Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

523.5
Use of the Macroscopic Balances to Solve Steady-State Problems
747
The phase compositions in each stage are assumed to satisfy an equilibrium relation such
as (see Eq. 23.1-19)
or, more generally,
y,,
=
f
(xJ,
where
f
(x)
is taken to be a known function.
Equations 23.5-32 and 33 (or its generalization) now permit determination of all composi-
tions in the portion of the column above the feed point, usually known as the rectifying section,
and similar calculations can be made for the stripping section, the portion below the feed point.
We may then determine the number
of
stages required for the separation under consideration
and the proper location of the feed stage.
First, however, we need to determine the stream rate ratios required in Eq. 23.5-32, and
we consider three special cases here:
(a)
Total
reflux.
This special mode of operation, in which
P
and Ware zero, is important, as
it provides the smallest possible number of stages that can yield the desired output composi-
tions. Here
Un
=
Dn-l
(23.5-34)
for all n, and the operating line is given by
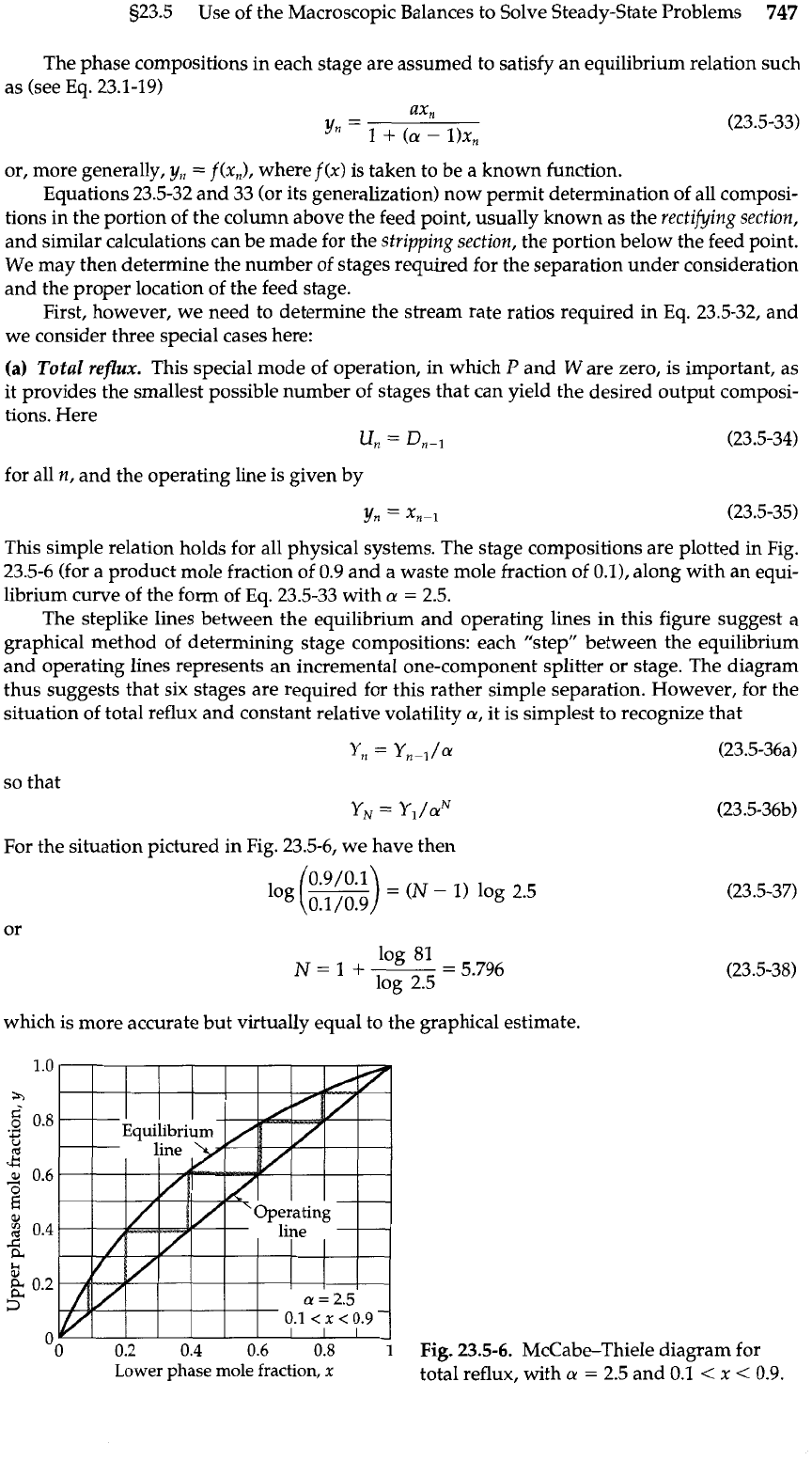
This simple relation holds for all physical systems. The stage compositions are plotted in Fig.
23.5-6 (for a product mole fraction of 0.9 and a waste mole fraction of 0.0, along with an equi-
librium curve of the form of Eq. 23.5-33 with
a
=
2.5.
The steplike lines between the equilibrium and operating lines in this figure suggest a
graphical method of determining stage compositions: each "step" between the equilibrium
and operating lines represents an incremental one-component splitter or stage. The diagram
thus suggests that six stages are required for this rather simple separation. However, for the
situation of total reflux and constant relative volatility
a,
it is simplest to recognize that
For the situation pictured in Fig. 23.5-6, we have then
0'9/0'1
=
(N
-
1)
log 2.5
log
(m)
log 81
N
=
1
+
-
=
5.796
log 2.5
which is more accurate but virtually equal to the graphical estimate.
0.2
0.4
0.6
0.8
1
Lower phase mole
fraction,
x
Fig.
23.5-6.
McCabe-Thiele diagram for
total reflux, with
a
=
2.5 and
0.1
<
x
<
0.9.
748
Chapter 23 Macroscopic Balances for Multicomponent Systems
If products are to be withdrawn, it is necessary to calculate the stream-rate ratios, and the
means for doing so vary with the specific operation considered.
(b)
Thermodynamic constraints: adiabatic cascades and minimum reflux.
For most of the
common stagewise operations, stream ratios are determined by thermodynamic constraints,
and these are thoroughly discussed in a wide variety of unit operations texts. We need not re-
peat this readily accessible information here, but we briefly consider distillation, the most
widely used of all, by way of example. In principle, stream ratios in distillation are deter-
mined by assuming adiabatic columns and a set of "enthalpy balances" (see last paragraph of
s15.1) corresponding to the mass balances just introduced.
However, it is very often permissible to assume equal molar heats of vaporizatio_n for the
various species and to neglect "sensible heats" (i.e., the
CpAT
contributions to
AH).
With
these simplifications the stream rates
U,
and
D,
are constants. We may then write for any po-
sition above the feed plate
U= D
+
P
and
y,_lU=x,D +ypP
(23.5-39/40)
and below the feed plate
D
=
U+
W
and
x,D
=
y,, ,U
+
x,W
(23.5-41/42)
Here the stage indices
n
and
rn
refer respectively to the upper or rectifying section (above the
feed point) and to the lower or stripping section of the column (below the feed point).
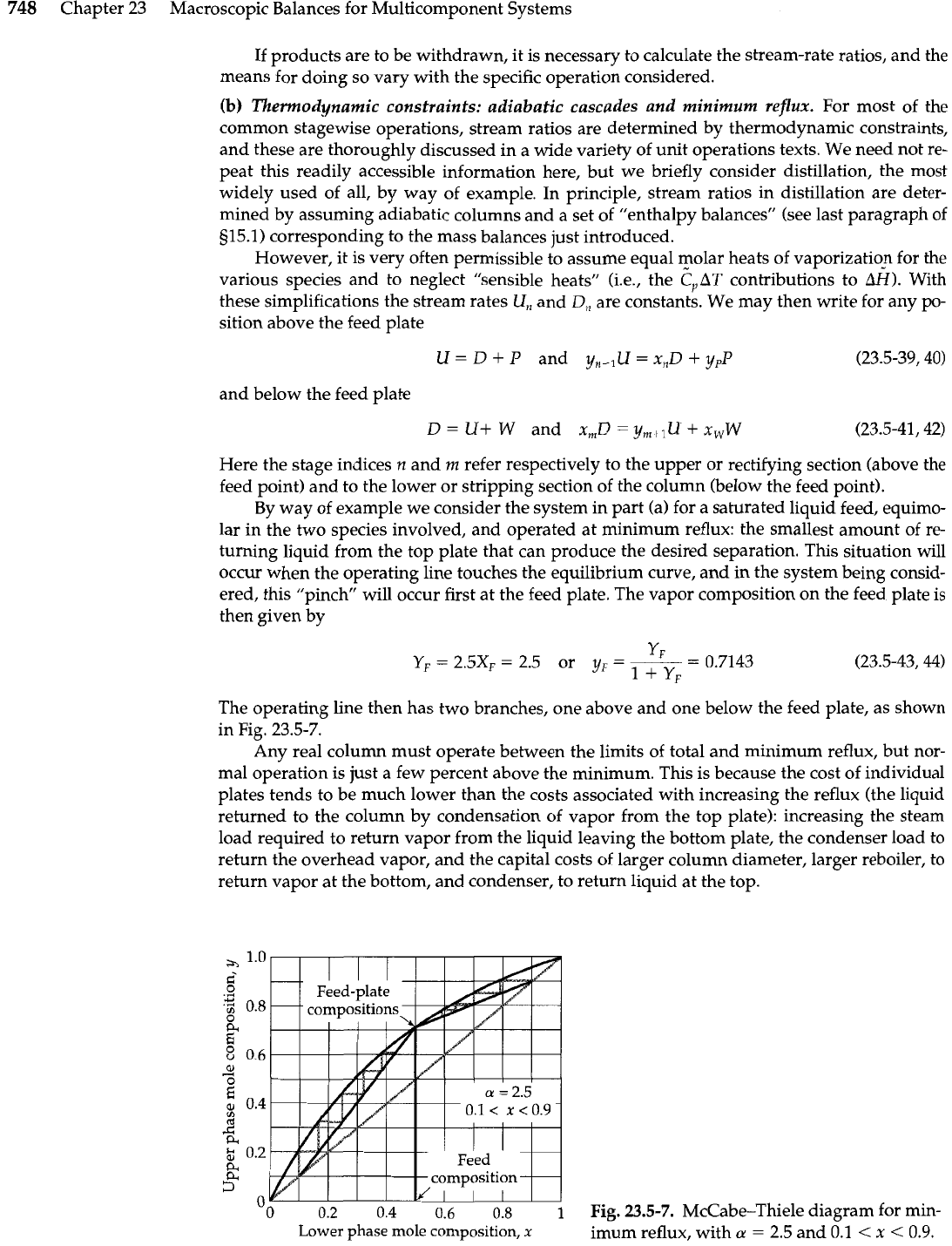
By way of example we consider the system in part (a) for a saturated liquid feed, equimo-
lar in the two species involved, and operated at minimum reflux: the smallest amount of re-
turning liquid from the top plate that can produce the desired separation. This situation will
occur when the operating line touches the equilibrium curve, and in the system being consid-
ered, this "pinch will occur first at the feed plate. The vapor composition on the feed plate is
then given by
The operating line then has two branches, one above and one below the feed plate, as shown
in Fig. 23.5-7.
Any real column must operate between the limits of total and minimum reflux, but nor-
mal operation is just a few percent above the minimum. This is because the cost of individual
plates tends to be much lower than the costs associated with increasing the reflux (the liquid
returned to the column by condensation of vapor from the top plate): increasing the steam
load required to return vapor from the liquid leaving the bottom plate, the condenser load to
return the overhead vapor, and the capital costs of larger column diameter, larger reboiler, to
return vapor at the bottom, and condenser, to return liquid at the top.
"0
0.2
0.4
0.6
0.8
1
Lower phase mole composition,
x
Fig.
23.5-7.
McCabe-Thiele diagram for min-
imum reflux, with
a
=
2.5 and 0.1
<
x
<
0.9.
g23.5
Use of the Macroscopic Balances to Solve Steady-State Problems
749
0.2 0.4 0.6 0.8
1
Mole fraction
of
lower phase,
x
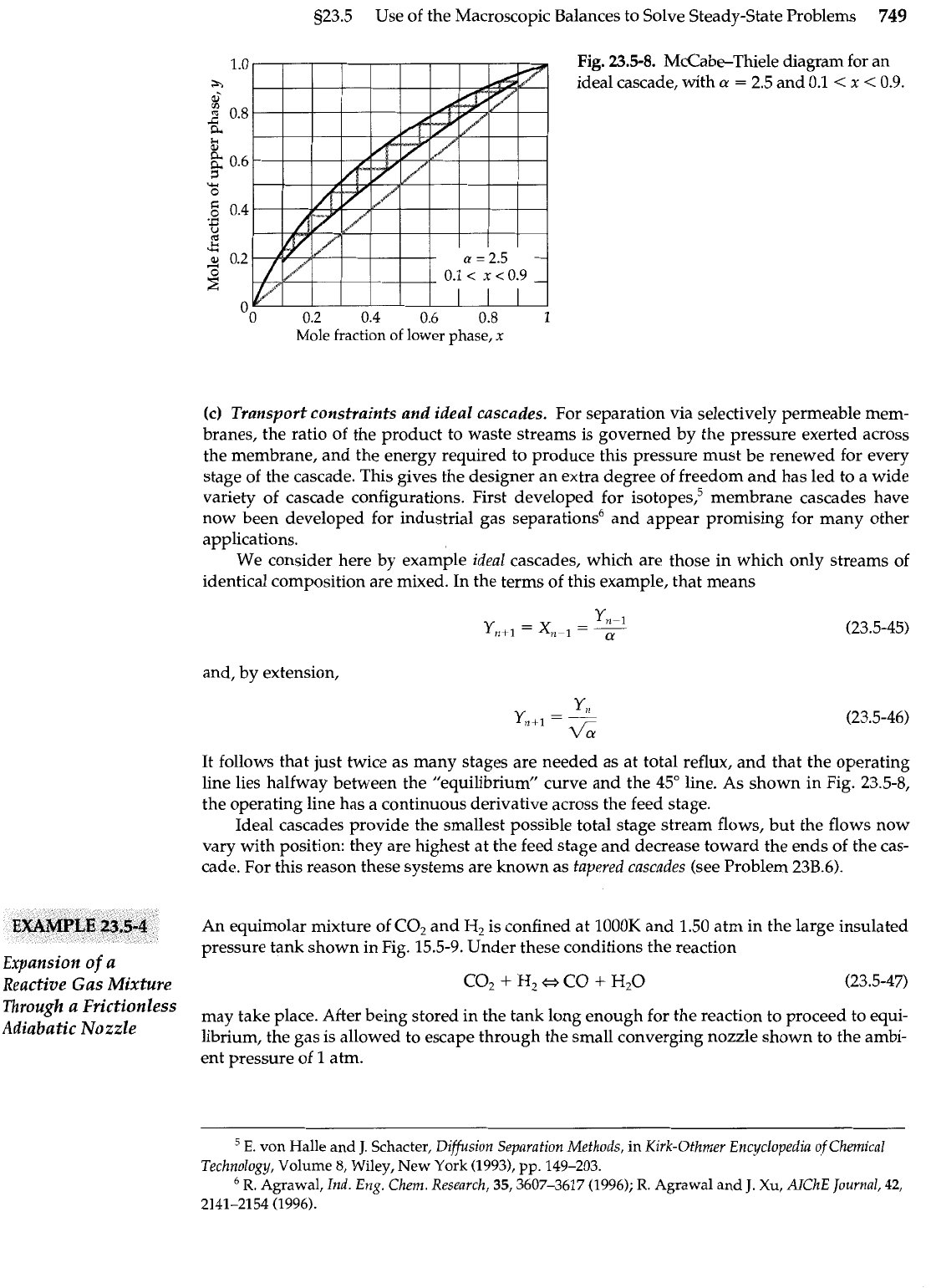
Fig.
23.5-8.
McCabe-Thiele diagram for an
ideal cascade, with
a
=
2.5 and 0.1
<
x
<
0.9.
(c)
Transport constraints and ideal cascades.
For separation via selectively permeable mem-
branes, the ratio of the product to waste streams is governed by the pressure exerted across
the membrane, and the energy required to produce this pressure must be renewed for every
stage of the cascade. This gives the designer an extra degree of freedom and has led to a wide
variety of cascade configurations. First developed for isotopes; membrane cascades have
now been developed for industrial gas separations6 and appear promising for many other
applications.
We consider here by example
ideal
cascades, which are those in which only streams of
identical composition are mixed. In the terms of this example, that means
and, by extension,
It follows that just twice as many stages are needed as at total reflux, and that the operating
line lies halfway between the "equilibrium" curve and the
45"
line. As shown in Fig. 23.5-8,
the operating line has a continuous derivative across the feed stage.
Ideal cascades provide the smallest possible total stage stream flows, but the flows now
vary with position: they are highest at the feed stage and decrease toward the ends of the cas-
cade. For this reason these systems are known as
tapered cascades
(see Problem 23B.6).
An equimolar mixture of CO, and
Hz
is confined at lOOOK and 1.50 atm in the large insulated
pressure tank shown in Fig. 15.5-9. Under these conditions the reaction
Expansion
of
a
Reactive Gas Mixture
C02
+
H,
&
CO
+
H20
(23.5-47)
ntrough
a
may take place. After being stored in the tank long enough for the reaction to proceed to equi-
Adiabatic Nozzle
librium, the gas is allowed to escape through the small converging nozzle shown to the ambi-
ent pressure of 1 atm.
E.
von Halle
and
J.
Schacter,
Diffirsion Separation Methods,
in
Kirk-Othmer Encyclopedia of Chemical
Technology,
Volume
8,
Wiley,
New
York
(1993),
pp.
149-203.
R.
Agrawal,
Ind.
Eng.
Ckem. Research,
35,3607-3617 (1996);
R.
Agrawal and
J.
Xu,
AlChE
Journal,
42,
2141-2154 (1996).

750
Chapter 23 Macroscopic Balances for Multicomponent Systems
SOLUTION
Estimate the temperature and velocity of the escaping gas through the nozzle throat
(a)
assuming that no appreciable reaction takes place during passage of gas through the nozzle,
and
(b)
assuming instant attainment of thermodynamic equilibrium at all points in the nozzle.
In each case, assume that the expansion is adiabatic and frictionless.
We begin by assuming quasi-steady-state operation, flat velocity profiles, and negligible
changes in potential energy. We also assume constant heat capacities and ideal gas behavior,
and we neglect diffusion in the direction of flow. We may then write the macroscopic energy
balance, Eq. 23.3-1, in the form
Here the subscripts
1
and 2 refer to conditions in the tank and at the nozzle throat, respec-
tively, and, as in Example 15.5-4, the fluid velocity in the tank is assumed to be zero.
To determine the enthalpy change, we equate d(iv2) from the d-form of the steady-state
energy balance (Eq. 23.3-1) to d($v2) of the d-form of the steady-state mechanical energy bal-
ance (Eq. 23.4-1) to get
This result also follows from Eq:
E
of Table 19.2-4. In addition to Eq. 23.5-49, we use the ideal
gas law and %n expression for
H(T),
obtained with the help of Table 17.1-1, Eq. 19.3-16, and
the relation
pH
=
ch,
to get
Here
x,
is the mole fraction of the species
a
at temperature T,and
&
is the molar enthalpy
of
species
a
at the reference temperature
To.
The evaluation of His discussed separately for the
two approximations.
Approximation (a):Assumption of
very
slow chemical reaction.
Here the
x,
are constant
at the equilibrium values for 1000K, and we may write Eq. 23.5-51 as
Hence we may write Eq. 23.5-49 in the form
-
Since
x,
and
C,,
are assumed constant, this equation may
to get
T (23.5-53)
be integrated from (p,, T,) to (p,,
TJ
We may now combine this expression with Eqs. 23.5-48 and 51 to obtain the desired expres-
sion for the gas velocity at plane 2:
523.5
Use of the Macroscopic Balances to Solve Steady-State Problems
751
By substituting numerical values into Eqs. 23.5-54 and 55, we obtain (see Problem 23A.1) T2
=
920K and
v,
=
1726 ft/s. It may be seen that this treatment is very similar to that presented in
Example 15.5-4. It is also subject to the restriction that the throat velocity must be subsonic;
that is, the pressure in the nozzle throat cannot fall below that fraction of
p,
required to pro-
duce sonic velocity at the throat (see Eq. 15B.6-2).
If
the ambient pressure falls below this criti-
cal value of p,, the throat pressure will remain at the critical value, and there will be a shock
wave beyond the nozzle exit.
Approximation
0:
Assumption of ve
y
rapid reaction. We may proceed here as in part
(a), except that the mole fractions x, must now be considered functions of the temperature de-
fined by the equilibrium relation
and the stoichiometric relations
The quantity
KJT)
in Eq. 23.5-56 is the known equilibrium constant for the reaction. It may be
considered as a function only of temperature, because of the assumed ideal gas behavior and
because the number of moles present is not affected by the chemical reaction. Equations 23.5-
57 and 58 follow from the stoichiometry of the reaction and the composition of the gas origi-
nally placed
in
the tank.
The expression for the final temperature is now considerably more complicated. For this
reaction, where C.,x,M, is constant, Eqs. 23.5-49 and 50 may be combined to give
where, with the heat capacities approximated as constar~ts,
In general, the integral in Eq. 23.5-60 must be evaluated numerically, since the x, and the
dx,/dT are all complicated functions of temperature governed by Eqs. 23.5-56 to 59. Once T2
has been determined from Eq. 23.5-61, however,
v,
may be obtained by use of Eqs. 23.548
and 51. By substituting numerical values into these expressions, we obtain (see Problem
23B.2) T2
=
937K and
v2
=
1752 ft /s.
We find, then, that both the exit temperature and the velocity from the nozzle are greater
when chemical equilibrium is maintained throughout the expansion. The reason for this is
that the equilibrium shifts with decreasing temperature in such a way as to release heat of re-
action to the system. Such a release of energy will occur with decreasing temperature in any
system at chemical equilibrium, regardless of the reactions involved. This is one consequence
of the famous rule of Le Chatelier. In this case, the reaction is endothermic as written and the
equilibrium constant decreases with falling temperature. As a result,
CO
and
H,O
are par-
tially reconverted to
H,
and C02 on expansion, with a corresponding release of energy.
It is interesting that in rocket engines the exhaust velocity, hence the engine thrust, are
also increased if rapid equilibration can be obtained, even though the combustion reactions
are strongly exothermic. The reason for this is that the equilibrium constants for these reac-
tions increase with falling temperature so that the heat of reaction is again released on expan-
sion. This principle has been suggested as a method for improving the thrust of rocket
engines. The increase in thrust potentially obtainable in this way is quite large.
This example was chosen for its simplicity. Note in particular that if a change in the num-
ber of moles accompanies the chemical reaction, then the equilibrium constant, and hence the
enthalpy, are functions of the pressure. In this case, which is quite common, the variables
p
752
Chapter 23 Macroscopic Balances for Multicomponent Systems
and
T
implicit in Eq. 23.5-60 cannot be separated, and a step-by-step integration of this equa-
tion is required. Such integrations have been performed, for example, for the prediction of the
behavior of supersonic wind tunnels and rocket engines, but the calculations involved are too
lengthy for here.
523.6
USE OF
THE
MACROSCOPIC BALANCES TO
SOLVE UNSTEADY-STATE PROBLEMS
In
523.5
the discussion was restricted to steady state. Here we move on to the transient
behavior of multicomponent systems. Such behavior is important in a large number of
practical operations, such as leaching and drying of solids, chromatographic separations,
and chemical reactor operations. In many of these processes heats of reaction as well as
mass transfer must be considered.
A
complete discussion of these topics is outside the
scope of this text, and we restrict ourselves to several simple examples. More extensive
discussions may be found elsewhere.'
EXAMPLE
23.6-1
Start-up
of
a Chemical
Reactor
SOLUTION
It is desired to produce
a
substance
B
from a raw material
A
in a chemical reactor of volume
V
equipped with a stirrer that is capable of keeping the entire contents of the reactor fairly
ho-
mogeneous. The formation of
B
is reversible, and the forward and reverse reactions may be
considered first order, with reaction-rate constants
kyB
and k;;, respectively. In addition,
B
un-
dergoes an irreversible first-order decomposition, with a reaction-rate constant kqc, to
a
third
component
C.
The chemical reactions of interest may be represented as
At zero time, a solution of
A
at a concentration
cA,
is introduced to the initially empty reactor
at a constant mass flow rate
w.
Develop an expression for the amount of
B
in the reactor, when it is just filled to its ca-
pacity
V,
assuming that there is no
B
in the feed solution and neglecting changes of fluid
properties.
We begin by writing the unsteady-state macroscopic mass balances for species
A
and
B.
In
molar units these may be expressed
as
Next we eliminate
MA,tot
from Eq. 23.6-3. First we differentiate this equation with respect to
t
to get
In this equation, we replace
dMA,tOt/dt
by the right side of
Eq.
23.6-2, and then use Eq. 23.6-3
to eliminate
MArtot.
In this way we obtain a linear second-order differential equation for
M,,,,
as a function of time:
'
W.
R.
Marshall, Jr., and
R.
L.
Pigford,
The Application of Differential Equations to Chemical Engineering
Problems,
University
of
Delaware Press, Newark, Del.
(1947);
B.
A.
Ogunnaike and
W.
H.
Ray,
Process
Dynamics, Modeling, and Control,
Oxford University Press
(1994).
s23.6 Use of the Macroscopic Balances to Solve Unsteady-State Problems
753
This equation is to be solved with the initial conditions
This equation can be integrated to give
where
111
111
2.+
=
-(k'l:,
+
kli,
+
kyc)
2
d(
kyA
+
kk',i,
+
ky;l2
-
4
~IB~IC
(23.6-9)
-
Equations 23.6-8 and
9
give the total mass of
B
in the reactor as a function of time, up to the
time at which the reactor is completely filled. These expressions are very similar to the equa-
tions obtained for the damped manometer in Example 7.7-2 and the temperature controller in
Example 15.5-2. It can be shown, however, that
s+
and
s-
are both real and negative, and
therefore
MB,tot
cannot oscillate (see Problem 23B.3).
EXAMPLE
23.62
Unsteady Operation
of
a Packed Column
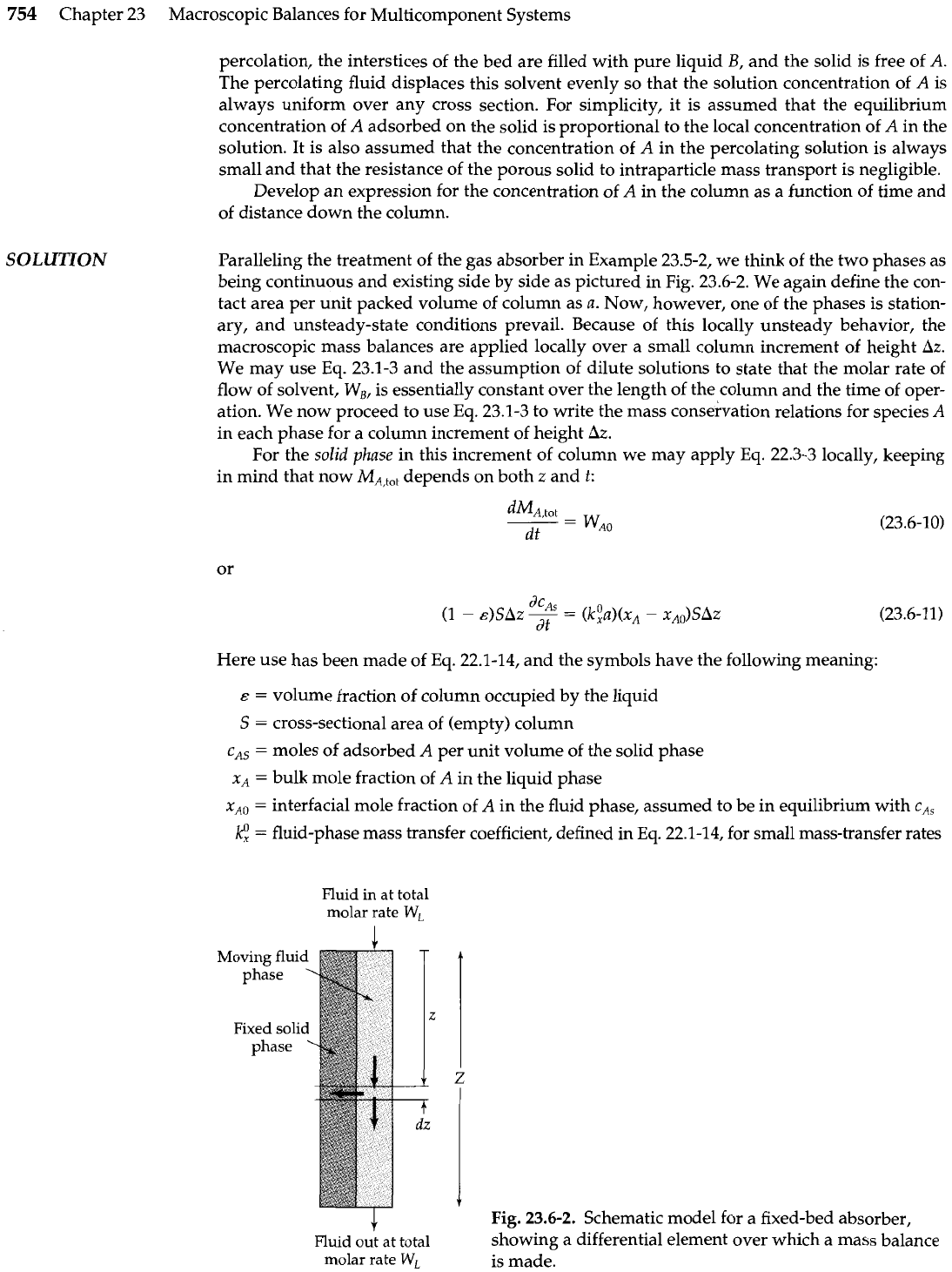
There are many industrially important processes in which mass transfer takes place between
a fluid and a granular porous solid: for example, recovery of organic vapors by adsorption on
charcoal, extraction of caffeine from coffee beans, and separation of aromatic and aliphatic
hydrocarbons by selective adsorption on silica gel. Ordinarily, the solid is held fixed, as indi-
cated in Fig. 23.6-1, and the fluid is allowed to percolate through it. The operation is thus in-
herently unsteady, and the solid must be periodically replaced or "regenerated," that is,
returned to its original condition by heating or other treatment. To illustrate the behavior of
such fixed-bed mass transfer operations, we consider as a physically simple case, the removal
of a solute from a solution by passage through an adsorbent bed.
In this operation, a solution containing a single solute
A
at mole fraction
x,,
in a solvent
B
is passed at a constant volumetric flow rate
w/p
through a packed tower. The tower pack-
ing consists of a granular solid capable of adsorbing
A
from the solution. At the start of the
Fluid phase
in:
Total molar flow rate
=
WL
Solute concentration
=
CAO
A-
T
Fluid phase out:
Total molar
flow
rate
=
WL
Solute concentration
=
cA(Z,
t)
(a)
Fig.
23.6-1.
A
fixed-bed
absorber:
(a)
pictorial
representation of equipment;
(b)
a typical effluent curve.
754
Chapter
23
Macroscopic Balances for Multicomponent Systems
SOLUTION
percolation, the interstices of the bed are filled with pure liquid
B,
and the solid is free of
A.
The percolating fluid displaces this solvent evenly so that the solution concentration of
A
is
always uniform over any cross section. For simplicity, it is assumed that the equilibrium
concentration of
A
adsorbed on the solid is proportional to the local concentration of
A
in the
solution. It is also assumed that the concentration of
A
in the percolating solution is always
small and that the resistance of the porous solid to intraparticle mass transport is negligible.
Develop an expression for the concentration of
A
in the column as a function of time and
of distance down the column.
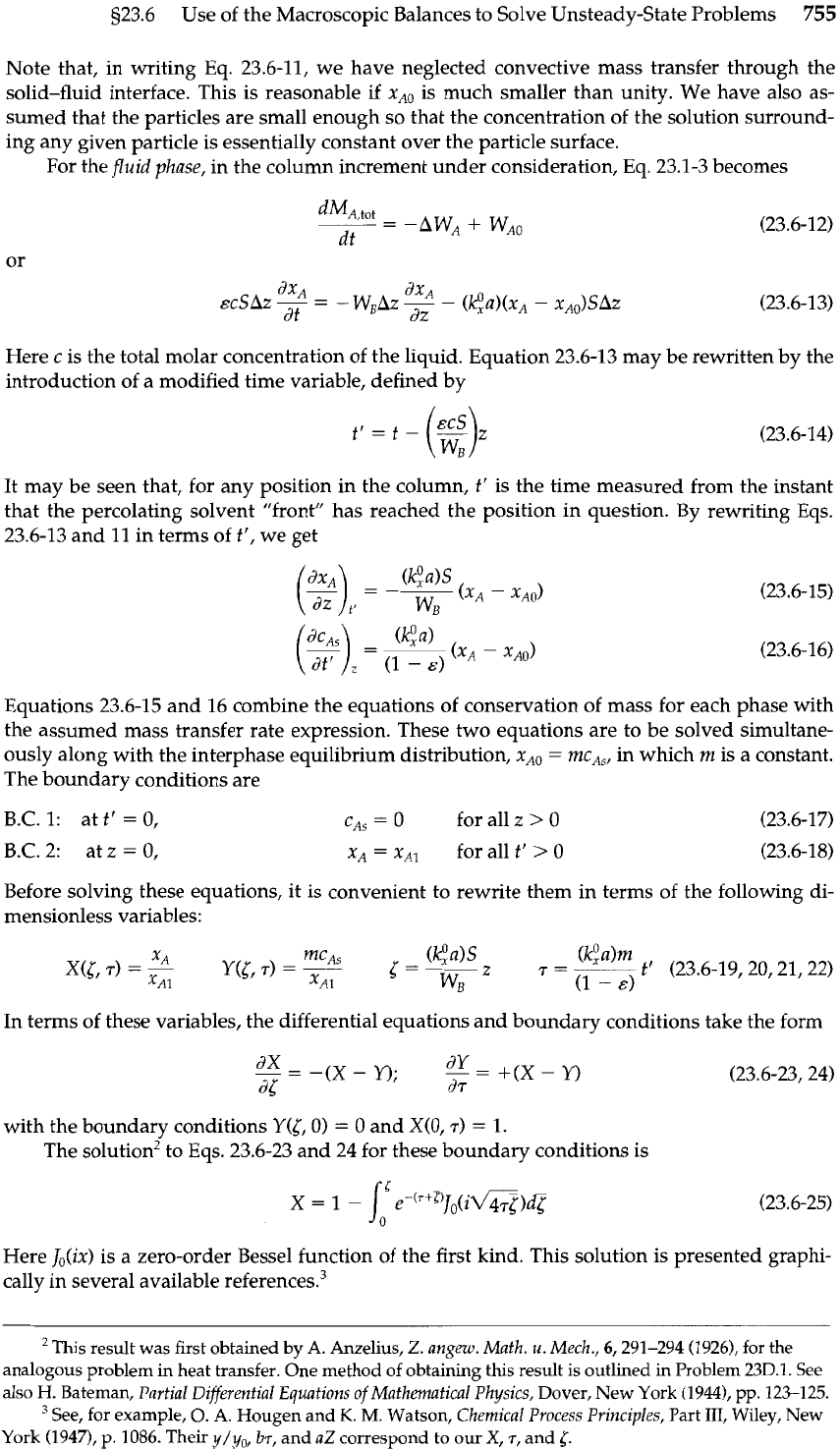
Paralleling the treatment of the gas absorber in Example 23.5-2, we think of the two phases as
being continuous and existing side by side as pictured in Fig. 23.6-2. We again define the con-
tact area per unit packed volume of column as
a.
Now, however, one of the phases is station-
ary, and unsteady-state conditions prevail. Because of this locally unsteady behavior, the
macroscopic mass balances are applied locally over a small column increment of height
Az.
We may use
Eq.
23.1-3 and the assumption of dilute solutions to state that the molar rate of
flow of solvent,
WE,
is essentially constant over the length of the column and the time of oper-
ation. We now proceed to use
Eq.
23.1-3 to write the mass conservation relations for species
A
in each phase for
a
column increment of height
Az.
For the
solid
phase
in this increment of column we may apply
Eq.
22.3-3 locally, keeping
in mind that now
MA,,,,
depends on both
z
and
t:
Here use has been made of
Eq.
22.1-14, and the symbols have the following meaning:
E
=
volume fraction of column occupied by the liquid
S
=
cross-sectional area of (empty) column
c,,
=
moles of adsorbed
A
per unit volume of the solid phase
x,
=
bulk mole fraction of
A
in the liquid phase
xAo
=
interfacial mole fraction of
A
in the fluid phase, assumed to be in equilibrium with
cA,
=
fluid-phase mass transfer coefficient, defined in
Eq.
22.1-14, for small mass-transfer rates
Fluid
in
at
total
molar rate
WL
1
Fig.
23.6-2.
Schematic model for a fixed-bed absorber,
Fluid
ou;
at total
showing a differential element over which a mass balance
molar rate
WL
is made.
g23.6
Use of the Macroscopic Balances to Solve Unsteady-State Problems
755
Note that, in writing Eq. 23.6-11, we have neglected convective mass transfer through the
solid-fluid interface. This is reasonable
if
xAO is much smaller than unity. We have also as-
sumed that the particles are small enough so that the concentration of the solution surround-
ing any given particle is essentially constant over the particle surface.
For the
fluid
phase, in the column increment under consideration, Eq. 23.1-3 becomes
Here c is the total molar concentration of the liquid. Equation 23.6-13 may be rewritten by the
introduction of a modified time variable, defined by
It may be seen that, for any position in the column,
t'
is the time measured from the instant
that the percolating solvent "front" has reached the position in question. By rewriting Eqs.
23.6-13 and
11
in terms of t', we get
Equations 23.6-15 and 16 combine the equations of conservation of mass for each phase with
the assumed mass transfer rate expression. These two equations are to be solved simultane-
ously along with the interphase equilibrium distribution, xAO
=
mcAsl in which
m
is a constant.
The boundary conditions are
B.C.
1:
at
t'
=
0,
B.C. 2: at
z
=
0,
cAs
=
0
for all
z
>
0
xA
=
XA,
for all
t'
>
0
Before solving these equations, it is convenient to rewrite them in terms of the following di-
mensionless variables:
In terms of these variables, the differential equations and boundary conditions take the form
with the boundary conditions Y(f; 0)
=
0
and X(0,
r)
=
1.
The solution2 to Eqs. 23.6-23 and 24 for these boundary conditions is
Here JO(ix) is a zero-order Bessel function of the first kind. This solution is presented graphi-
cally in several available
reference^.^
'
This result was first obtained
by
A. Anzelius,
Z. angew. Math.
u.
Mech.,
6,291-294 (1926),
for the
analogous problem in heat transfer. One method of obtaining this result is outlined in Problem
23D.1.
See
also H. Bateman,
Partial Differential Equations of Mathematical Physics,
Dover, New York
(19441,
pp.
123-125.
See, for example,
0.
A.
Hougen and
K.
M.
Watson,
Chemical Process Principles,
Part
111,
Wiley, New
York
(1947),
p.
1086.
Their
y/yo,
b~,
and
aZ
correspond to
our
X,
7,
and
l.

756
Chapter 23 Macroscopic Balances for Multicomponent Systems
For many complex systems, complete descriptions are either infeasible or unnecessary, and it
is sufficient to obtain only a few basic characteristics. Specifically, we may ask how one may
The
of
Low-
determine the system volume
V
and the volume flow rate
Q
through it from observations of
Order Moments
short tracer pulses of mass
m
introduced at the inlet and then measured at the outlet. Con-
sider for this purpose the macroscopically steady flow through a closed system of arbitrary
geometry, but with a single inlet and outlet, such as that suggested in Fig. 7.0-1, except that
there are no moving surfaces. The flow and diffusional behavior are arbitrary, except that the
tracer distribution must be described by the diffusion equation (Eq. 19.1-7 with Eq. 17.7-3 in-
serted for the mass flux)
in which
p,
is the local tracer concentration and
9Ts
is the pseudobinary diffusivity for the
tracer moving through the solution that fills the system. Turbulent systems may be included
by using time-smoothed quantities and an effective turbulent diffusivity.
In developing the macroscopic balances we shall need to use the condition that there is
no flow or diffusion through the walls of the enclosure
(n
.
v)
=
0 and (n
VpT)
=
0 (23.6-27,281
and that the diffusive flux of the tracer is small compared to the convective flux at the inlet
and outlet to the system
Here
n
is the outwardly directed unit normal vector. We take the inlet tracer concentration to
be zero up to
t
=
0 and also after some finite time
t
=
t,.
In practice the concentration pulse
duration should be quite short.
SOLUTION
The analysis4 is based on the moments
I"')
of the tracer concentration with respect to time, de-
fined by:
We now multiply Eq. 23.6-26 by
t"
and integrate with respect to time over the range of
nonzero exit tracer concentration
When the first term is integrated by parts and we make use of the notation introduced in
Eq.
23.6-30, we get
for all systems that give finite moments. We now have a hierarchy of equations for the
I'"'
in
terms of the lower-order moments, and the structure of these equations is very convenient.
In physical terms, it was first noted by Spalding5 that Eq. 23.6-32 has the same form as the
diffusion equation with chemical reaction, Eq. 19.1-16, but with the concentration replaced
by
I(")
and the reaction term replaced by
nI'"-~".
Hence we can integrate these equations over the
entire volume of the flow system and thereby develop a new set of macroscopic balances.
E.
N.
Lightfoot,
A.
M.
Lenhoff,
and
R.
I.
Rodrigues,
Chem.
Eng.
Sci.,
36,954-956 (1982).
D.
B.
Spalding,
Chem.
Eng.
Sci.,
9,74-77
(1958).
