Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.


923.1
The Macroscopic Mass Balances
727
In this chapter we summarize the macroscopic balances for the more general situa-
tion described above. Each of these balances will now contain one extra term, to account
for mass, momentum, or energy transport across the bounding surfaces. The balances
thus obtained are capable of describing industrial mass transfer processes, such as ab-
sorption, extraction, ion exchange, and selective adsorption. Inasmuch as entire treatises
have been devoted to these topics, all we try to do here is to show how the material dis-
cussed in the preceding chapters paves the way for the study of mass transfer opera-
tions. The reader interested in pursuing these topics further should consult the available
textbooks and treatises.'-"
The main emphasis on this chapter is on the mass balances for mixtures. For that
reason,
523.1
is accompanied by five examples, which illustrate problems arising in envi-
ronmental science, isotope separation, economic evaluation, and biomedical science. In
ss23.2
to
23.4
the other macroscopic balances are given. In Table
23.5-1
they are summa-
rized for systems with multiple inlets and outlets. The last two sections of the chapter il-
lustrate applications of the macroscopic balances to more complex systems.
523.1
THE
MACROSCOPIC MASS BALANCES
The statement of the law of conservation of mass of chemical species
a
in a multicompo-
nent macroscopic flow system is
This is a generalization of
Eq.
7.1-2.
Here
ma,,,
is the instantaneous total mass of
a
in the
system, and
-Awn
=
w,,
-
w,,
=
p,l(v,)S,
-
pa2(v2)S2
is the difference between the mass
rates of flow of species
a
across planes
1
and
2.
The quantity
w,,,
is the mass rate of addi-
tion of species
a
to the system by mass transfer across the bounding surface. Note that
w,,,
is positive when mass is
added
to the system, just as
Q
and
W,
are taken to be posi-
tive
in
the total energy balance when heat is added to the system and work is done
on
the system by moving parts. Finally, the symbol
r,,t,,
stands for the net rate of produc-
tion of species
a
by homogeneous and heterogeneous reactions within the system.'
Recall that in Table
15.5-1
the molecular and eddy transport of momentum and
en-
ergy across surfaces
1
and
2
in the direction of flow were neglected with respect to the
convective transport. The same is done everywhere in this chapter-in
Eq.
23.1-1
and in
the other macroscopic balances presented here.
W.
L.
McCabe,
J.
C.
Smith, and
P.
Harriot,
Unit Operations of Chemical Engineering,
McGraw-Hill,
New York, 6th edition
(2000).
T.
K.
Sherwood,
R.
L.
Pigford, and
C.
R.
Wilke,
Mass Transfer,
McGraw-Hill, New York (1975).
R.
E.
Treybal,
Mass Transfer Operations,
3rd edition, McGraw-Hill, New York (1980).
C.
J.
King,
Separation Processes,
McGraw-Hill, New York (1971).
C.
D.
Holland,
Multicomponent Distillation,
McGraw-Hill, New York (1963).
T.
C.
Lo, M. H.
I.
Baird, and
C.
Hanson, eds.,
Handbook of Solvent Extracfion,
Wiley-Interscience,
New York (1983).
R.
T.
Yang,
Gas Separations
by
Adsorption Processes,
Butterworth, Boston (1987).
J.
D.
Seader and
E.
J.
Henley,
Separation Process Principles,
Wiley, New York (1998).
The quantities
ma,,,,
w,,,
and
r,,,,
may be expressed as integrals:
in which
n
is the outwardly directed unit normal vector, and
So
is that portion of the bounding surface
on which mass transfer occurs. The integrands in
r,,,,
are
the net rates of production of species
a
by
homogeneous and heterogeneous reactions, respectively.
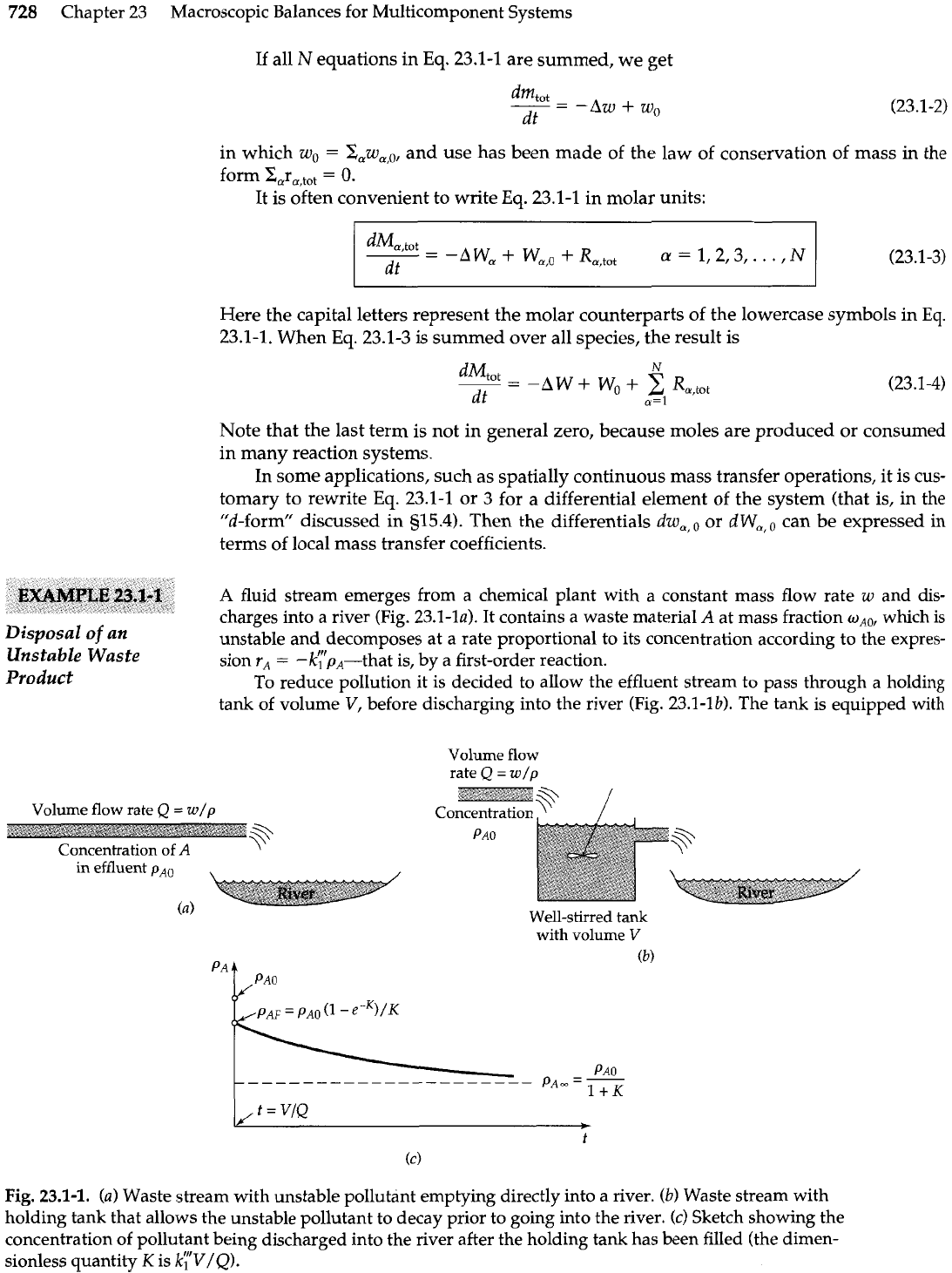
728
Chapter
23
Macroscopic Balances for Multicomponent Systems
If all
N
equations in Eq. 23.1-1 are summed, we get
in which w,
=
Saw,,,,
and use has been made of the law of conservation of mass in the
form Z,r,,,,
=
0.
It
is often convenient to write Eq. 23.1-1 in molar units:
Here the capital letters represent the molar counterparts of the lowercase symbols in
Eq.
23.1-1. When Eq. 23.1-3 is summed over all species, the result is
Note that the last term is not in general zero, because moles are produced or consumed
in many reaction systems.
In some applications, such as spatially continuous mass transfer operations, it is cus-
tomary to rewrite Eq. 23.1-1 or
3
for a differential element of the system (that is, in the
"d-form" discussed in
515.4).
Then
the differentials
dw,,
,
or
d
W,,
can
be expressed in
terms of local mass transfer coefficients.
A
fluid stream emerges from a chemical plant with a constant mass flow rate
w
and dis-
charges into a river
(Fig.
23.1-la). It contains a waste material
A
at mass fraction
w,,,
which is
Disposal
of
an
unstable and decomposes at a rate proportional to its concentration according to the expres-
Unstable Waste
sion
r,
=
-k';bA-that is, by a first-order reaction.
Product
To reduce pollution it is decided to allow the effluent stream to pass through a holding
tank of volume
V,
before discharging into the river (Fig. 23.1-lb). The tank is equipped
with
Volume flow rate
Q
=
w/p
p
Concentration of
A
-+
in effluent
PAO
(a)
Volume
flow
rate
Q
=
w/p
Concentration,
Well-stirred
tank
with volume
V
(b)
Fig.
23.1-1.
(a)
Waste stream with unstable pollutant emptying directly into a river.
(b)
Waste stream with
holding tank that allows the unstable pollutant to decay prior to going into the river.
(c)
Sketch showing the
concentration of pollutant being discharged into the river after the holding tank has been filled (the dimen-
sionless quantity
K
is
k;"V/Q).

523.1 The Macroscopic Mass Balances
729
SOLUTION
an efficient stirrer that keeps the fluid in the tank at very nearly uniform composition. At time
t
=
0
the fluid begins to flow into the empty tank. No liquid flows out until the tank has been
filled up to the volume V.
Develop an expression for the concentration of the fluid in the tank as a function of time,
both during the tank-filling process and after the tank has been completely filled.
(a)
We begin by considering the period during which the tank is being filled-that is the pe-
riod t
5
pV/w, where
p
is the density of the fluid mixture. We apply the macroscopic mass
balance of Eq. 23.1-1 to the holding tank. The quantity mA,tot on the left side is wto, at time t.
The mass rate of flow entering the tank is wwAo, and there is no outflow during the tank-filling
stage. No
A
is entering or leaving through a mass transfer interface. The mass rate of produc-
tion of species
A
is
Y,,~,,
=
(wt/p)(-k;"pA)
=
-k~m,,t,t. Therefore the macroscopic mass balance
for species
A
during the filling period is
This first-order differential equation can be solved with the initial condition that mA,tot
=
0
at
t
=
0
to give
This may be written
in
terms of the instantaneous mass fraction of
A
in the tank by using the
relation mA,tot
=
wtw,:
The mass fraction of
A
at the instant when the tank is full,
o,,
is then given by
in which
K
=
kYpv/w
=
k;"V/Q.
(b)
The mass balance on the tank after it has been filled is
or, in dimensionless form, with
T
=
(w/pV)t,
This first-order differential equation can be solved with the initial condition that
w,
=
w,,
at
r
=
1
to give
This shows that as time progresses the mass fraction of the pollutant being discharged into
the river decreases exponentially, with a limiting value of
The curve for the mass concentration as a function of time after the filling of the tank is shown
in Fig. 23.1-l(c). This curve can be used to determine conditions such that the effluent concen-
tration will be in the permitted range. Equation 23.1-12 can be used to decide on the size of
holding tank that is required.

730
Chapter 23 Macroscopic Balances for Multicomponent Systems
product
Fig.
23.1-2.
Binary splitter, in which a feed stream is split into
P,
y,
Y
a product stream and a waste stream.
EXAMPLE
23.1-2
Bind
y
Splitters
Describe the operation of a binary splitter, one of the commonest and simplest separation de-
vices (see Fig. 23.1-2). Here a binary mixture of
A
and
B
enters the apparatus in a feed stream
at a molar rate F, and by some separation mechanism it is split into a product stream with a
molar rate P and a waste stream with molar rate
W.
The mole fraction of
A
(the desired com-
ponent) in the feed stream is
z,
and the mole fractions in the product and waste streams are
y
and x, respectively.
SOL
UTION
We start by writing the steady-state macroscopic mass balances for component
A
and for the
entire fluid as
ZF =yP
+
XW
(23.1-13)
F=P+W (23.1-14)
It is customary to define the ratio
0
=
P/F
of the molar rates of the product and feed streams
as the cut. Equation 23.1-13 then becomes, after eliminating
W
by use of Eq. 23.1-14,
Normally the cut
0
and the feed composition z are taken to be known.
We now need
a
relation between the feed and waste compositions, and it is conventional
to write an equation relating the compositions of the two outgoing streams:
Here
a
is known as the separation facfor, also usually taken as known, and which characterizes
the separation capability of the splitter. Here
Y
and
X
are the mole ratios defined by
Y
x=- and
X
=
-
x
1-x
(23.1-17,18)
1-Y
In terms of the mole fractions, Eq. 23.1-16 may be written as
Equations 23.1-15 and 19 (or 20) describe completely the splitter operation.
For vapor-liquid splitting-that is, equilibrium distillation-it is typical to define the
ideal splitter in terms of an operation in which the product and waste streams are in equilib-
rium. For this situation,
a
is the relative volatility, and for thermodynamically ideal systems, it
is
just the ratio of the component vapor pressures. Even for nonideal systems,
a
changes rela-
tively slowly with composition.
For real splitters one can then define
a
in terms of an empirical correction factor-for ex-
ample, the eficiency-defined by
a
=
Ea*
(23.1-21)
where
a*
is the separation factor for the ideal model, and
E
is a correction factor that accounts
for the failure of the actual system to meet the ideal behavior.
We thus find that, for a given feed composition, the enrichment (y
-
z)/z produced by the
splitter is a function of the cut
0
and the separation factor
a.
The enrichment can be calculated
from the following equation, which is obtained by combining Eqs. 23.1-15 and 20:
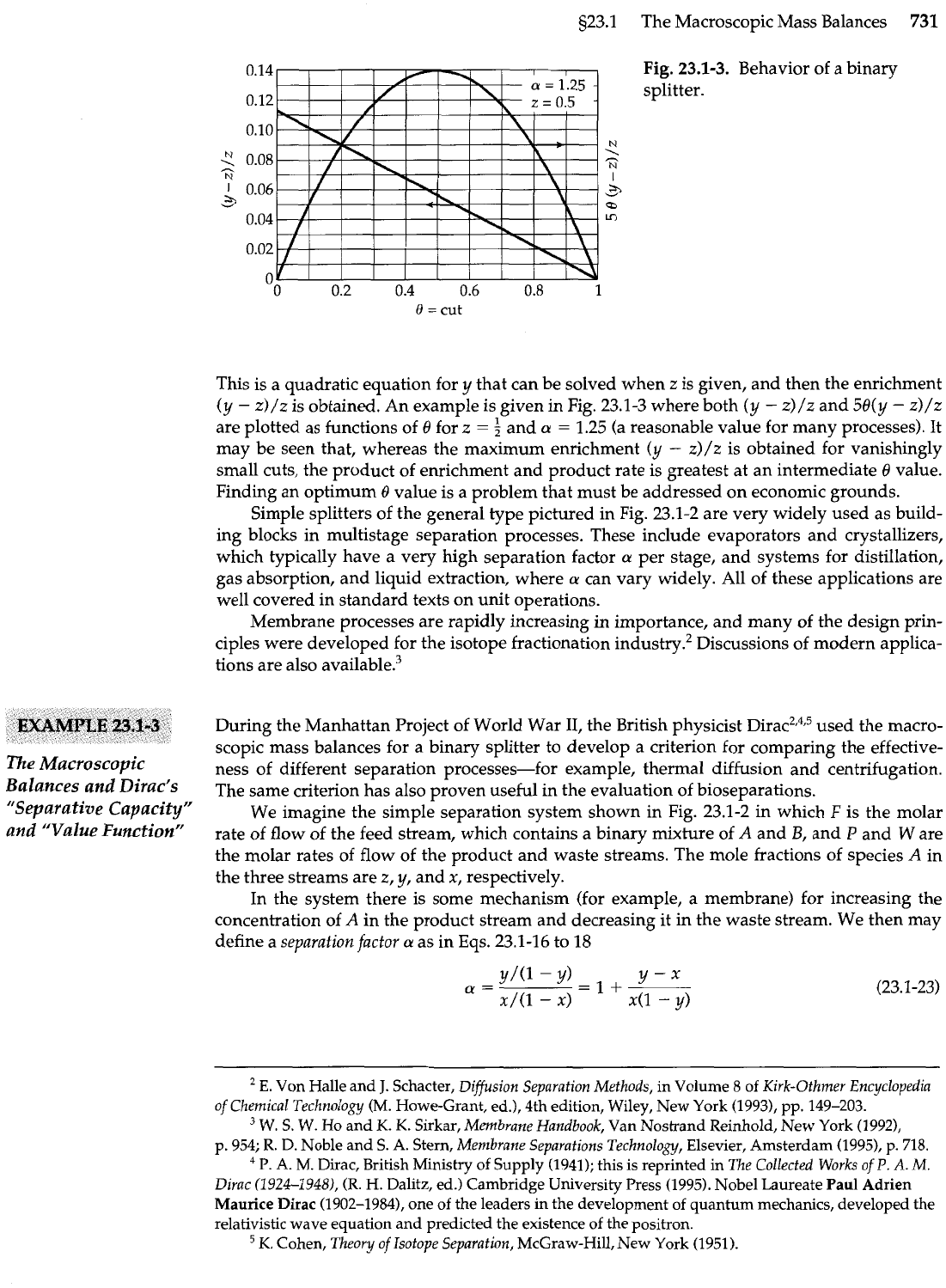
s23.1 The Macroscopic Mass Balances
731
Fig.
23.1-3.
Behavior of a binary
splitter.
N
1
N
I
3
0
In
0
=
cut
This is a quadratic equation for
y
that can be solved when
z
is given, and then the enrichment
(y
-
z)/z is obtained. An example is given in Fig. 23.1-3 where both
(y
-
z)/z
and
58(y
-
z)/z
are plotted as functions of
8
for
z
=
5
and
a
=
1.25 (a reasonable value for many processes). It
may be seen that, whereas the maximum enrichment
(y
-
z)/z is obtained for vanishingly
small cuts, the product of enrichment and product rate is greatest at an intermediate
9
value.
Finding an optimum
8
value is a problem that must be addressed on economic grounds.
Simple splitters of the general type pictured in Fig. 23.1-2 are very widely used as build-
ing blocks in multistage separation processes. These include evaporators and crystallizers,
which typically have a very high separation factor
a
per stage, and systems for distillation,
gas absorption, and liquid extraction, where
a
can vary widely. All of these applications are
well covered in standard texts on unit operations.
Membrane processes are rapidly increasing
in
importance, and many of the design prin-
ciples were developed for the isotope fractionation industry.' Discussions of modern applica-
tions are also a~ailable.~
EXAMPLE
23.1-3
The Macroscopic
Balances and Dirac's
"Separative Capacity"
and
"Value Function"
During the Manhattan Project of World War
11,
the British physicist Dira~~,~,~ used the macro-
scopic mass balances for a binary splitter to develop a criterion for comparing the effective-
ness of different separation processes-for example, thermal diffusion and centrifugation.
The same criterion has also proven useful in the evaluation of bioseparations.
We imagine the simple separation system shown in Fig. 23.1-2 in which
F
is the molar
rate of flow of the feed stream, which contains
a
binary mixture of
A
and
B,
and
P
and Ware
the molar rates of flow of the product and waste streams. The mole fractions of species
A
in
the three streams are z,
y,
and x, respectively.
In the system there is some mechanism (for example, a membrane) for increasing the
concentration of
A
in the product stream and decreasing it in the waste stream. We then may
define a
separation
factor
a
as in Eqs. 23.1-16 to 18
E. Von Halle and
J.
Schacter,
Diffusion Separation Methods,
in Volume
8
of
Kirk-Othmer Encyclopedia
of
Chemical Technology
(M.
Howe-Grant, ed.), 4th edition, Wiley, New York (19931, pp. 149-203.
W.
S. W. Ho and
K.
K.
Sirkar,
Membrane Handbook,
Van Nostrand Reinhold, New York (1992),
p. 954;
R.
D. Noble and S.
A.
Stern,
Membrane Separations Technology,
Elsevier, Amsterdam (19951, p.
718.
P.
A.
M.
Dirac, British Ministry of Supply (1941); this is reprinted in
The Collected Works of
P.
A.
M.
Dirac
(1924-19481,
(R.
H.
Dalitz, ed.) Cambridge University Press (1995). Nobel Laureate
Paul
Adrien
Maurice
Dirac (1902-1984), one of the leaders in the development of quantum mechanics, developed the
relativistic wave equation and predicted the existence of the positron.
K.
Cohen,
Theory of Isotope Separation,
McGraw-Hill, New York (1951).

732
Chapter 23 Macroscopic Balances for Multicomponent Systems
SOLUTION
We have written this in
a
second form, because we will consider only systems in which there
is only a slight enrichment of species
A,
so that
a
-
1
is a very small quantity. When Eq. 23.1-
23 is solved for
y
as a function of x we then get
Next we define the Dirac separative capacity
A
of the system as the net increase in "value"
(this could, for example, be the monetary value) of the streams that are participating in the
system:
in which v(x) is the Dirac value function. (In the separation science literature, the separative ca-
pacity is often given the symbol
6U.)
Show how the separative capacity and value function can be obtained by using the defin-
ition in Eq. 23.1-25 along with the mass balances for the system.
The total mass balance and the mass balance for species
A
are:
We now divide Eq. 23.1-27 by
F,
and then use Eq. 23.1-26 to eliminate
W.
Then introducing
the quantity
8
=
P/F
(called the "cuV), we get
Next we divide Eq. 23.1-25 by
F
and introduce
8
to get
Inasmuch as the differences between the concentrations of the streams are quite small, we can
expand v(y) and v(x) about
z
and get
where the primes indicate differentiation with respect to
z.
When these expressions are put
into Eq. 23.1-29 and we use Eq. 23.1-28, we get
When we use
Eq.
23.1-24, this last equation becomes
We now assume that the separative capacity of the system is virtually independent of concen-
tration. Therefore we set the concentration-dependent factor in Eq. 23.1-33 equal to unity, so
that
is the final expression for the separative capacity. According to this expression, the separative
capacity has
a
maximum when the system is operated at
0
=
f.
It remains to obtain the Dirac value function, which must satisfy the differential equation
g23.1 The Macroscopic Mass Balances
733
When this equation is integrated, we get
The two integration constants may be assigned arbitrarily, and several different choices have
been used. However, the most common choice is v($)
=
0
and v1(;)
=
0.
This leads to
which is the symmetrical solution, in the sense that v(l
-
z)
=
v(z) and vl(l
-
z)
=
-vl(z).
The value function v(z) and the separative capacity
A
have proven useful in comparing
separations made in different kinds of equipment as well as different concentration ranges.
From an economic standpoint v(z) as given by Eq. 23.1-37 has been found useful for determin-
ing price differences for isotope mixtures of differing purity.
EXAMPLE
23.1-4
Compartmental
One of the simplest and most useful applications of the species macroscopic mass balance is
compartmental analysis, in which a complex system is treated as a network of perfect mixers,
each of constant volume, connected by ducts of negligible volume, with no dispersion occur-
Analysis
ring in the connecting ducts. Imagine mixing units, labeled
1,
2,3,
.
.
.
,
n,
. . .
,
N,
containing
various species (labeled with indices
a,
P,
y,
.
.
.).
Then the mass concentration
p,,
of species
a
in unit
n
changes with time according to the equation
Here
V,
is the volume of unit n,
Q,,
is the volumetric flow rate of solvent flow from unit
m
to
unit
n,
and r,, is the rate of formation of species
a
per unit volume in unit n.
Show how such a model can be specialized to describe the removal of toxic metabolic
products (that is, the toxic materials resulting from the human metabolism) from a patient by
hemodialysis. Hemodialysis is the periodic removal of toxic metabolites achieved by contacting
the blood and a dialysis fluid in countercurrent flow, separated by a cellophane membrane
that
is
permeable to the metabolite.
SOLUTION
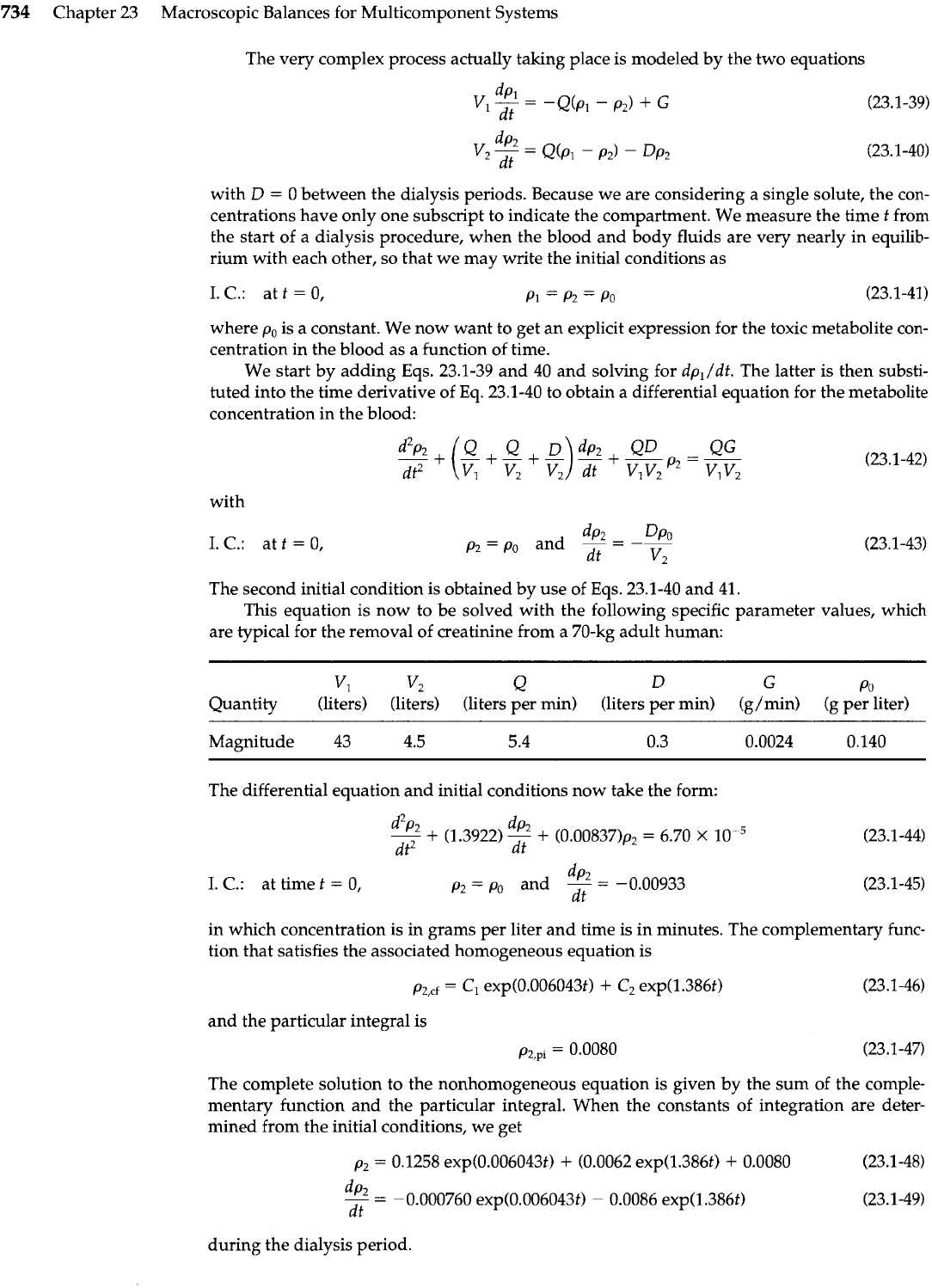
The simple two-compartment model of Fig. 23.1-4 has been found to be adequate for repre-
senting the hemodialysis system. Here the large block, or compartment
1
(labeled "body") rep-
resents the combined body fluids, except for those in the blood, which are represented by
compartment
2.
The blood circulates via a branching system of vessels through compartment
1
at a volumetric rate
Q,
and in the process extracts solute across the vessel walls. This process is
highly efficient, and a single solute is assumed to leave compartment
1
at concentration
p,,
equal to the concentration throughout that compartment. At the same time, the solute is being
formed within the body fluids at a constant rate
GI
and during dialysis it is being extracted
from the blood by the dialyzer at a rate
Dp,.
The proportionality constant
D
is known as the
"dialyzer clearance" and is fixed by the dialyzer design and operating conditions.
Fig.
23.1-4.
Two-compartment model
used to analyze the functioning of a
dialyzer.
734
Chapter 23 Macroscopic Balances for Multicomponent Systems
The very complex process actually taking place is modeled by the two equations
with
D
=
0 between the dialysis periods. Because we are considering a single solute, the con-
centrations have only one subscript to indicate the compartment. We measure the time
t
from
the start of a dialysis procedure, when the blood and body fluids are very nearly in equilib-
rium with each other, so that we may write the initial conditions as
1.
C.: at
t
=
0,
PI
=
P2
=
Po
(23.1-41)
where
p,
is a constant. We now want to get an explicit expression for the toxic metabolite con-
centration in the blood as a function of time.
We start by adding Eqs. 23.1-39 and 40 and solving for
dp,/dt.
The latter is then substi-
tuted into the time derivative of Eq. 23.1-40 to obtain a differential equation for the metabolite
concentration in the blood:
with
I.
C.:
at
t
=
0,
The second initial condition is obtained by use of Eqs. 23.1-40 and 41.
This equation is now to be solved with the following specific parameter values, which
are typical for the removal of creatinine from
a
70-kg adult human:
v1
v2
Q
D
G
Po
Quantity (liters) (liters) (liters per min) (liters per min) (g/min)
(g
per liter)
Magnitude 43 4.5 5.4 0.3 0.0024 0.140
The differential equation and initial conditions now take the form:
I.
C.:
at time
t
=
0,
P2
=
Po
and
dp2
=
-0.00933 (23.1-45)
in which concentration is in grams per liter and time is in minutes. The complementary func-
tion that satisfies the associated homogeneous equation is
P,,~,
=
C,
exp(0.006043t)
+
C2
exp(1.386t) (23.1-46)
and the particular integral is
p2,pi
=
0.0080
The complete solution to the nonhomogeneous equation
is
given by the sum of the comple-
mentary function and the particular integral. When the constants of integration are deter-
mined from the initial conditions, we get
during the dialysis period.
s23.1 The Macroscopic Mass Balances
735
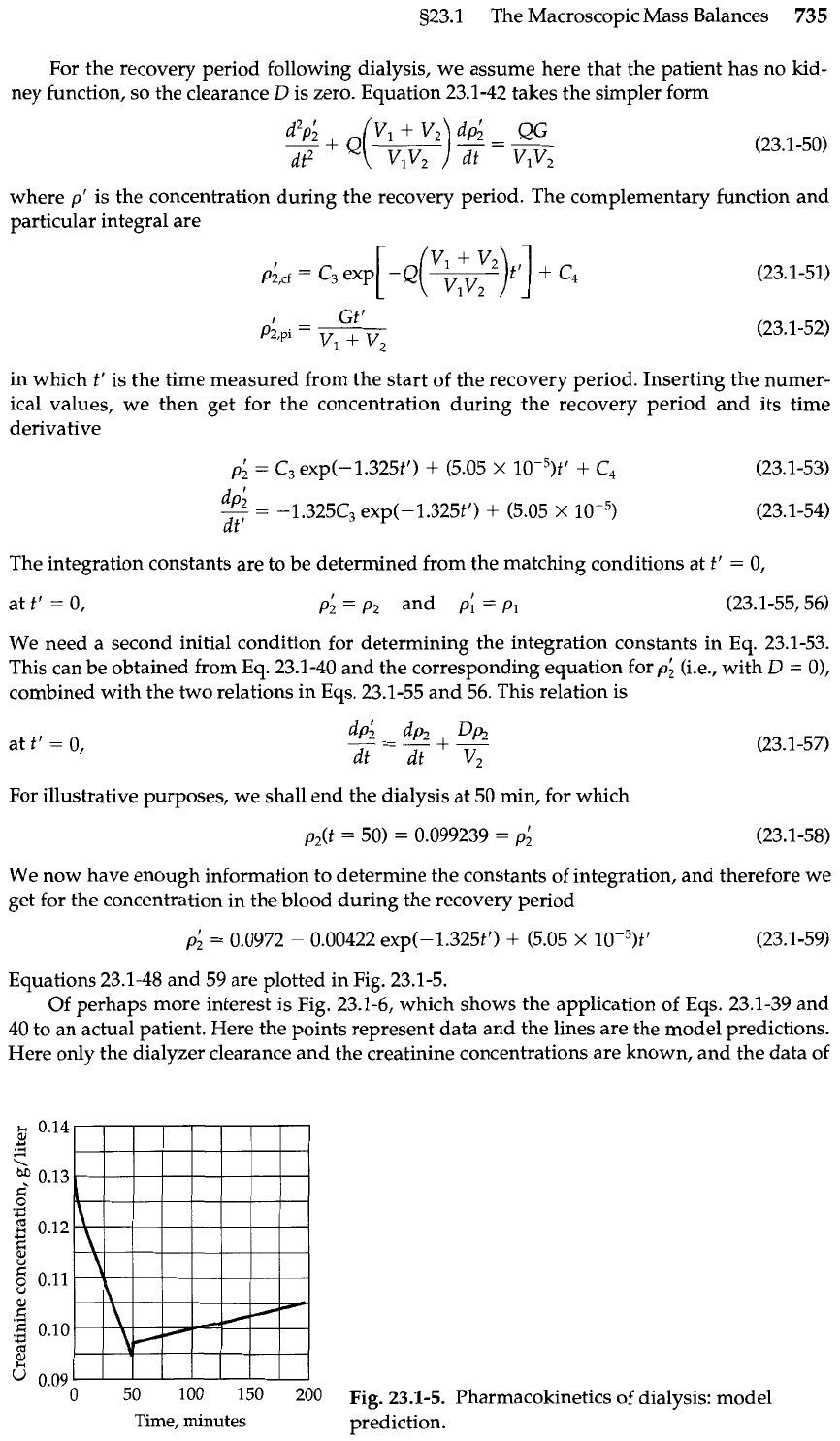
For the recovery period following dialysis, we assume here that the patient has no
kid-
ney function, so the clearance
D
is zero. Equation 23.1-42 takes the simpler form
where
p'
is the concentration during the recovery period. The complementary function and
particular integral are
I
Gt'
=
v1
+
v2
in which
t'
is the time measured from the start of the recovery period. Inserting the numer-
ical values, we then get for the concentration during the recovery period and its time
derivative
The integration constants are to be determined from the matching conditions at
t'
=
0,
We need a second initial condition for determining the integration constants in Eq. 23.1-53.
This can be obtained from Eq. 23.1-40 and the corresponding equation for
p;
(i.e., with
D
=
O),
combined with the two relations in Eqs. 23.1-55 and 56. This relation is
For illustrative purposes, we shall end the dialysis at 50 min, for which
We now have enough information to determine the constants of integration, and therefore we
get for the concentration in the blood during the recovery period
Equations 23.1-48 and 59 are plotted in Fig. 23.1-5.
Of
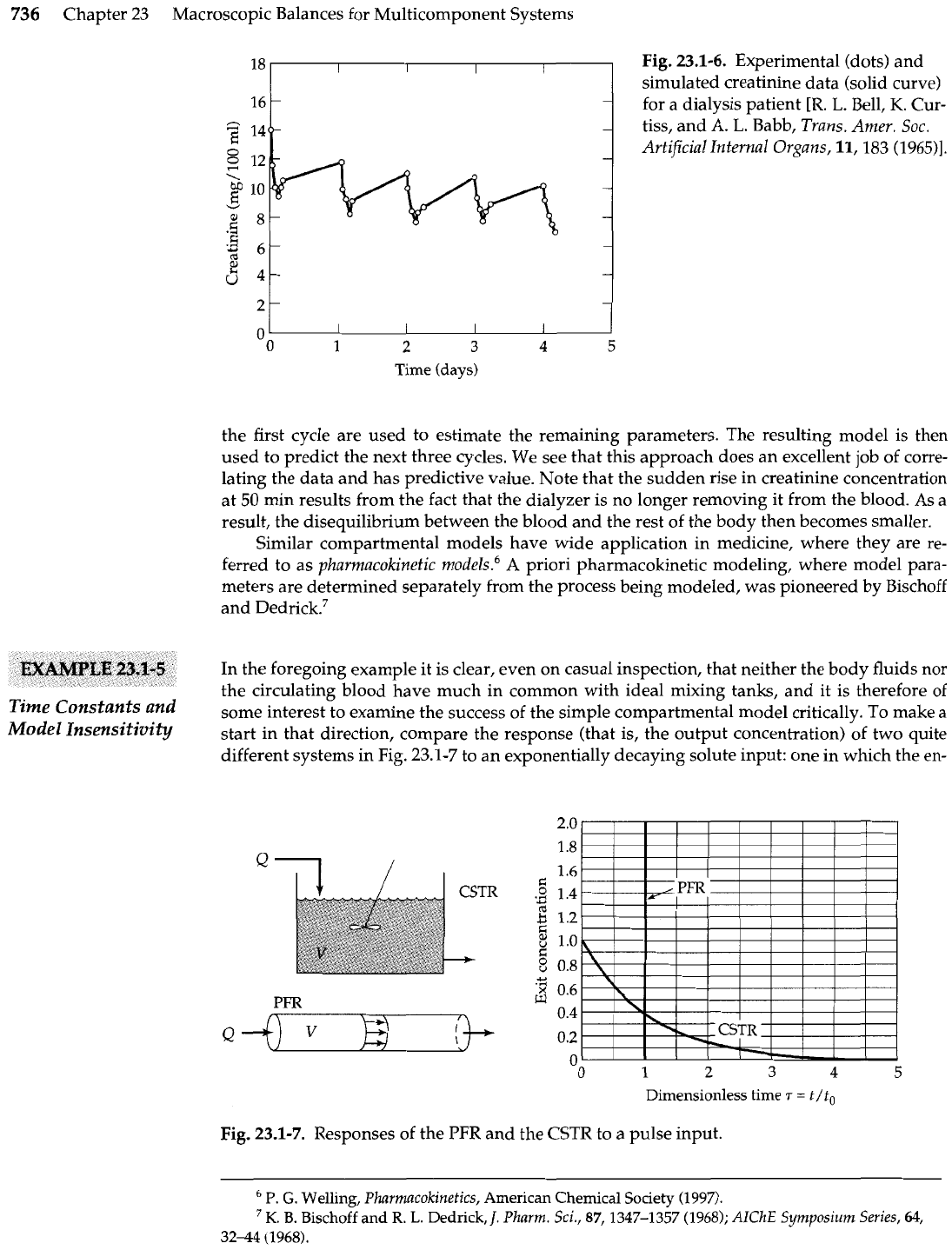
perhaps more interest is Fig. 23.1-6, which shows the application of Eqs. 23.1-39 and
40 to an actual patient. Here the points represent data and the lines are the model predictions.
Here only the dialyzer clearance and the creatinine concentrations are known, and the data of
0.14
Y
.3
4
\
0.13
5
.r(
+
E
0.12
Y
G
8
g
0.11
2
:g
0.10
Y
a
2
0.09
0 50 100
200
Fig.
23.1-5.
Pharmacokinetics of dialysis: model
Time,
minutes
prediction.
736
Chapter 23 Macroscopic Balances for Multicomponent Systems
18
Fig.
23.1-6. Experimental (dots) and
simulated creatinine data (solid curve)
16
for a dialysis patient
[R.
L.
Bell,
K.
Cur-
2
14
tiss, and
A.
L.
Babb,
Trans. Amer. Soc.
o
Artificial Internal Organs,
11, 183
(1965)l.
12
\
g
10
V
28
.*
F;
.a
6
rd
5
4
I
I
I
I
1
2
3
4
5
Time (days)
the first cycle are used to estimate the remaining parameters. The resulting model is then
used to predict the next three cycles. We see that this approach does an excellent job of corre-
lating the data and has predictive value. Note that the sudden rise in creatinine concentration
at
50
min results from the fact that the dialyzer is no longer removing it from the blood. As
a
result, the disequilibrium between the blood and the rest of the body then becomes smaller.
Similar compartmental models have wide application in medicine, where they are re-
ferred to as
pharmacokinetic model~.~
A
priori pharmacokinetic modeling, where model para-
meters are determined separately from the process being modeled, was pioneered by Bischoff
and Dedri~k.~
In
the foregoing example it is clear, even on casual inspection, that neither the body fluids nor
the circulating blood have much in common with ideal mixing tanks, and it is therefore of
Time
and
some interest to examine the success of the simple compartmental model critically. To make
a
Ahdel Insensitivity
start in that direction, compare the response (that is, the output concentration) of two quite
different systems in Fig. 23.1-7 to an exponentially decaying solute input: one in which the en-
PFR
Dimensionless time
r
=
t/tO
Fig.
23.1-7. Responses of the PFR and the CSTR to a pulse input.
P.
G.
Welling,
Pharmacokinetics,
American Chemical Society (1997).
K.
B.
Bischoff
and
R.
L.
Dedrick,
J.
Pkarm. Sci.,
87,
1347-1357 (1968);
AIChE
Symposium Series,
64,
32-44 (1968).
