Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.


522.8
Transfer Coefficients at High Net Mass Transfer Rates
707
A
falling film system is shown in Fig. 22.8-4. The time of travel from the liquid inlet
to the liquid outlet (the "exposure time") is sufficiently short that the diffusing species
does not penetrate very far into the liquid. In such a situation, we can (from a mathemat-
ical point of view) regard the falling film as infinitely thick. We may then take over the
results from Example 20.1-1.
Equation 20.1-23 gives the concentration profiles for a corresponding unsteady-state
system with large net mass transfer rate, and an analogous equation can be written
down for the temperature profiles:
T
-
To
erf(qT
-
9,)
+
erf
(0,
rIT
=
- -
Tm
-
TO
1
+
erf p,
Here
Q
=
y/m
and
q,
=
y/G
are dimensionless distances from the interface,
and p in each formula is a dimensionless molar average velocity at the interface:
From these results and the definitions for the transfer coefficients in Eqs. 22.8-1 and 2, we
may now get the rate factors
4,
the flux ratios
R,
and the correction factors 0, defined in
the preceding subsection:
From the definitions in Eqs. 22.8-1 and 2 and the profiles in Eqs. 22.8-11 and 12, we can
also get the expressions for the transfer coefficients at low net mass transfer rates:
z
=
distance into liquid
film
Liquid
Liquid
in
out
Fig.
22.8-4.
Diffusion into a
falling liquid film. Here
tmp
is
the total time of exposure of a
typical element of volume near
the surface.

708
Chapter 22 Interphase Transport in Nonisothermal Mixtures
The corresponding coefficients at high net mass transfer rates can be obtained by multi-
plying by the correction factor in Eq. 22.8-16.
From the last two equations we get the relation
A
similar relation, with an exponent of
$
(instead of
3)
is obtained from the Chilton-Col-
burn relations given in Eqs. 22.3-23 to 25. The latter are valid for flows adjacent to rigid
boundaries, whereas Eq. 22.8-19 pertains to fluid-fluid systems with no velocity gradient
at the interface.
The proportionality of
k,,,,,
to the square root of the diffusivity, given in
Eq.
22.8-17,
has been confirmed experimentally for the liquid phase in several gas-liquid mass trans-
fer systems, including short wetted-wall columns, packed columns, and liquids around
gas bubbles in certain instances. The penetration model has also been applied to absorp-
tion with chemical reactions (see Example 20.1-2).
The Flat-Plate Boundary Layer Model
The steady-state transport in the boundary layer along a flat plate for a fluid with con-
stant physical properties was discussed in g20.2. The eneral expression for the profiles,
n(q, A, K), was given in Eq. 20.2-43. There q
=
y
e
vJ2vx is a dimensionless position co-
ordinate measured from the plate,
A
is the physical property group (i.e., 1, Pr, or
Sc),
and
K
=
(v,/v,)w is a dimensionless net mass flux from the plate.
Once again we introduce the notations defined in Table 22.8-1. Then for the bound-
ary layer calculation we have
In the boundary layer calculation it was assumed that the heat capacities of both species
are identical.
The momentum, heat, and mass fluxes for the flat plate are given in Fig. 22.8-5. Then
in the following two figures, Figs. 22.8-6 and 22.8-7, two plots are given, comparing the
correction factors,
8,
for the film model, the penetration model, and the boundary layer
model. The boundary layer model gives a dependence on
A
that is not found in the other
models, because this model includes the effect of the tangential velocity profiles on the
temperature and concentration profiles. The film model predicts the smallest depen-
dence of the transfer coefficients on net mass-transfer rate.
Correction factors considerably different from
1
arise when either
$J
or
R
is of mag-
nitude
1
or greater for
T
or
XA;
see Figures 22.8-6, 7 and 8, and the relation
0
=
+/R.
Large net interfacial mass fluxes, by these measures, are common when the mass trans-
fer is mechanically driven as in ultrafiltration (Example 22.8-5) and transpiration cooling
(Problem 20B.7(c)). Large net mass fluxes can also occur in vaporization, condensation,
melting and other changes of state, and in heterogeneous chemical reactions, when ac-
companied by correspondingly large temperature differences or radiation intensities to
transfer the requisite latent heat or energy of reaction. More moderate net fluxes, and
correction factors near unity, are common in multistage and packed-column separation
processes, where the differences of temperature and composition within a separation
stage or flow cross-section are normally rather small. The energy flux ratio
R,
is an im-
portant criterion for assessing the net-flux corrections, as illustrated in Examples 22.8-2
and 3.

s22.8
Transfer Coefficients at High Net Mass Transfer Rates
709
l+RforR<O
Asymptote for
4
+
-m:
R
+
-1
Fig.
22.8-6.
The variation of the
transfer coefficients with mass
transfer rate as predicted by
various models. The line for
A
+
w
holds for the nonsepa-
rated, steady state boundary-
layer regions on rigid surfaces,
whatever their geometry.
0.
10
8
6
4
2
1
0.8
0.6
0.4
141
0.2
0.1
0.08
0.06
0.04
0.02
0.01
A
I
1 1 11 11111
I
1
1
11 11111 1 1 1 11 111
0.01 0.02 0.05 0.1 0.2 0.5 1.0 2 5 10 20 50 100
I
RI
Fig.
22.8-5.
Heat and mass fluxes between a flat plate and a laminar
boundary layer
[W.
E.
Stewart, ScD thesis, Massachusetts Institute of
/
,
, , , , , , , , , , , , , , ,
.
, , , , , , , , ,
For positive R, read positive
+
from lower curves..-
For negative R, read negative
+
on upper curves.
--
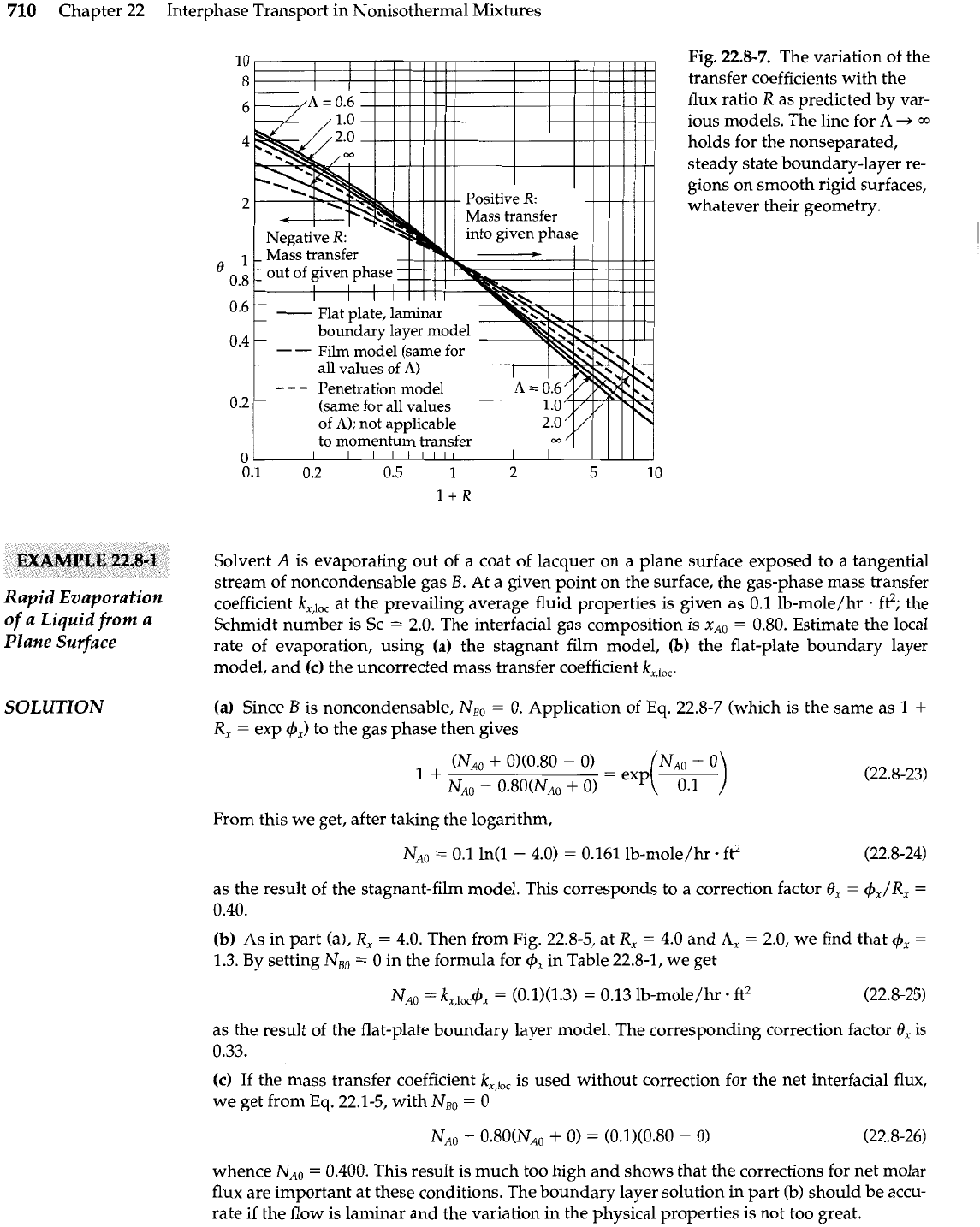
710
Chapter
22
Interphase Transport in Nonisothermal Mixtures
Fig.
22.8-7.
The variation of the
transfer coefficients with the
flux ratio
R
as predicted by var-
ious models. The line for
A
+=
03
holds for the nonseparated,
steady state boundary-layer re-
gions on smooth rigid surfaces,
whatever their geometry.
EXAMPLE
22.8-1
Rapid Evaporation
of
a
Liquid
from a
Solvent
A
is evaporating out of a coat of lacquer on a plane surface exposed to a tangential
stream of noncondensable gas
B.
At a given point on the surface, the gas-phase mass transfer
coefficient
k,,,,,
at the prevailing average fluid properties is given as 0.1 lb-mole/hr
.
ft2; the
Schmidt number is Sc
=
2.0. The interfacial gas composition is
XAO
=
0.80. Estimate the local
Plane
Surfnce
rate of evaporation, using
(a)
the stagnant film model,
(b)
the flat-plate boundary layer
model, and
(c)
the uncorrected mass transfer coefficient
k,,,,,.
SOLUTION
(a)
Since
B
is noncondensable,
NBo
=
0. Application of
Eq.
22.8-7 (which is the same as 1
+
R,
=
exp
4,)
to the gas phase then gives
From this we get, after taking the logarithm,
as the result of the stagnant-film model. This corresponds to a correction factor
8,
=
+,/R,
=
0.40.
(b)
As in part (a),
R,
=
4.0. Then from Fig. 22.8-5, at
R,
=
4.0 and
A,
=
2.0, we find that
4,
=
1.3. By setting
NBo
=
0 in the formula for
4,
in Table 22.8-1, we get
as the result of the flat-plate boundary layer model. The corresponding correction factor
0,
is
0.33.
(c)
If the mass transfer coefficient
k,,l,,
is used without correction for the net interfacial flux,
we get from
Eq.
22.1-5, with
NBO
=
0
whence
NAO
=
0.400. This result is much too high and shows that the corrections for net molar
flux are important at these conditions. The boundary layer solution in part
(b)
should be accu-
rate if the flow is laminar and the variation in the physical properties is not too great.

g22.8 Transfer Coefficients at High Net Mass Transfer Rates
711
Adjust the results of Example 22.3-1 for the net molar flux by applying the correction factors
6,
and 19, from the film model and from the flat-plate boundary layer model.
Correction Factors in
Droplet Evaporation
SOLUTION
In Example 22.3-3 the molar flux ratio R, at any point on the surface of the drop is
From Eq. 22.8-9 (film model) or Fig. 22.8-7 (flat-plate boundary layer model), the predicted
correction factor
6,
is about 0.99 at all points on the drop. Hence the corrected mass transfer
rate is (by adjustment of Eq. 22.3-31)
This result differs only slightly from that obtained in Example 22.3-1. Thus the assumption of
a small mass-transfer rate was satisfactory under the given conditions.
Extend the analysis of Example 22.3-2 to include the corrections for net mass-transfer rate,
using the stagnant film model.
Wet-Bulb
Performance
Corrected for
ass-
Transfer Rate
SOLUTION
By
rewriting the energy balance, Eq. 22.3-32, for any point on the wick, we obtain for finite
mass-transfer rate
Multiplication of both sides by CpA/(AiiArVaphioc) gives, since
Nm
=
0,
NAO&
-
&(T-
-
To)
RT=~-
bloc
~~A,uap
The right-hand member of this equation is easily calculated if
TO,
T,,
and
p
are given.
Next we write the expression
r#~
=
ln(1
+
R) for both heat and mass transfer, taking into
account the fact that
NBO
=
0:
Solving both equations for NA, and equating the resulting expressions gives
hloc
In(1
+
RJ
=
-
ln(1
+
R,)
(22.8-33)
kx,locC,A
Then substituting the expressions for R, and RT from Table 22.8-1 yields
This equation shows that
xAo
and
To
will be constant over the surface of the wick if
h,,/(k&,,,) is constant and thus equal to h,,,/(k,,l<,A). This constancy is assumed here for
712
Chapter 22 Interphase Transport in Nonisothermal Mixtures
EXAMPLE
22.8-4
Comparison of
Film
and Penetration
Models for Unsteady
Evaporation in a
Long Tube
simplicity. Such an assumption is particularly satisfactory for the water-air system, for which
Pr and Sc are nearly equal. With this substitution,
Eq.
22.8-34 becomes
This solution simplifies exactly to Eq. 22.3-35 at low mass-transfer rates.
For the numerical problem in Example 22.3-2, the following values apply:
xAo
=
0.0247
CPA
=
8.03 Btu/lb-mole
.
F
for water vapor at 105°F
h,/k,,
=
5.93
Btu/lb-mole
.
F
from the Chilton-Colburn analogy (Eq. 22.3-25)
Insertion of these values into Eq. 22.8-35 gives
Solving this equation, we get
x,,
=
0.0034 (22.8-37)
This result differs only slightly from the value 0.0033 obtained in Example 22.3-2 and justifies
the previous omission of the correction factors under the given conditions.
Numerical studies indicate that the simple
Eq.
22.3-34 gives a close approximation to
Eq.
22.8-35 for the air-water system under all likely wet-bulb conditions. Eqs. 22.3-32 and 33
overestimate the mass transfer rate almost equally, and when these equations are combined,
the errors largely compensate.
Compare the effects of net mass transfer for the unsteady evaporation system described in
Example 20.1-1 with the predictions of
(a)
the generalized penetration model, and
(b)
the
stagnant-film model introduced above. The latter calculation amounts to a quasi-steady-state
treatment of this time-dependent system.
SOLUTION
We begin by noting that for this system
x,,
=
0 and
N,,
=
0. It follows from Eq. 22.8-la and
Table 22.8-1 that
The correction factor 8, is thus the ratio of the flux corrected for net mass transfer to the un-
corrected flux.
(a)
The penetration model.
We note that the concentration gradient at the liquid surface can
be obtained by differentiating Eq. 20.1-16 and rewriting the result in terms of
x,
and
z.
The
re-
sult is
2 exp(-q2)
x,,
1
+
erf
cp
.\/=
For negligible net mass transfer,
cp
=
0. Thus, the ratio of the mass flux in the presence of net
mass transfer to the flux in the absence of net mass transfer is

s22.8 Transfer Coefficients at High Net Mass Transfer Rates
713
Table
22.8-2
Comparison of Film and Penetration Models.
8, from penetration model
8, from film model
X~~
(Eq. 22.8-41) (Eq. 22.8-42)
in agreement with Eqs. 22.8-14 and 16. To get
8,
as a function of
x,,,
we may use Fig. 22.8-7, or
use Eq. 20.1-17 to write
8,
=
(1
-
xAO)+(xAO) (penetration model) (22.8-41)
where +(xAO) is the quantity defined just after Eq.
20.1-22
and given in Table 20.1-1.
(b)
The stagnant-film model.
The film model result may be obtained from Eq. 22.8-9 in the
form 8
=
(1
/R) ln(1
+
R) to obtain
1
-
XAO
X~O
lxAO)
(film model)
0,
=
-
ln
-
Numerical values for both models are provided in Table 22.8-2 and also in
Fig.
22.8-7.
It is seen that the penetration model predicts a stronger correction 8, for net mass transfer
than does the film model. This is in part because the net flow thickens the boundary layer, an
effect that the film model does not consider. It may also be noted that this example is a realis-
tic use of the penetration model, as there is little effect of solute concentration on the physical
properties in this simple isothermal system.
A
much different situation is seen in the next
example.
Ultrafiltration of proteins is a concentration process, in which water from an aqueous protein
solution is forced through a membrane impermeable to the protein but permeable to water
Concentration
and small solutes such as inorganic salts. Protein then accumulates in a polarization layer, or
Polarization in
region of high protein concentration adjacent to the membrane surface, as indicated in Fig.
Ultrafiltration
22.8-8. Determine the relation between water permeation velocity and the transmembrane
Protein
boundary layer
Membrane
\
/n
PP
-
Po
Fig.
22.8-8.
A
spinning-
pa
disk ultrafilter.

714
Chapter 22 Interphase Transport in Nonisothermal Mixtures
SOLUTION
pressure difference. Describe the effect of net mass transfer on the mass transfer coefficient for
protein transport. Assume that the membrane is completely impermeable to protein so that
the net transport of protein across the membrane surface is zero.
For simplicity we choose a spinning-disk geometry as shown in Fig. 22.8-8, for which the pro-
tein concentration will be a function only of the distance
y
from the disk surface and not of ra-
dial position5 (see Problem 19D.4). However, we will have to consider the dependence of
density, viscosity, and protein-water diffusivity on the protein concentration, and we will
need the concept of osmotic pres~ure.~
The basis for our solution is the concept of
hydraulic permeability
of the filtration
membrane:
Here
v,
is the velocity, or volumetric
flux,
of the solvent leaving the downstream surface of
the membrane. Equation 22.8-43 defines
KH,
the hydraulic permeability of the membrane. The
quantities
p,
and
p6
are the hydrodynamic pressures against the membrane as indicated in
Fig. 22.8-8, and
7~
is the
osmotic pressure
at the upstream surface of the membrane. The inclu-
sion of
.rr
recognizes that it is really the total thermodynamic potential that drives the trans-
membrane transport (this point will be discussed further in Chapter 24.)
For this situation, the interfacial protein velocity is zero, so that a solvent mass balance
across the protein boundary layer gives
in which
y
is the distance from the upstream membrane surface into the protein boundary
layer. The quantity
p'S'
is the density of the pure solvent, and
pso
=
PS(y=O
and
vso
=
vSy(y=O
are
the mass concentration and velocity of solvent at the upstream membrane surface.
The osmotic pressure
?.r
is a function of the protein concentration
p,,
and we will provide
an example of this in Problem 22C.1. We find then that the water flux across the membrane
depends on the protein concentration at the membrane surface as well as the hydrodynamic
pressure drop across the membrane. This concentration, in turn, can be related to
v,
through
the membrane impermeability condition for the protein and the definition of the mass trans-
fer coefficient. Then at
y
=
0,
we describe the impermeability of the membrane to protein by
where
k;,
has been defined analogously to
k;..
Combination with
Eq.
22.8-44 then gives
This equation may now be solved for the filtrate velocity:
Here
p,
=
pp,
+
pso
and
0
=
&/kp
is a mass transfer correction factor, analogous to
O,,
which
now must include the effects of property changes as well as the net velocity correction intro-
duced in Table 22.8-1. We return to a discussion of this quantity below (see
Eq.
22.8-48). The
term
p,,
is the solution density at the upstream membrane surface.
We can now calculate the desired quantities,
v,
and the transmembrane pressure differ-
ence, if we have sufficient information about the transport and equilibrium properties. Here
we consider the approaching protein concentration
pp,
to be given, and for convenience we
D.
R.
Olander,
J.
Heat Transfer,
84,185
(1972).
R.
J.
Silbey and
R.
A. Alberty, Physical Chemistry, 3rd edition, Wiley,
New
York (20011,
p.
206.

522.8 Transfer Coefficients at High Net Mass Transfer Rates
715
begin by selecting values of the protein concentration p,, at the membrane surface over the
range between p,, and the solubility limit of the protein:
(i)
For any chosen value of pp,, we can calculate the corresponding value of
v,
from Eq.
22.8-47 with appropriate values for
k,
and
8.
These values also permit calculation of
osmotic pressure
7~
from the appropriate equilibrium relationship.
(ii)
We may then calculate the transmembrane pressure difference required for this flow
from
Eq.
22.8-43 and an appropriate value of
K,.
The strong effects of protein concentration on system properties mean that the solution must
be obtained numerically.
We content ourselves here to summarize the results of Kozinski and Lightfoot7 for
bovine serum albumin; they were the first to make such calculations and seem still to have
provided the best documentation. In their publications it is shown that the effective mass
transfer coefficient can be expressed as the product of two factors, one accounting for the con-
centration effects and another taking account of the additional effect of property variations:
where, over the parameter space investigated,
and
Equations 22.8-49 to 52 must be considered empirical. Equation 22.8-47 overpredicts
us
for
small polarization levels, but for that situation the effect of osmotic pressure on flow is small.
The subscript re1 means "relative to the free-stream value."
The mass transfer coefficient in the limit of slow mass transfer and small property varia-
tions is given7 as
v(m)
kpL
=
0.6205(&)1/2(-)1'3
Sh,
=
Shl,,
=
-
9pJw)
v(a)
%ps(m)
in which
L
is the disk diameter and
IR
is the rate of rotation in radians per unit time. The inde-
pendence of mass transfer rate on disk size is the reason that this geometry is so popular for
careful mass transfer studies. Other geometries are considered briefly by Kozinski and Light-
A comparison of a priori predictions from the above model with experimental data is
shown in Fig. 22.8-9, where we see that the two agree well. This good agreement may result
in part because the albumin molecules behave much like incompressible particles at the high
solvent ionic strength at which the data were taken. It may also be seen that osmotic effects
are negligible below pressure drops of about 5 psi; here the predicted behavior is indistin-
guishable from that of the protein-free solvent, essentially water. It is only in this unimpor-
tant region that
Eq.
22.8-48 is unreliable. Details of the calculations are provided in Problem
22C.1.
The effect of increasing pressure difference across the protein boundary layer is quite dif-
ferent from that for a nonselective membrane. At first, the concentration boundary layer gets
thinner, as would be expected, and the mass transfer coefficient
&,
increases. However, with
A. A.
Kozinski, PhD thesis, University of Wisconsin (1971);
A.
A.
Kozinski and
E.
N.
Lightfoot,
AIChE
Journal,
18,1030-1040 (1972).

716
Chapter 22 Interphase Transport in Nonisothermal Mixtures
Fig.
22.8-9.
Protein ultrafiltration
with a spinning disk at 273 rpm.
0.1
0.08
.2
\
6
0.06
2
2
ar
0.04
.I
E
Transmembrane pressure drop, psi
+
4
0.02
further increase in the pressure difference the boundary layer thickness,
k;
and
19,
all approach
asymptotic limits. In practice, these asymptotes are closely approached before the effect of po-
larization becomes appreciable, relative to the membrane flow resistance, and these asymp-
totes suffice to predict the relation between the transmembrane pressure difference and
transmembrane flow.
The behavior can be seen more clearly inserting Eq. 22.8-48 and 49 and the approximate
formula
into Eq. 22.8-47. Then, to a surprisingly good approximation, Eq. 22.8-47 takes the form
06
lo
20
30
40
1
The quantity in the first set of parentheses has the form of the simple film model, but with
k,
multiplied by 1.39. It is probably
Eq.
22.8-55 that has made the simple film model attractive to
many for correlating ultrafiltration and reverse osmosis data. However, neglect of the multi-
plier 1.39 has caused corresponding underestimation of
v,,
even before addressing the effects
of property variations.
Bovine serum
albumin, 2.2 g/lOO ml
522.9
MATRIX APPROXIMATIONS FOR MULTICOMPONENT
MASS TRANSPORT
Multicomponent mass transport occurs widely in chemical, physiological, biological,
and environmental processes and is analyzed by various mathematical methods. Here
we review some matrix approximation methods for mass transport by convection and
ordinary diffusion in multicomponent gases.
A
fuller treatment, including mass trans-
port in liquids, is given in the text by Taylor and Krishna.'
Multicomponent mass transport problems are commonly approximated by lin-
earization-that is,
by
replacing the variable properties in the governing equations with
constant reference values. This approach is a useful complement to purely numerical
methods, especially for complex flows, and can give good predictions when the property
R.
Taylor and
R.
Krishna,
Multicomponent Mass Transfer,
Wiley,
New
York
(1993).
