Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

523.1 The Macroscopic Mass Balances
737
SOLUTION
tering fluid moves through in plug flow (a plug flow reactor, PFR), and another that acts as a
perfect mixer (or continuous stirred tank reactor, CSTR). As shown in Fig. 23.1-7, the responses
to a pulse input are quite different for the PFR and the CSTR. Assume steady flow at a volu-
metric flow rate Q through each system, and further assume that the tracer being followed is
too dilute to affect the flow behavior of the carrier solvent. Assume that no reaction is occurring.
For both systems we assume that the concentration is initially zero throughout and that the
concentration of species
a
in the inlet stream is
pa
=
PO
exp(- t/to)
(23.1 -60)
where p, and t, are constants, specific to the problem.
For the PFR, the exit stream concentration shows only a time delay and decay, and we
may write at once for
X
=
p,/po
X
=
0 for t
<
t,,, (23.1-61)
where t,,,
=
V/Q is the mean solute residence time, a second time constant imposed on the sys-
tem. The result for longer time is
which is of more interest to us here.
For the CSTR we begin with the basic differential equation
with the initial condition that
X
=
0
at t
=
0.
This first-order linear differential equation has
the solution
in which
a
=
to/t,,, and
.r
=
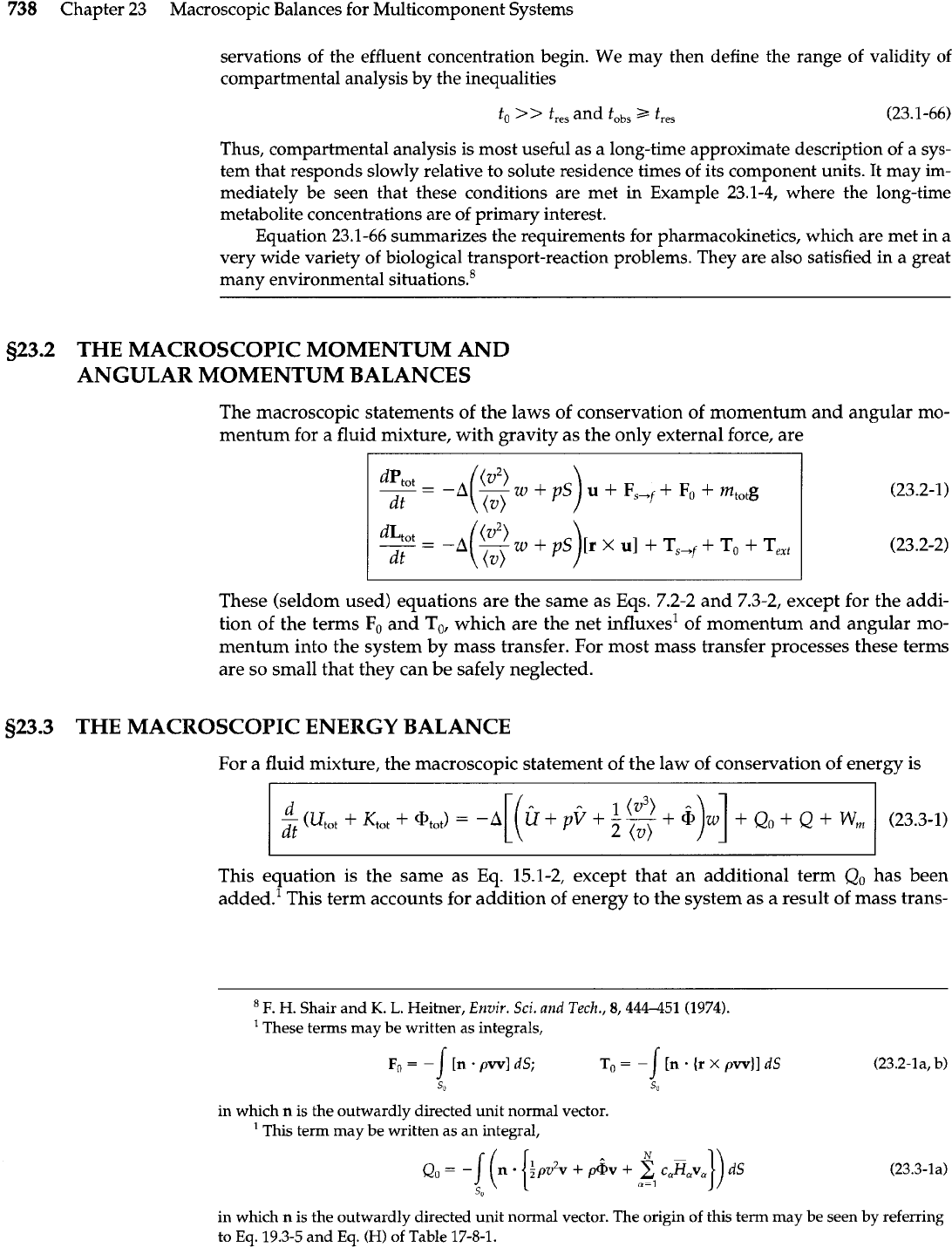
t/to. Exit concentrations are plotted in Fig. 23.1-8 as functions of
the dimensionless time
r
=
t/t, for each reactor and for
l/a
=
t,,,/to of 0.1 and 1.0.
It may be seen that for
l/a
=
t,,,/t,
=
1.0
the two reactors produce much different efflu-
ent concentrations, as one might expect. However, for t,,,/to
<<
1
and t significantly greater
than t,,,, the effluent curves for the two reactors are virtually indistinguishable. This is the re-
gion of validity for compartmental analysis, and we see that in addition to the time constants
imposed by the system itself, there is also another time constant
tabs,
the time at which the ob-
PFR
Fig.
23.1-8.
Response of the
PFR
and the CSTR to an exponentially
decaying input.
738
Chapter
23
Macroscopic Balances for Multicomponent Systems
servations of the effluent concentration begin. We may then define the range of validity of
compartmental analysis by the inequalities
Thus, compartmental analysis is most useful as a long-time approximate description of a sys-
tem that responds slowly relative to solute residence times of its component units. It may im-
mediately be seen that these conditions are met
in
Example 23.1-4, where the long-time
metabolite concentrations are of primary interest.
Equation 23.1-66 summarizes the requirements for pharmacokinetics, which are met in a
very wide variety of biological transport-reaction problems. They are also satisfied in a great
many environmental situations.'
523.2 THE MACROSCOPIC MOMENTUM AND
ANGULAR MOMENTUM BALANCES
The macroscopic statements of the laws of conservation of momentum and angular mo-
mentum for a fluid mixture, with gravity as the only external force, are
These (seldom used) equations are the same as Eqs. 7.2-2 and 7.3-2, except for the addi-
tion of the terms
Fo
and To, which are the net influxes' of momentum and angular mo-
mentum into the system by mass transfer. For most mass transfer processes these terms
are so small that they can be safely neglected.
523.3 THE MACROSCOPIC ENERGY BALANCE
For a fluid mixture, the macroscopic statement of the law of conservation of energy is
I
I
This equation is the same as
Eq.
15.1-2, except that an additional term Qo has been
added.' This term accounts for addition of energy to the system as a result of mass trans-
'
F.
H. Shair and
K.
L.
Heitner,
Envir. Sci. and
Tech.,
8,444-451 (1974).
These terms may be written as integrals,
F,
=
-
[n
.
pw]
dS;
I
To
=
-
[n
.
{r
x
pw]]
dS
I
(23.2-1
a, b)
so
50
in which
n
is the outwardly directed unit normal vector.
This term may be written as an integral,
in which n is the outwardly directed unit normal vector. The origin of this term may be seen by referring
to
Eq.
19.3-5 and
Eq.
(H)
of
Table 17-8-1.
s23.5
Use of the Macroscopic Balances to Solve Steady-State Problems
739
fer. It may be of considerable importance, particularly if material is entering through the
bounding surface at a much higher or lower temperature than that of the fluid inside the
flow system, or if it reacts chemically in the system.
When chemical reactions are occurring, considerable heat may be released or ab-
sorbed. This heat of reaction is automatically taken into account in the calculation of the
enthalpies of the entering and leaving streams (see Example 23.5-1).
In some applications, in which the energy transfer rates across the surface are func-
tions of position, it is more convenient to rewrite Eq. 23.3-1 in the d-form-that is, over a
differential portion of the flow system as described in 915.4. Then the increment of heat
added, dQ, is expressible in terms of a local heat transfer coefficient.
523.4
THE MACROSCOPIC MECHANICAL ENERGY BALANCE
A
careful examination of the derivation of the mechanical energy balance in
g7.8
shows
that the result obtained there applies to mixtures as well as to pure fluids. If we now in-
clude the surface
So,
then we get
I
I
This is the same as Eq. 7.4-2, except for the addition of the term
B,,
which accounts for
the mechanical energy transport across the mass transfer boundary.' The use of this
equation is illustrated in Example 22.5-3.
823.5
USE OF
THE
MACROSCOPIC BALANCES
TO SOLVE STEADY-STATE PROBLEMS
The macroscopic balances are summarized in Table 23.5-1 for systems with more than
one entry and exit plane. The terms with subscript
0
describe the addition or removal
of mass, momentum, angular momentum, energy, and mechanical energy at mass-
transfer surfaces. Usually these balances are not used in their entirety, but it is conve-
nient to have a complete listing of them for problem-solving purposes. For steady-state
problems, the left sides of the equations may be omitted. As we saw in Chapters
7
and
15, considerable intuition is required in using the macroscopic balances, and some-
times it is necessary to supplement the equations with experimental observations.
EXAMPLE
23.5-1
Energy
Balances for a
Sulfur Dioxide
Converter
Hot gases from a sulfur burner enter a converter, in which the sulfur dioxide present is to be
oxidized catalytically to sulfur trioxide, according to the reaction SO2
+
;o,
S
SO3. How
much heat must be removed from the converter per hour to permit a 95% conversion of the
SO, for the conditions shown in Fig. 23.5-I? Assume that the converter is large enough for the
components of the exit gas to be
in
thermodynamic equilibrium with one another. That is, the
partial pressures of the exit gases are related by the equilibrium constraint
In terms
of
a surface integral, this term is given
by

740
Chapter
23
Macroscopic Balances for Multicomponent Systems
Table
23.5-1 Unsteady-State Macroscopic Balances for Nonisothermal
Multicomponent Systems
Mass
of
species
a:
d
-
dt
%,tot
=
Zw.1
-
Zw,,
+
w,,
+
r.tot
=
1.2,3,.
.
.N
Momentum:
Angular momentum:
Mechanical energy:
(Total) energy:
Notes:
(a)
Bw,,
=
w,,,
+
walb
+
wale
+
. .
,
where
w,,,
=
p,,,v,,S,,,
and so on; Equations (A) and
(B)
can be written
in molar units by replacing the lowercase symbols by capital letters and adding to Eq. (A) the term
Z,R,,t,,
to
account for the fact that moles need not be conserved in a chemical reaction.
(b)
h,
and
h,
are elevations above an arbitrary datum plane.
(c)
HI
and
H2
are enthalpies per unit mass (for the mixture) relative to some arbitrarily chosen reference
state; see Example 19.3-1.
(d) All equations are written for compressible flow; for incompressible flow,
E,
=
0. The quantities
E,
and
E,
are defined in Eqs. 7.3-3 and 4.
(e)
u,
and
u2
are unit vectors in the direction of flow.
2
1
T2=?
c----,
I
h
=
1-00 atm
I
-
SO3-rich gas
1
(;==
-
+
Coolant out
.-/'
SO,, 7.80 lb-moles hr-'
02,
10.80 lb-moles hr-I
N2, 81.40 lb-moles
hr-'
f
Converter
Coolant
in
i
TI
=
440°C
pl
=
1.05 atm
Fig.
23.5-1. Catalytic oxidation
of sulfur dioxide.

523.5 Use of the Macroscopic Balances to Solve Steady-State Problems
741
SOLUTION
Approximate values of
K,
for this reaction are
It is convenient to divide this problem into two parts:
(a)
first we use the mass balance and
equilibrium expression to find the desired exit temperature, and then
(b)
we use the energy
balance to determine the required heat removal.
(a)
~etermination' of
T,.
We begin by writing the steady-state macroscopic mass balance,
Eq. 23.1-3, for the various constituents in the two streams in the form:
In addition, we take advantage of the two stoichiometric relations
We can now get the desired molar flow rates through surface 2:
Next, substituting numerical values into the equilibrium expression
Eq.
23.5-1 gives
This value of
K,
corresponds to an exit temperature
T2
of about 510°C, according to the equi-
librium data given above.
(b)
Calculation
of
the required heat removal.
As indicated by the results of Example 15.3-1,
changes in kinetic and potential energy may be neglected here in comparison with changes in
enthalpy. In addition, for the conditions of this example, we may assume ideal gas behavior.
Then, for each constituent,
=
~I,(T).
We may then write the macroscopic energy balance,
Eq. 23.3-1, as
For each of the individual constituents we may write
=
H",
+
-
P)
Here
H",
is the standard enthalpy of formation2 of species
a
from its constituent elements
at the enthalpy reference temperature
To,
and
(C,,),,,
is the enthalpy-mean heat capacityz
of the species between
T
and
To.
For the conditions of this problem, we may use the
'
See
0.
A.
Hougen,
K.
M.
Watson, and
R.
A.
Ragatz,
Chemical
Process
Principles,
Part II,2nd edition,
Wiley, New York (1959),
pp.
1017-1018.
See, for example,
0.
A.
Hougen,
K.
M.
Watson, and
R.
A.
Ragatz,
Chemical Process Principles,
Part
I,
2nd edition, Wiley, New York (19591,
pp.
257,296.
742
Chapter 23 Macroscopic Balances for Multicomponent Systems
EXAMPLE
23.5-2
Height of
a
Packed-
Tower ~bsorber?
SOLUTION
following2 numerical values for these physical properties (the last two columns are ob-
tained from Eq. 23.5-12):
[~al/~:more. Cl
H::
from 25°C to
cal/g-mole
(w,H,),
(
w,H,),
Species at 25°C 440°C 510°C Btu/hr Btu/hr
SO2
-
70,960
11
.05 11.24 -931,900 -44,800
SO3 -94,450
-
15.87 0 1,158,700
02
0 7.45 7.53 60,100 46,600
N2
0 7.12 7.17 433,000 509,500
Totals -438,800
-
647,400
Substitution of the preceding values into Eq. 23.5-11 gives the required rate of heat
removal:
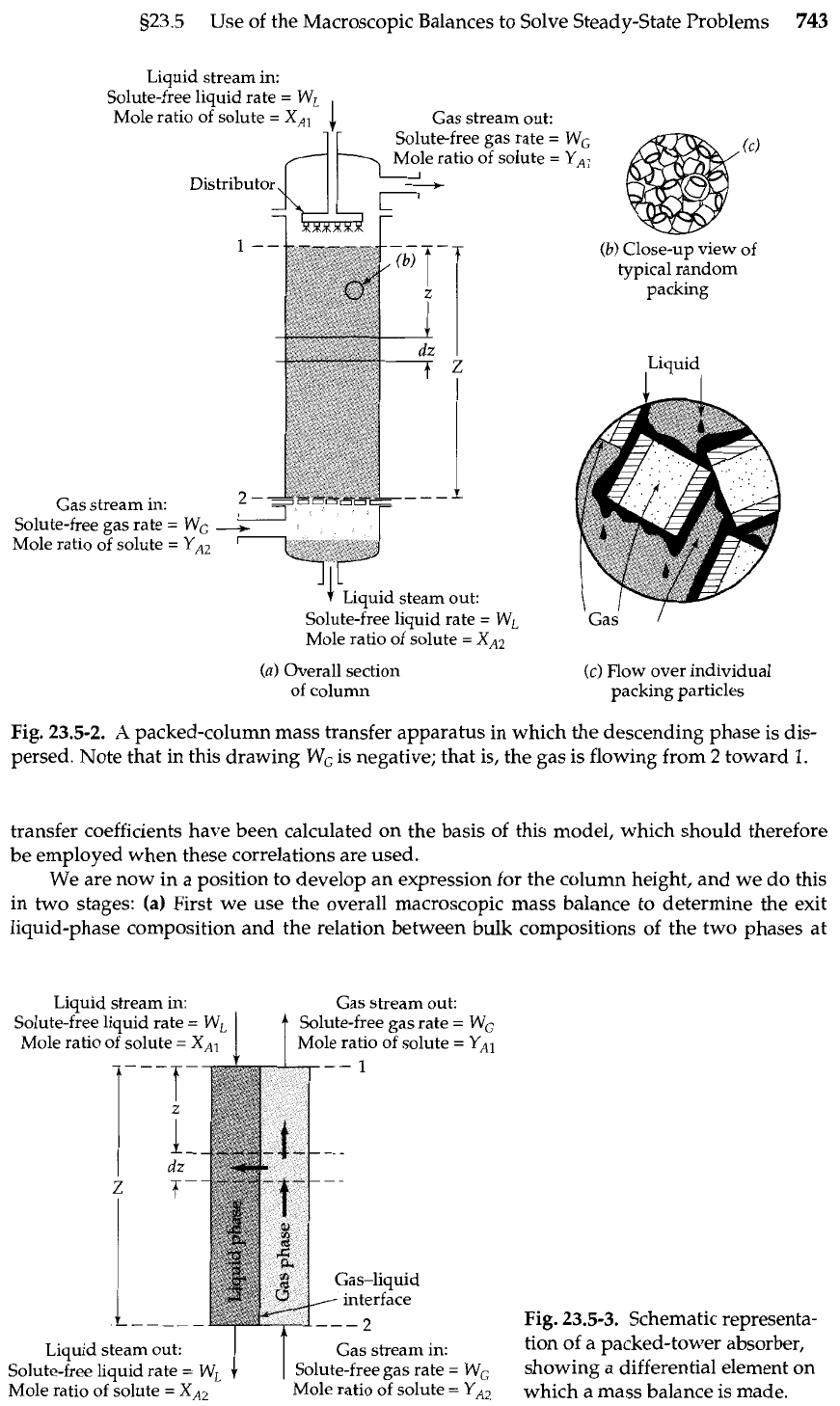
It is desired to remove a soluble gas
A
from a mixture of
A
and an insoluble gas
B
by contact-
ing the mixture with a nonvolatile liquid solvent
L
in the apparatus shown in Fig. 23.5-2. The
apparatus consists essentially of a vertical pipe filled with a randomly arranged packing of
small rings of a chemically inert material. The liquid
L
is sprayed evenly over the top of the
packing and trickles over the surfaces of these small rings. In so doing, it is intimately con-
tacted with the gas mixture that is passing up the tower. This direct contacting between the
two streams permits the transfer of
A
from the gas to the liquid.
The gas and liquid streams enter the apparatus at molar rates of
-
WG
and W,, respec-
tively, on an A-free basis. Note that the gas rate is negative, because the gas stream is flowing
from plane 2 to plane
1
in this problem. The molar ratio of
A
to
G
in the entering gas stream is
YA2
=
yA2/(1
-
yA2),
and the molar ratio of
A
to
L
in the entering liquid stream is
X,,
=
xAl/(l
-
xAl). Develop an expression for the tower height
z
required to reduce the molar ratio
YA
in the gas stream from
YA2
to
Y,,,
in terms of the mass transfer coefficients in the two
streams and the stream rates and compositions.
Assume that the concentration of A is always small in both streams, so that the operation
may be considered isothermal and so that the high mass-transfer rate corrections to the mass
transfer coefficients are not needed, and the mass transfer coefficients,
k:
and
k!,
defined in the
second line of Eq. 22.2-14 can be used.
Since the behavior of a packed tower is quite complex, we replace the true system by a hypo-
thetical model. We consider the system to be equivalent to two streams flowing side-by-side
with no back-mixing, as shown in Fig. 23.5-3, and in contact with one another across an inter-
facial area
a
per unit volume of packed column (see Eq. 22.1-14).
We further assume that the fluid velocity and composition of each stream are uniform
over the tower cross section, and neglect both eddy and molecular transport in the flow direc-
tion. We also consider the concentration profiles in the direction of flow to be continuous
curves, not appreciably affected by the placement of the individual packing particles.
The model resulting from these simplifying assumptions is probably not a very satisfac-
tory description of a packed tower. The neglect of back-mixing and fluid-velocity nonunifor-
mity are probably particularly serious. However, the presently available correlations for mass
J.
D.
Seader and
E.
J.
Henley,
Separation
Process Principles,
Wiley,
New
York
(1998).
523.5
Use of the Macroscopic Balances to Solve Steady-State Problems
743
Liquid stream in:
Solute-free liquid rate
=
WL
Mole ratio of solute
=
Xal
1
Gas stream out:
Solute-free gas rate
=
WG
Mole ratio of solute
=
YAl
Distributor
&-
Gas stream in:
Solute-free gas rate
=
WG
-
Mole ratio of solute
=
YA2
'/'Liquid steam out:
Solute-free liquid rate
=
WL
Mole ratio of solute
=
XA2
(a)
Overall section
of column
(b)
Close-up view of
typical random
packing
(c)
Flow over individual
packing particles
Fig.
23.5-2.
A
packed-column mass transfer apparatus in which the descending phase is dis-
persed. Note that in this drawing
WG
is negative; that is, the gas is flowing from
2
toward
1.
transfer coefficients have been calculated on the basis of this model, which should therefore
be employed when these correlations are used.
We are now in a position to develop an expression for the column height, and we do this
in two stages:
(a)
First we use the overall macroscopic mass balance to determine the exit
liquid-phase composition and the relation between bulk compositions of the two phases at
Liquid stream in:
Gas stream out:
Solute-free liquid rate
=
WL
It
Solute-free gas rate
=
WG
Mole ratio of solute
=
XAl
Mole ratio of solute
=
YAl
Liquid steam out:
I
I
Gas stream in:
Solute-free liquid rate
=
WL
Solute-free gas rate
=
WG
Mole ratio of solute
=
XA2
Mole ratio of solute
=
YA2
Fig.
23.5-3.
Schematic representa-
tion of a packed-tower absorber,
showing a differential element on
which
a
mass balance is made.

744
Chapter 23 Macroscopic Balances for Multicomponent Systems
each point in the tower.
(b)
We then use these results along with the differential form of the
macroscopic mass balance to determine the interfacial conditions and the required tower
height.
(a)
Overall macroscopic mass balances.
For the solute A we write the macroscopic mass bal-
ance of Eq. 23.1-3 for each stream of the system between planes
l
and
2
as
liquid
stream
gas stream
Here the subscripts A1 and Ag refer to the solute A in the liquid and gas streams, respectively.
Since the number of moles leaving the liquid stream must enter the gas stream across the in-
terface, WAlr0
=
-
W,,,,, and Eqs. 23.5-14 and 15 may be combined to give
This can now be rewritten in terms of the compositions of the entering and leaving streams by
setting WAl2
=
WLXA2, and so on, and then rearrangement gives
Thus we have found the concentration of
A
in the exit liquid stream.
By replacing plane 2 by a plane at a distance
z
down the column, Eq. 23.5-17 may be used
to obtain an expression relating bulk stream compositions at any point in the tower:
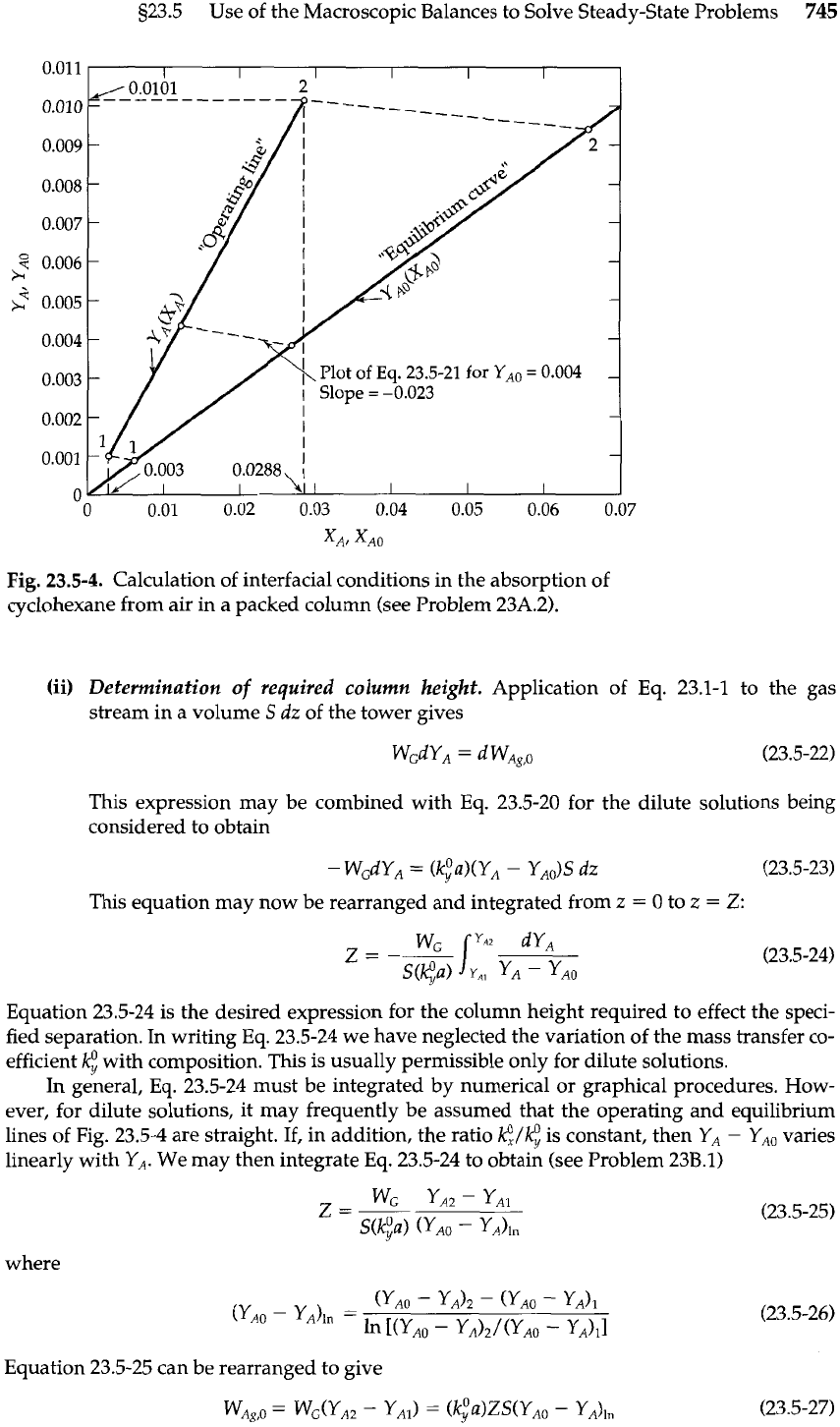
Equation 23.5-18 (the "operating line") is shown in Fig. 23.5-4 along with the equilibrium dis-
tribution for the conditions of Problem 23A.2.
(b)
Application of the macroscopic-balances
in
the d-form.
We now apply Eq. 23.1-3 to a dif-
ferential increment
dz
of the tower, first to estimate the interfacial conditions and then deter-
mine the tower height required for a given separation.
(i)
Determination of interfacial conditions.
Because only
A
is transferred across the in-
terface, we may write, according to the second line of Eq. 22.1-14 (which presumes
low concentrations of A and small mass-transfer rates):
Here
a
is the interfacial area per unit volume of the packed bed tower,
S
is the cross-
sectional area of the tower,
XAO
and yAO are the interfacial mole fractions of A in the
liquid and gas phases, respectively, and xA and
yA
are the corresponding bulk con-
centrations (the index
b
is being omitted here, so that XA,
yA,
XA, and
YA
are all bulk
compositions).
Then, since (for the dilute solutions considered here) xA
=
XA/(XA
+
1)
=
XA
and yA
=
YA/(YA
+
1)
=
YA,
Eqs. 23.5-19 and 20 may be combined to give
This equation enables us to determine
YAO
as a function of
YA.
For any
YA,
one may
locate XA on the operating line (mass balance). One then draws a straight line of
slope -(@a)/($a) through the point
(YA,
XA), as shown in Fig. 23.5-4. The intersec-
tion of this line with the equilibrium curve then gives the local interfacial composi-
tions
(YAO,
XAO).
523.5 Use of the Macroscopic Balances to Solve Steady-State Problems
745
Fig.
23.5-4.
Calculation of interfacial conditions in the absorption of
cyclohexane from air in a packed column (see Problem 23A.2).
(ii)
Determination of required column height.
Application of Eq. 23.1-1 to the gas
stream in
a
volume
S
dz of the tower gives
WG~YA
=
~WA,O (23.5-22)
This expression may be combined with Eq. 23.5-20 for the dilute solutions being
considered to obtain
-
WGdYA
=
(kyOa)(YA
-
YAO)S dz
(23.5-23)
This equation may now be rearranged and integrated from
z
=
0
to
z
=
Z:
Equation 23.5-24 is the desired expression for the column height required to effect the speci-
fied separation. In writing Eq. 23.5-24 we have neglected the variation of the mass transfer co-
efficient
k:
with composition. This is usually permissible only for dilute solutions.
In general, Eq. 23.5-24 must be integrated by numerical or graphical procedures. How-
ever, for dilute solutions, it may frequently be assumed that the operating and equilibrium
lines of Fig. 23.5-4 are straight.
If,
in addition, the ratio
@/k;
is constant, then
YA
-
Y,,
varies
linearly with Y,. We may then integrate Eq. 23.5-24 to obtain (see Problem 23B.1)
where
Equation 23.5-25 can be rearranged to give
746
Chapter
23
Macroscopic Balances for Multicomponent Systems
Comparison of Eq.
23.5-27
and Eq.
15.4-15
shows the close analogy between packed towers
and simple heat exchangers. Expressions analogous to Eq.
23.5-24
but containing the overall
mass transfer coefficient
K!
may also be derived (see Problem
23B.1).
Again, we may use the
final results, Eqs.
23.5-25
or
27,
for either cocurrent or countercurrent flow. Keep in mind,
however, that the simplified model used to describe the packed tower is not as reliable as the
corresponding one used for heat exchangers.
EXAMPLE
23.5-3
Linear
Cascades
We saw in Example
23.1-2
that the degree of separation possible in a simple binary splitter
can be quite limited, and it is therefore often desirable to combine individual splitters in
a
countercurrent cascade such as that shown in Fig.
23.5-5.
Here the feed to any splitter stage
is
the sum of the waste stream from the splitter immediately above it and the product from the
splitter immediately below.
Show how such an arrangement can increase the degree of separation relative to that ob-
tained in a single splitter.
SOLUTION
For the system as a whole we can write a mass balance for the desired product and for the so-
lution as a whole. That is, we treat the entire system as a splitter and write
It will be assumed here that all of quantities in these equations are given, so that the problem
is specified as far as the overall mass balances are concerned. It remains for us to determine
the number of stages required to meet these conditions.
We begin by writing a set of mass balances over the top portion of the column, here the
top two stages for illustrative purposes (see Fig.
23.5-5):
Here
U,
and
D,
are the upflowing and downflowing streams from stage n, and
y,
and
x,,
are
the corresponding mole fractions of the desired solute. When
P
is eliminated between Eqs.
23.5-3
and
31,
we get
This equation gives the relation between the compositions of the downflowing and upflowing
streams passing each other at any column cross section above the feed stage, in terms of the cor-
responding flow rates. This relation, when shown on an
x-y
plot (which
is
called a McCabe-Tkiele
diag~arn~,~) is known as the operating line for the system. We concentrate for the moment on com-
positions and return later to the problem of determining stream rate ratios.
F$4
I
5
Fig.
23.5-5.
A
linear cascade. Upward flows are shown by solid
:+
lines, and downward flows by dashed lines.
W.
L.
McCabe
and
E.
W.
Thiele,
Ind.
Eng.
Chem.,
17,605411
(1925).
