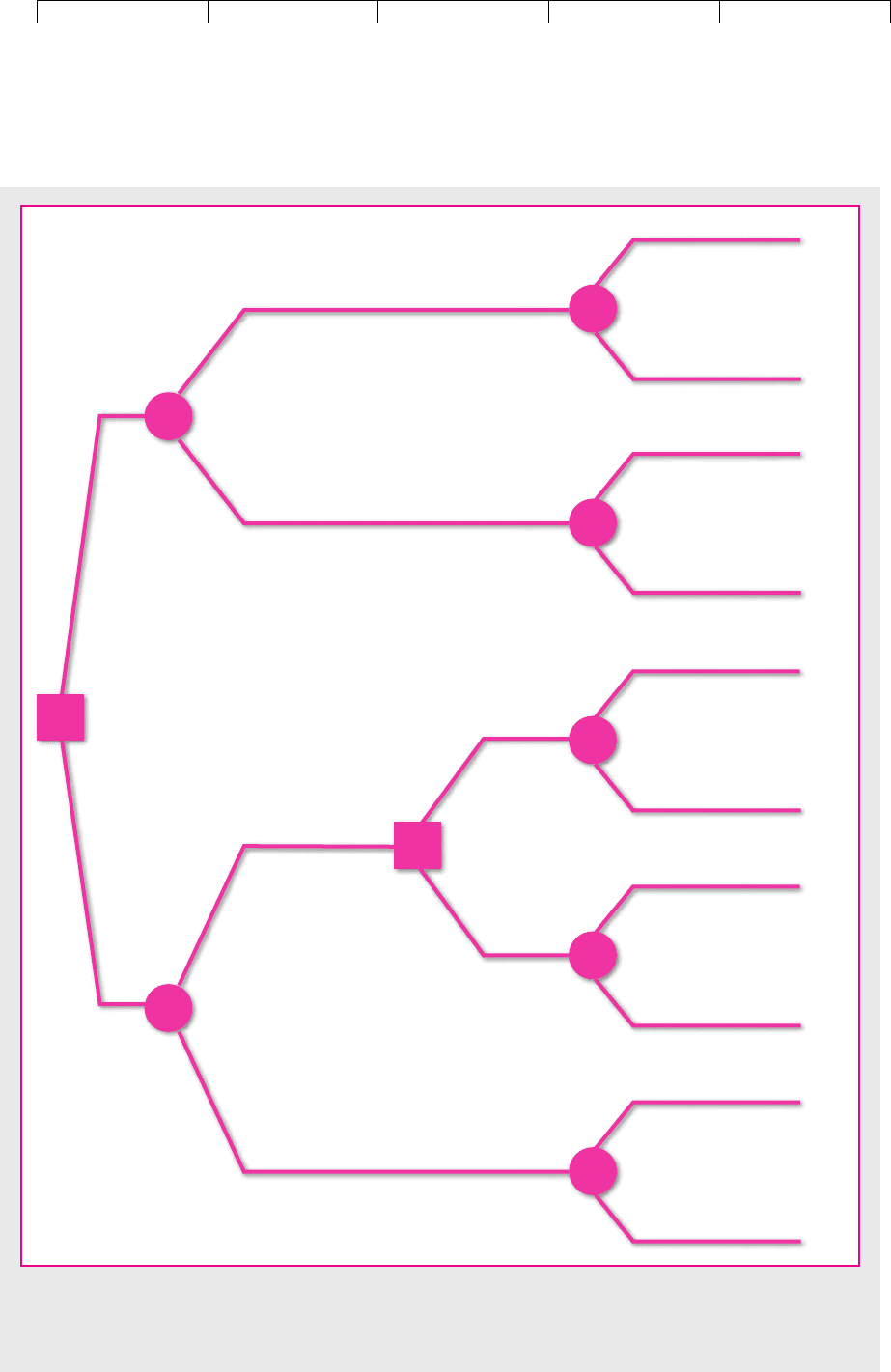
Figure 10.8 displays these choices. The square on the left marks the company’s
initial decision to purchase a turboprop for $550,000 or a piston aircraft for
$250,000. After the company has made its decision, fate decides on the first year’s
demand. You can see in parentheses the probability that demand will be high or
low, and you can see the expected cash flow for each combination of aircraft and
demand level. At the end of the year the company has a second decision to make
if it has a piston-engine aircraft: It can either expand or sit tight. This decision point
is marked by the second square. Finally fate takes over again and selects the level
of demand for year 2. Again you can see in parentheses the probability of high or
low demand. Notice that the probabilities for the second year depend on the first-
period outcomes. For example, if demand is high in the first period, then there is
an 80 percent chance that it will also be high in the second. The chance of high
CHAPTER 10
A Project Is Not a Black Box 273
18
The use of decision trees was first advocated by J. Magee in “How to Use Decision Trees in Capital In-
vestment,” Harvard Business Review 42(September–October 1964), pp. 79–96. Real options were first
identified in S. C. Myers, “Determinants of Corporate Borrowing,” Journal of Financial Economics 5
(November 1977), pp. 146–175.
Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
274 PART III Practical Problems in Capital Budgeting
Turboprop
–$550
Piston
–$250
Low demand (.2)
High demand (.8)
High demand (.6)
$150
Low demand (.4)
$30
$220
$960
Low demand (.6)
High demand (.4)
$140
$930
Low demand (.2)
High demand (.8)
High demand (.6)
$100
Expand
–$150
Do not
expand
Low demand (.4)
$50
$100
$800
Low demand (.6)
High demand (.4)
$100
$220
Low demand (.2)
High demand (.8)
$180
$410
FIGURE 10.8
Decision tree for Magna Charter. Should it buy a turboprop or a smaller piston-engine plane? A second piston plane can
be purchased in year 1 if demand turns out to be high. (All figures are in thousands. Probabilities are in parentheses.)

Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
demand in both the first and second periods is .6 .8 .48. After the parentheses
we again show the profitability of the project for each combination of aircraft and
demand level. You can interpret each of these figures as the present value at the end
of year 2 of the cash flows for that and all subsequent years.
The problem for Ms. Magna is to decide what to do today. We solve that prob-
lem by thinking first what she would do next year. This means that we start at the
right side of the tree and work backward to the beginning on the left.
The only decision that Ms. Magna needs to make next year is whether to expand
if purchase of a piston-engine plane is succeeded by high demand. If she expands,
she invests $150,000 and receives a payoff of $800,000 if demand continues to be
high and $100,000 if demand falls. So her expected payoff is
low demand)
If the opportunity cost of capital for this venture is 10 percent,
19
then the net pres-
ent value of expanding, computed as of year 1, is
If Ms. Magna does not expand, the expected payoff is
low demand)
The net present value of not expanding, computed as of year 1, is
Expansion obviously pays if market demand is high.
Now that we know what Magna Charter ought to do if faced with the expansion
decision, we can roll back to today’s decision. If the first piston-engine plane is
bought, Magna can expect to receive cash worth $550,000 in year 1 if demand is
high and cash worth $185,000 if it is low:
NPV 0
364
1.10
331, or $331,000
1.8 4102 1.2 1802364, or $364,000
1probability low demand payoff with
1Probability high demand payoff with high demand2
NPV 150
660
1.10
450, or $450,000
1.8 8002 1.2 1002660, or $660,000
1probability low demand payoff with
1Probability high demand payoff with high demand2
CHAPTER 10 A Project Is Not a Black Box 275
19
We are guilty here of assuming away one of the most difficult questions. Just as in the Vegetron mop
case in Chapter 9, the most risky part of Ms. Magna’s venture is likely to be the initial prototype proj-
ect. Perhaps we should use a lower discount rate for the second piston-engine plane than for the first.
High demand (.6)
$550,000
Invest
$250,000
Low demand (.4)
$185,000
$100,000 cash flow
plus $450,000 net
present value
$50,000 cash flow
plus net present value of
= $135,000
(.4
×
220)
+
(.6
×
100)
1.10
Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
The net present value of the investment in the piston-engine plane is therefore
$117,000:
If Magna buys the turboprop, there are no future decisions to analyze, and
so there is no need to roll back. We just calculate expected cash flows and
discount:
Thus the investment in the piston-engine plane has an NPV of $117,000; the in-
vestment in the turboprop has an NPV of $96,000. The piston-engine plane is the
better bet. Note, however, that the choice would be different if we forgot to take ac-
count of the option to expand. In that case the NPV of the piston-engine plane
would drop from $117,000 to $52,000:
The value of the option to expand is, therefore,
The decision tree in Figure 10.8 recognizes that, if Ms. Magna buys one piston-
engine plane, she is not stuck with that decision. She has the option to expand by
buying an additional plane if demand turns out to be unexpectedly high. But Fig-
ure 10.8 also assumes that, if Ms. Magna goes for the big time by buying a turbo-
prop, there is nothing that she can do if demand turns out to be unexpectedly low.
That is unrealistic. If business in the first year is poor, it may pay for Ms. Magna to
sell the turboprop and abandon the venture entirely. In Figure 10.8 we could rep-
resent this option to bail out by adding an extra decision point (a further square)
if the company buys the turboprop and first-year demand is low. If that happens,
Ms. Magna could decide either to sell the plane or to hold on and hope demand re-
covers. If the abandonment option is sufficiently valuable, it may make sense to
take the turboprop and shoot for the big payoff.
Pro and Con Decision Trees
Any cash-flow forecast rests on some assumption about the firm’s future invest-
ment and operating strategy. Often that assumption is implicit. Decision trees force
the underlying strategy into the open. By displaying the links between today’s and
117 52 65, or $65,000
52, or $52,000
.63.814102 .2118024 .43.412202 .6110024
11.102
2
NPV 250
.611002 .41502
1.10
550
102
1.10
670
11.102
2
96, or $96,000
.63.819602 .2122024 .43.419302 .6114024
11.102
2
NPV 550
.611502 .41302
1.10
NPV 250
.615502 .411852
1.10
117, or $117,000
276 PART III Practical Problems in Capital Budgeting

Brealey−Meyers:
Principles of Corporate
Finance, Seventh Edition
III. Practical Problems in
Capital Budgeting
10. A Project is Not a Black
Box
© The McGraw−Hill
Companies, 2003
tomorrow’s decisions, they help the financial manager to find the strategy with the
highest net present value.
The trouble with decision trees is that they get so _____ complex so _____
quickly (insert your own expletives). What will Magna Charter do if demand is
neither high nor low but just middling? In that event Ms. Magna might sell the tur-
boprop and buy a piston-engine plane, or she might defer expansion and aban-
donment decisions until year 2. Perhaps middling demand requires a decision
about a price cut or an intensified sales campaign.
We could draw a new decision tree covering this expanded set of events and
decisions. Try it if you like: You’ll see how fast the circles, squares, and branches
accumulate.
Life is complex, and there is very little we can do about it. It is therefore unfair
to criticize decision trees because they can become complex. Our criticism is re-
served for analysts who let the complexity become overwhelming. The point of
decision trees is to allow explicit analysis of possible future events and decisions.
They should be judged not on their comprehensiveness but on whether they
show the most important links between today’s and tomorrow’s decisions. Deci-
sion trees used in real life will be more complex than Figure 10.8, but they will
nevertheless display only a small fraction of possible future events and decisions.
Decision trees are like grapevines: They are productive only if they are vigor-
ously pruned.
Decision trees can help identify the future choices available to the manager
and can give a clearer view of the cash flows and risks of a project. However, our
analysis of the Magna Charter project begged an important question. The option
to expand enlarged the spread of possible outcomes and therefore increased the
risk of investing in a piston aircraft. Conversely, the option to bail out would
narrow the spread of possible outcomes, reducing the risk of investment. We
should have used different discount rates to recognize these changes in risk, but
decision trees do not tell us how to do this. But the situation is not hopeless.
Modern techniques of option pricing can value these investment options. We
will describe these techniques in Chapters 20 and 21, and turn again to real op-
tions in Chapter 22.
Decision Trees and Monte Carlo Simulation
We have said that any cash-flow forecast rests on assumptions about future in-
vestment and operating strategy. Think back to the Monte Carlo simulation model
that we constructed for Otobai’s electric scooter project. What strategy was that
based on? We don’t know. Inevitably Otobai will face decisions about pricing, pro-
duction, expansion, and abandonment, but the model builder’s assumptions about
these decisions are buried in the model’s equations. The model builder may have
implicitly identified a future strategy for Otobai, but it is clearly not the optimal
one. There will be some runs of the model when nearly everything goes wrong and
when in real life Otobai would abandon to cut its losses. Yet the model goes on pe-
riod after period, heedless of the drain on Otobai’s cash resources. The most unfa-
vorable outcomes reported by the simulation model would never be encountered
in real life.
On the other hand, the simulation model probably understates the project’s po-
tential value if nearly everything goes right: There is no provision for expanding to
take advantage of good luck.
CHAPTER 10
A Project Is Not a Black Box 277
