Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

634 29. NONWOOD FIBER USE IN PULP AND PAPER
phloem consists of fibers, sieve tube elements,
companion cells, and parenchyma. In monocots
these bundles are scattered in the stem throughout
the ground tissue, whereas in dicots the bundles
are all about the same distance from the center and
form a ring of bundles around the pith. In dicots,
the bundles unite as the stem grows outward; in
many species the bundles are no longer distinct
(Fig. 29-2). The scattering of the xylem remains
in those monocots that undergo secondary growth.
The outer layer of cells is the epidermis.
The layer just inside the epidermis is the cortex.
The layer inside the cortex but outside the vascular
tissue is the pericycle. Epidermal cells have
sinuous or toothed margins and may have projec-
tions (in cells called trichomes) that are helpful in
identification of samples.
Useful fibers
Useful fiber (that provides the strength for
paper made from the pulp) is derived from the
vascular tissue of monocots of barley, Hordeum
spp.;
rice, Oryza spp.; esparto, Stipa tenacissima;
wheat, Triticum spp.; bamboo, Phyllostachys(Fig.
29-3);
sugar cane, Saccharum officinarum; and
others.
Bast, fibers from the phloem of dicots, is
derived from hemp, Cannabis sativa;
kenaf.
Hibiscus cannabinus; flax, Linum usitatissimum
(Fig. 29-3); and others. Fiber may obtained from
the pericycle or cortex of some dicots. Cotton is
the seed hair of the cotton plant (Fig. 29-3). Fiber
from the vascular tissue of leafs is obtained from
sisal (Agave sisalina) and manila hemp {Musa
textilis).
Nonwood
fiber
identification
A variety of nonwood fibers can be identified
by a series of specialized stains. For example,
several are available for hemp and flax. Acidified
potassium dichromate swells flax somewhat faster
than hemp. Cyanine stains these materials differ-
ently. Other separations exist for cotton, linen,
and wood; cotton, flax, jute, and hemp; animal
and plant fibers; etc. (Standard T 401 and others).
Straw
morphology
considerations
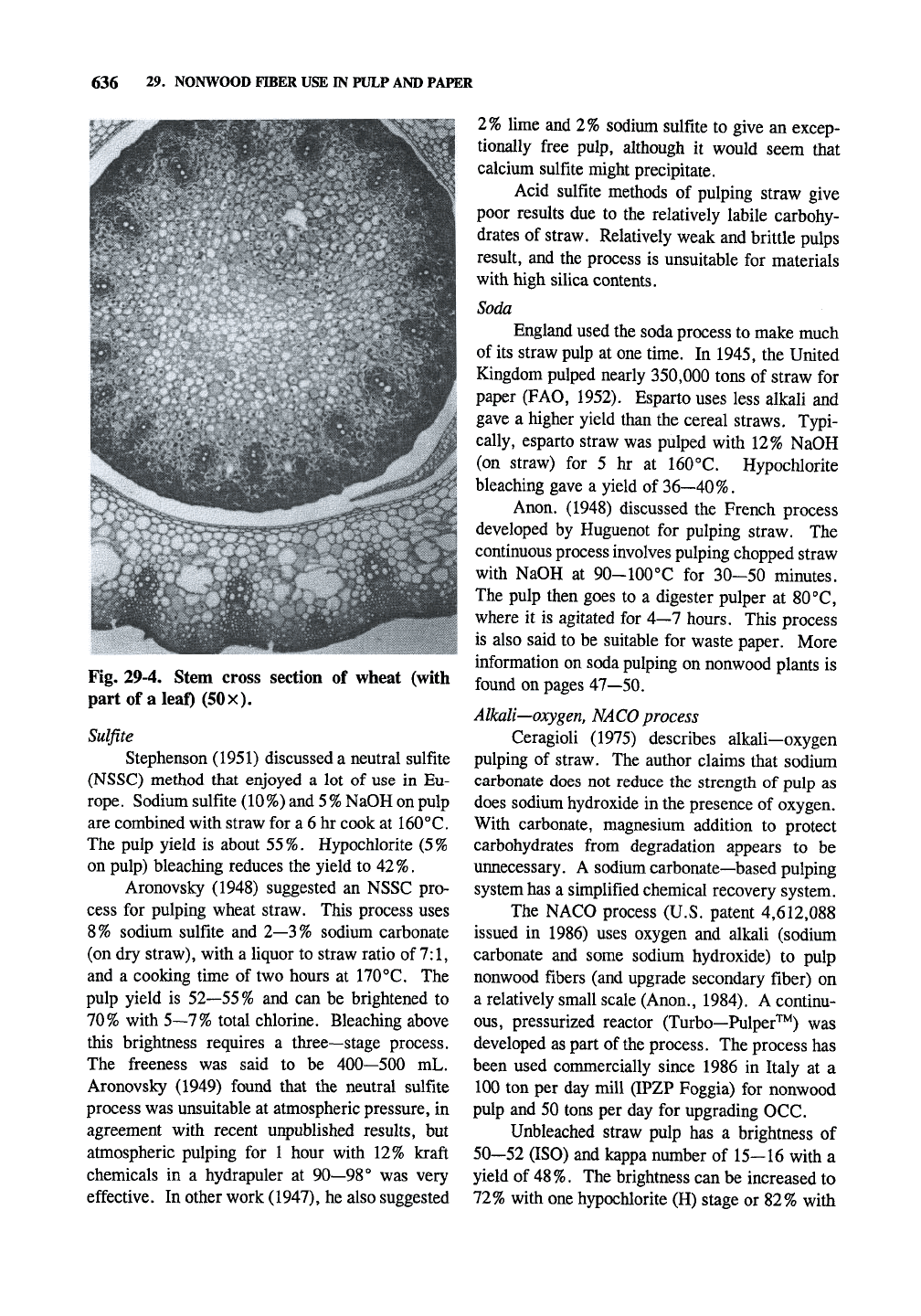
Fig. 29-4 shows a cross section of the straw
stem. The usable fibers are the dark fibers near
the edge of the stem consisting of phloem and
other tissue.
According to Huamin (1988), parenchyma
cells occupy 38% of wheat straw based on the
cross—sectional area of the stem, so the mass
percentage may be lower. These cells are small
and thin—walled, contribute to decreased pulp
freeness, and do not add much to the strength of
paper. They cook more slowly than the fiber cells
and use more cooking chemicals. The lignin,
cellulose, and xylan composition of the various
cell types is very similar.
Petersen (1991) found the oj—cellulose
content of the internode of straw of four grains to
be 37—42% while the leaves had only 28—30%
a—cellulose. The fibers are about 15—20%
longer in the nodes compared to the leaves. The
author suggests that whole straw should not be
pulped anymore than one would pulp trees with
leaves and all. The internode is 35% of orchard
grass (including the inflorescence) and 43 to 48%
of many other grasses.
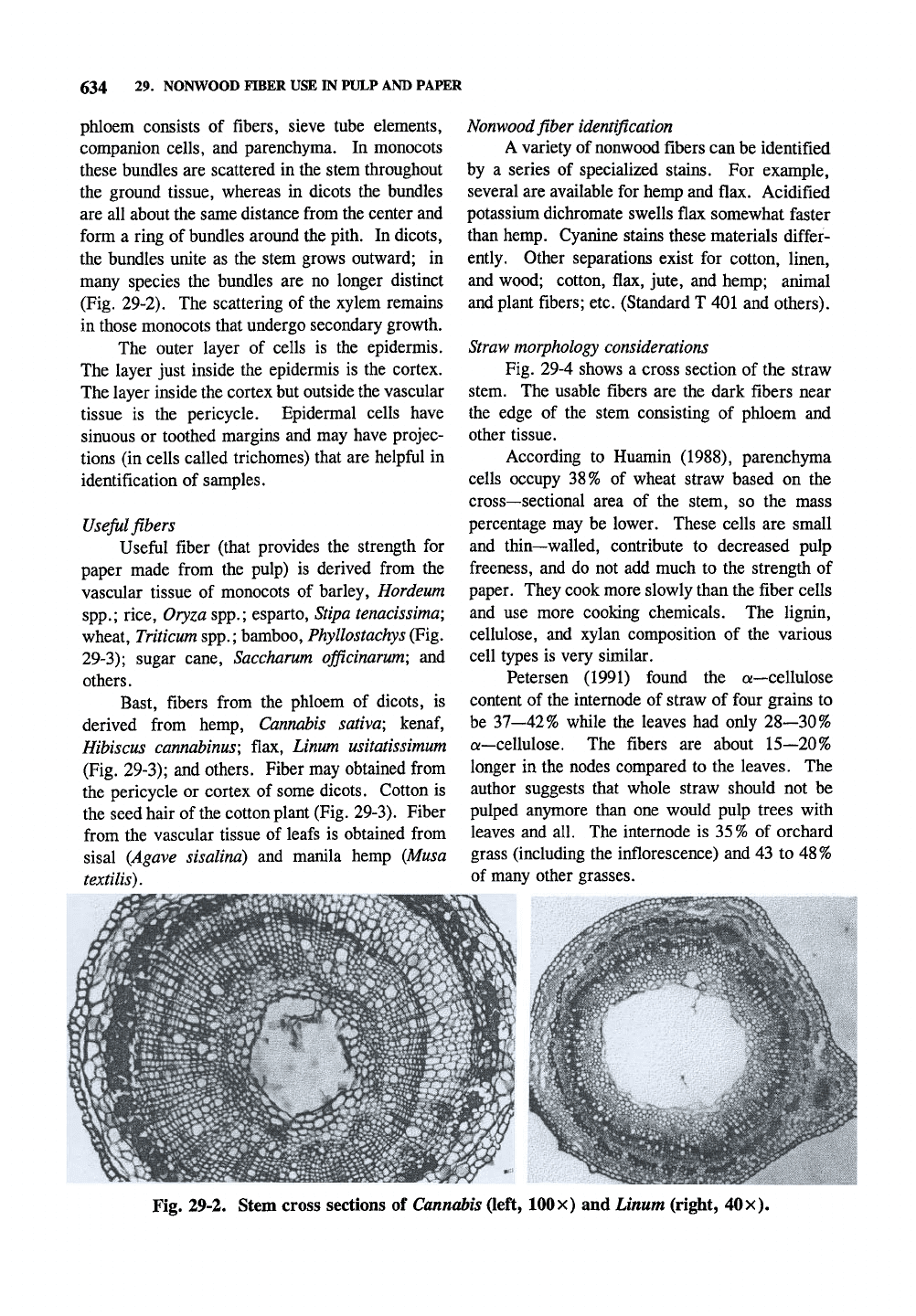
Fig. 29-2. Stem cross sections of Cannabis (left,
100
x) and Linum (right,
40
x).
INTRODUCTION 635
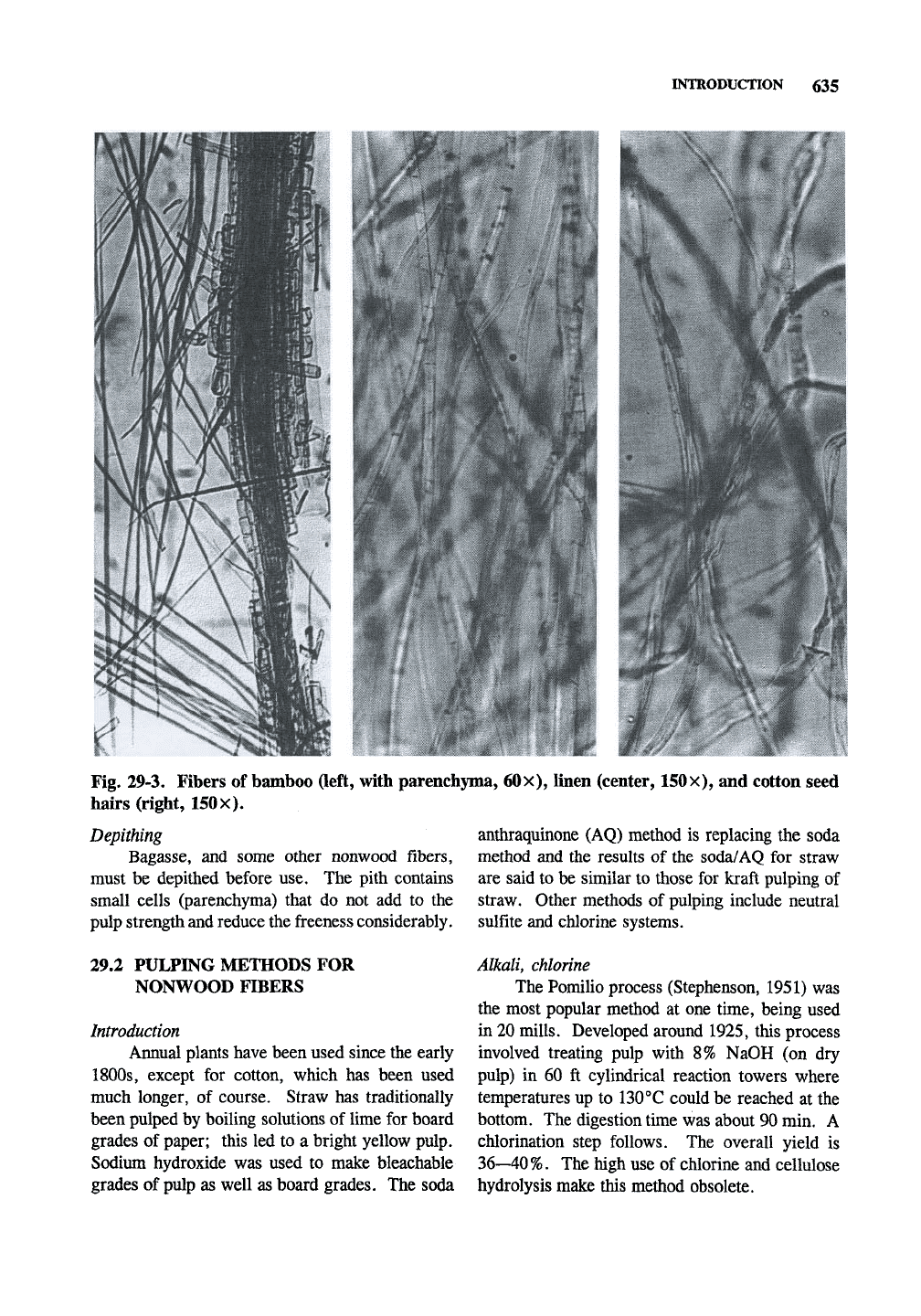
Fig. 29-3. Fibers of bamboo (left, with parenchyma, 60x), linen (center, 150X), and cotton seed
hairs (right, 150x).
Depithing
Bagasse, and some other nonwood fibers,
must be depithed before use. The pith contains
small cells (parenchyma) that do not add to the
pulp strength and reduce the freeness considerably.
29.2 PULPING METHODS FOR
NONWOOD FIBERS
Introduction
Annual plants have been used since the early
1800s, except for cotton, which has been used
much longer, of course. Straw has traditionally
been pulped by boiling solutions of lime for board
grades of paper; this led to a bright yellow pulp.
Sodium hydroxide was used to make bleachable
grades of pulp as well as board grades. The soda
anthraquinone (AQ) method is replacing the soda
method and the results of the soda/AQ for straw
are said to be similar to those for kraft pulping of
straw. Other methods of pulping include neutral
sulfite and chlorine systems.
Alkali, chlorine
The Pomilio process (Stephenson, 1951) was
the most popular method at one time, being used
in 20 mills. Developed around 1925, this process
involved treating pulp with 8% NaOH (on dry
pulp) in 60 ft cylindrical reaction towers where
temperatures up to 130°C could be reached at the
bottom. The digestion time was about 90 min. A
chlorination step follows. The overall yield is
36--40%.
The high use of chlorine and cellulose
hydrolysis make this method obsolete.
636 29. NONWOOD FIBER USE IN PULP AND PAPER
Fig. 29-4. Stem cross section of wheat (with
part of a leaf) (50x).
Sulfite
Stephenson (1951) discussed a neutral sulfite
(NSSC) method that enjoyed a lot of use in Eu-
rope.
Sodium sulfite (10%) and 5% NaOH on pulp
are combined with straw for a 6 hr cook at 160°C.
The pulp yield is about 55%. Hypochlorite (5%
on pulp) bleaching reduces the yield to 42%.
Aronovsky (1948) suggested an NSSC pro-
cess for pulping wheat straw. This process uses
8% sodium sulfite and 2—3% sodium carbonate
(on dry straw), with a liquor to straw ratio of
7:1,
and a cooking time of two hours at 170°C. The
pulp yield is 52—55% and can be brightened to
70%
with 5—7% total chlorine. Bleaching above
this brightness requires a three—stage process.
The freeness was said to be 400—500 mL.
Aronovsky (1949) found that the neutral sulfite
process was unsuitable at atmospheric pressure, in
agreement with recent unpublished results, but
atmospheric pulping for 1 hour with 12% kraft
chemicals in a hydrapuler at 90—98° was very
effective. In other work (1947), he also suggested
2%
lime and 2% sodium sulfite to give an excep-
tionally free pulp, although it would seem that
calcium sulfite might precipitate.
Acid sulfite methods of pulping straw give
poor results due to the relatively labile carbohy-
drates of straw. Relatively weak and brittle pulps
result, and the process is unsuitable for materials
with high silica contents.
Soda
England used the soda process to make much
of its straw pulp at one time. In 1945, the United
Kingdom pulped nearly 350,000 tons of straw for
paper (FAO, 1952). Esparto uses less alkali and
gave a higher yield than the cereal straws. Typi-
cally, esparto straw was pulped with 12% NaOH
(on straw) for 5 hr at 160°C. Hypochlorite
bleaching gave a yield of 36—40%.
Anon. (1948) discussed the French process
developed by Huguenot for pulping straw. The
continuous process involves pulping chopped straw
with NaOH at 90-100°C for 30—50 minutes.
The pulp then goes to a digester pulper at 80°C,
where it is agitated for 4—7 hours. This process
is also said to be suitable for waste paper. More
information on soda pulping on nonwood plants is
found on pages 47—50.
Alkali—oxygen,
NACO
process
Ceragioli (1975) describes alkali—oxygen
pulping of straw. The author claims that sodium
carbonate does not reduce the strength of pulp as
does sodium hydroxide in the presence of oxygen.
With carbonate, magnesium addition to protect
carbohydrates from degradation appears to be
unnecessary. A sodium carbonate—based pulping
system has a simplified chemical recovery system.
The NACO process (U.S. patent 4,612,088
issued in 1986) uses oxygen and alkali (sodium
carbonate and some sodium hydroxide) to pulp
nonwood fibers (and upgrade secondary fiber) on
a relatively small scale (Anon., 1984). A continu-
ous,
pressurized reactor (Turbo—Pulper'^'^) was
developed as part of the process. The process has
been used commercially since 1986 in Italy at a
100 ton per day mill (IPZP Foggia) for nonwood
pulp and 50 tons per day for upgrading OCC.
Unbleached straw pulp has a brightness of
50—52 (ISO) and kappa number of 15—16 with a
yield of
48%.
The brightness can be increased to
72%
with one hypochlorite (H) stage or 82% with
PULPING METHODS FOR NONWOOD FIBERS 637
two H stages (6—7% as active chlorine). Ozone
bleaching gives a brightness of
75—78 %.
Pulping
is carried out at 7—8% consistency, 90 psig, and
130—135 °C for a minimum of one hour.
29.3 CONSIDERATIONS FOR NONWOOD
FIBER USE
Ash and silica both interfere with the pulping
(and chemical recovery of pulping chemicals) of
many nonwood fibers. The cellulose, hemicellu-
lose,
lignin, and extractive contents are fundamen-
tal to pulping. Silica is present at elevated levels;
while wood has less than 0.1%, many annual
crops such as straws have 0.5%—5% or more.
The chemistry of
silica
An introduction to the chemistry of silica will
serve as a basis for the behavior of silica during
pulping operations. Silicon has an atomic weight
of 28.09 and has a valence of 4 in the oxide
forms.
Silicon dioxide
(SiOj,
also called silica or
silicic anhydride) is 46.75% silicon and occurs in
nature as a variety of minerals such as the quartz
minerals and cristobalite. The Si—O bond is
partially ionic (about 50%). SiOj occurs in crys-
talline and amorphous forms. The crystallized
form is said to be inert to alkali.
SiOj is insoluble in acids and water. It is
attacked by HF and ultimately converted to the
volatile gas SiF4. (This is the basis of some straw
pretreatments with HF for silica removal.) The
amorphous form is solubilized in alkali as the salts
of silicic acid. Silicic acid has the formula of
HjSiOj and is the basis of silica gel desiccant and
opal. The soluble ion under alkaline conditions is
SiOa^- or [Si02(OH)2]2-.
Dean (1985) lists the two acid ionization
constants of silicic acid and the solubility product
of CaSiOj at 25
°C.
The pK^ for silicic acid is
9.77 and the pA^ is 11.80. (Methods that precipi-
tate silica from black liquor using COj must
reduce the pH to about 9.0 to obtain
near—complete precipitation of silica.) The p^^p
for CaSiOj is 7.60; ^sp = 2.5 X 10-^ corre-
sponding to a solubility of 0.0184 g/L.
The chemical composition of straw
Aronovsky et al. (1943) analyzed a variety of
straw materials as well (Table 29-1). The mois-
ture contents were 6.6—8.4%. Barley is known to
have a relatively high content of
pectins
and gums,
making a high water extractives level, although
others have reported much lower results for water-
soluble materials. Among the straws, the low ash
content, high cellulose content, and long fibers of
rye grain make it particularly useful for pulp.
Mineral
composition,
especially silica
Delga (1947) investigated the mineral (and
elemental) composition of cereal stalks. They are
high in soluble ash (1.54—4.7%). Potassium was
0.77—3.44% and silicon (not silica) was 0.35 to
0.73%.
The highest level of sodium occurred in
oat straw (0.85%), while the highest level of
calcium was 0.26% in rye grain straw. Sulfur and
phosphorus contents were low. Oat straws grown
in two different soils did not vary in composition.
Fahmy and Fadl (1958) found Egyptian wheat
straw to contain 8.34% ash and 3.44% silica. The
leaf (sheath) is more concentrated with 14.9% ash
and 6.90% silica while the stem is 5.90% ash and
2.24% silica. Wet or dry sorting of the
prehydrolyzed raw material was said to remove
the silica—rich epidermis with a 4% loss of
material. Some work indicates that ethanol—ben-
zene extraction allows silica to be removed with a
second extraction step.
Silica is about 2.5—3.5% in bamboo; it is
more concentrated in the nodes, where it may
occur as nearly pure silica, than in the internode
region. Bagasse is about 1.5% silica. The silica
content of straw leaves is higher than that of the
stems.
Table 29-1. Chemical composition (%) of cereal
straws after Aronovsky (1943).
Extractives
EtOH—ben2
Cold water
Hot water
1%
NaOH
Lignin
Pentosans
Qf-cellulose
Ash
Nitrogen
Rice
:. 4.6
10.6
13.3
49.1
11.9
24.5
36.2
16.1
0.6
Barley
4.7
16.0
16.1
47.0
14.5
24.7
33.8
6.4
1.1
Wheat
3.7
5.8
7.4
41.0
16.7
28.2
39.9
6.6
0.4
Rye
3.2
8.4
9.4
37.4
19.0
30.5
37.4
4.3
0.7
Oat
4.4
13.2
15.3
41.8
17.5
27.1
39.4
7.2
0.5

638 29. NONWOOD FffiER USE IN PULP AND PAPER
These facts point out that if straw can be
preprocessed to remove leaves and nodes, then
many advantages may be realized.
Pulping and silica content
Fahmy and Fadl (1959) determined that the
duration of alkaline pulping of wheat and rice
straws was the most important parameter of the
ash and silica content of the final pulp. Other
variables have very little influence. For example,
rice straw cooked at 150°C for 0.5 hour was
lower in ash than that cooked for 4 hours, with the
effect more pronounced for leaves than for stalks.
This may partly be due to a lower pulp yield with
increased cooking time. Bleached pulps from rice
stalks cooked at 120°C for 0.5 hour had 0.064%
silica and 0.15% ash.
Sodium sulfite pulping of bamboo with kraft
green liquor removes less than 10% of the silica
from straw compared to the 60—70% removed
during kraft pulping. The sulfite process leads to
high—strength and high—yield pulps.
Alkali chemical recovery and silica
Black liquor from straw pulping has about
5500 Btu/lb (on solids) compare to 6600 Btu/lb for
black liquor from wood. A lower residual alkali
(about 3 to 4 g/L) causes lignin and silica to
precipitate during liquor concentration, making
scaling and high viscosities big problems. Liquor
viscosity is much higher than with wood pulp. A
long time ago, Rinman developed a technique of
adding some Ca(0H)2 to the pulping liquor so that
calcium silicate would precipitate onto fibers
during the cook.
Grubshein (1961) pointed out that during
causticization some of the sodium silicate is con-
verted to insoluble calcium silicate. This decreas-
es the causticizing efficiency and increases the
lime mud volume. He concluded that the answer
is removal of silica from the black liquor by one
of two methods: 1) treat the black liquor with
lime or 2) treat the black liquor with flue gases to
lower the pH and precipitate silicic acid. Method
1 is covered in the patent by Gruen (1953), who
suggested
the
precipitation with CaO occur near or
above the boiling point of the black liquor for a
short period of time (5—10 min) to decrease the
amount of organic material precipitated. The
process was patented in Germany by Schwalbe
(1929).
(One mill in South Africa uses ferric
oxide and alumina to precipitate silica.) The
CO2 method is more common since costly lime is
not used. Also, scaling will be lower in subse-
quent evaporation. The use of CO2 precipitation
with kraft liquors would probably increase the
TRS emissions in the recovery boiler since this
process is akin to direct contact evaporation.
The experimental findings of Lengyel (1960)
indicate that 6 to 8 g/L silica in black liquor can
be evaporated without corrosion provided that
addition of excess NaOH is used to dissolve
incrustations that form. Silica at this level does
not overly interfere with causticization but does
increase the quantity of lime mud by about 100%
and begins to retard the sedimentation rate.
Corrosion and scaling are aggravated by allowing
the black liquor to stand motionless for prolonged
periods of time. Black liquor with more than 8
g/L silica should be treated with CO2 (favored) or
CaO (less favored). Lime mud can be purged if
calcium silicate builds up in the system.
Sawheny (1988) reports that silica precipitates
from soda black liquor from pH 10.2 to 9.1. At
the lower pH large amounts of lignin also precipi-
tate.
The solubilities are also temperature depen-
dent. In pilot plant tests involving several species
of nonwood plants, careful carbonation (with a
bubble reactor) of black liquor containing 6 g/L
silica followed by filtering in a filter press resulted
in 90% of the silica being removed as a solid
containing 70% silica. The author claims rapid
precipitation/sedimentafion of large silica particles.
Ibrahim (1988) indicates that precipitation of
silica from black liquor is most effective if the
black liquor is preconcentrated to at least 8%
solids. Either CaO or CO2 would precipitate over
95%
of the silica under these conditions. (The
concentration is often about 4% solids off the
brown stock washers.) Long settling times (6
hours) were needed. The long time period often
means that the pH drops and lignin may continue
to precipitate. The precipitate should be washed
to recover usefiil alkali. Centrifugal separation of
the silica precipitate was the most efficient precipi-
tation method. The best precipitation of silica was
achieved at pH 9—10 (at 50°C) with a flue gas
(with CO2 concentration of 6—8%) application of
50—150 m^ per m^ of black liquor.
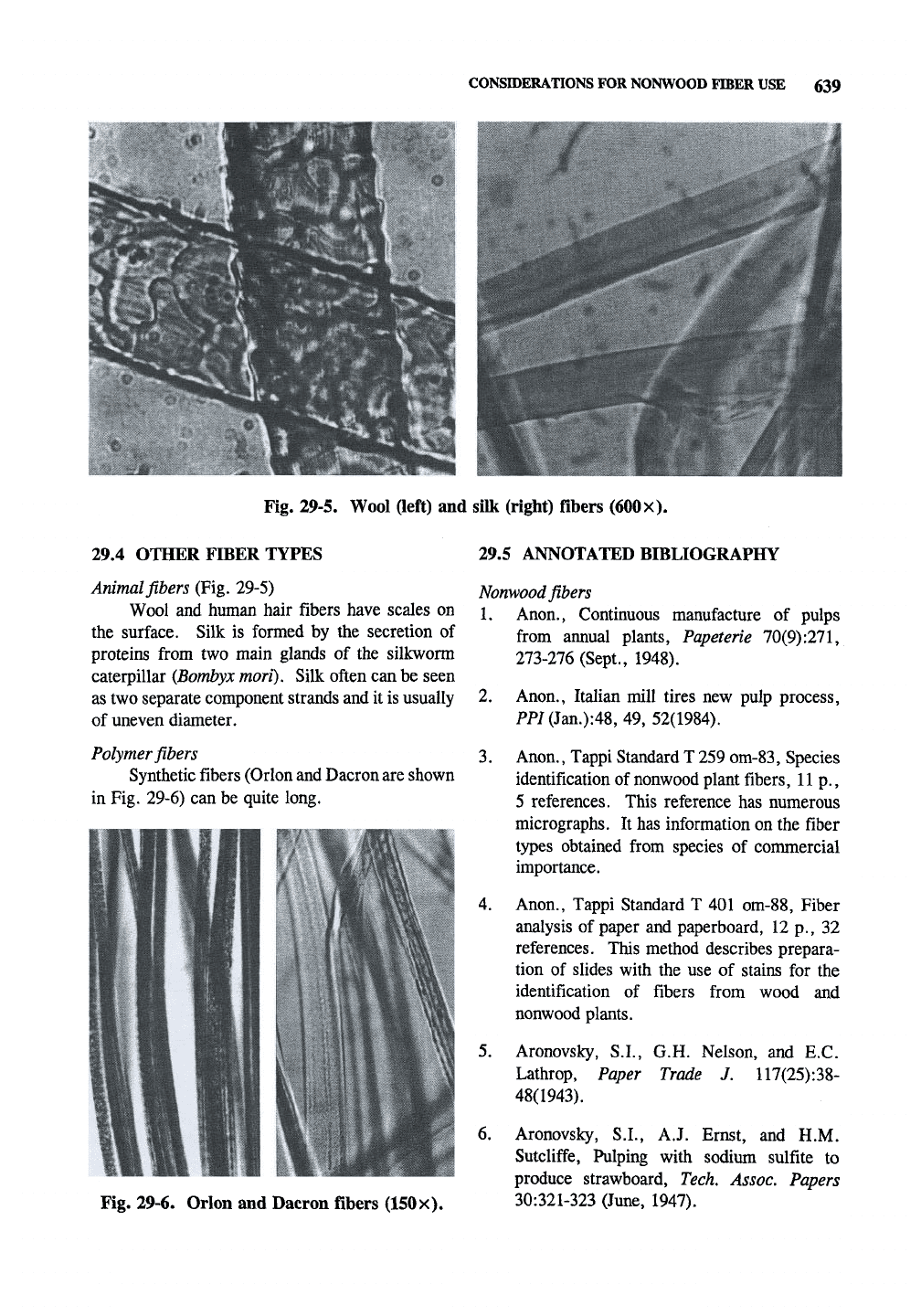
CONSIDERATIONS FOR NONWOOD FIBER USE 639
:
mM.
Fig. 29-5. Wool (left) and silk (right) fibers
(600
x).
29.4 OTHER FIBER TYPES
Animal fibers (Fig. 29-5)
Wool and human hair fibers have scales on
the surface. Silk is formed by the secretion of
proteins from two main glands of the silkworm
caterpillar (Bombyx mori). Silk often can be seen
as two separate component strands and it is usually
of uneven diameter.
Polymer fibers
Synthetic fibers (Orion and Dacron
are
shown
in Fig. 29-6) can be quite long.
Fig. 29-6. Orion and Dacron fibers (150x).
29.5 ANNOTATED BIBLIOGRAPHY
Norwood fibers
1.
Anon., Continuous manufacture of pulps
from annual plants, Papeterie 70(9):271,
273-276 (Sept., 1948).
2.
Anon., Italian mill tires new pulp process,
PP/(Jan.):48, 49, 52(1984).
3.
Anon., Tappi Standard T 259
om-83,
Species
identification of nonwood plant fibers, lip.,
5 references. This reference has numerous
micrographs. It has information on the fiber
types obtained from species of commercial
importance.
4.
Anon., Tappi Standard T 401 om-88. Fiber
analysis of paper and paperboard, 12 p., 32
references. This method describes prepara-
tion of slides with the use of stains for the
identification of fibers from wood and
nonwood plants.
5.
Aronovsky, S.I., G.H. Nelson, and E.G.
Lathrop, Paper Trade J, 117(25):38-
48(1943).
6. Aronovsky, S.I., A.J. Ernst, and H.M.
Sutcliffe, Pulping with sodium sulfite to
produce strawboard, Tech, Assoc, Papers
30:321-323 (June, 1947).

640 29. NONWOOD FIBER USE IN PULP AND PAPER
7.
Aronovsky, S.I., G.H. Nelson, A.J. Ernst,
H.M. Sutcliffe and E.G. Lathrop, Paper
Trade J.
127(11):
154-162(1948) and Tech,
Assoc. Papers 31:291-299(1948).
8. Aronovsky, S.I. and E.G. Lathrop, A new
mechano-chemical process for pulping agri-
cultural residues,
Tappi
32(4):145-149(April,
1949).
9. Atchison, J.E., World capacities for
nonwood plant fiber pulping increasing faster
than wood pulping capacities,
Tappi
Proceed-
ings, 1988
Pulping
Conference,
pp 25-45.
10.
Gasey, J.P., Pulp and Paper:
Chemistry
and
Chemical Technology, 2nd, Ed., Vol. 1,
Interscience, New York, 1960. Pages 398-
425 discuss the pulping of various nonwood
fibers.
11.
Geragioli, G., Wheat straw pulping by alkali-
oxygen processes—cooking variables and
pulp processes, pp. 227-252 (1975).
12.
Dean, J. A.,
Lange 's Handbook
of
Chemistry,
13th ed., McGraw-Hill, New York, 1985.
13.
Delga, J., Ghemical composition of the stalks
of cereals, Mem. services
chim.
etat (Paris)
33:7-73 (1947).
14.
Fahmy, Y. and M. Fadl, Digestion of wheat
straw for the economical production of puri-
fied pulps of low silica content, Textil-
Rundschau 13(12):709-719(1958).
15.
Fahmy, Y. and M. Fadl, Solubility of ash
and silicic acid during alkaline pulping of
cereal straws. Das Papier 13(13/14):311-
314(1959).
16.
FAG,
Tropical Woods and Agricultural
Resi-
dues as Sources of
Pulp,
Rome, Italy 1952,
190 p. Sulfite pulping of Parana-pine (23
p.),
chemical pulps from Australian eucalyp-
tus (6 p.), and agricultural residues (47 p.)
are some highlights of this work.
17.
Grubshein, B.D., Recovery of lime in the
alkaline pulping of straw and reed, Bumazh.
Prom.
36(8):
12-13(1961).
Russian.
18.
Gruen, B. H. E., U.S. patent 2,628,155
(Feb.
10, 1953).
19.
Huamin, Z., Separation of fibrous cells and
parenchymatous cells from wheat straw and
the characteristics in soda-AQ pulping, 1988
International
Non-wood Fiber Pulping and
Papermaking
Conference,
Ghina,
pp
469-478.
20.
Misra, D.K., Pulping and bleaching of
nonwood fibers, in Pulp and Paper:
Chemis-
try and Chemical Technology, Vol. 1, 3rd
ed., Gasey, J.P., Ed., Wiley, New York,
1980,
pp. 504-568.
21.
Petersen, P.B., Industrial application of
straw, 1991 Wood and Pulping Chemistry,
Tappi Press, Atlanta, pp. 179-183.
22.
Stephenson, J.N., Ed. Preparation of Stock
for Paper Making, Vol. 2, McGraw-Hill,
New York, 1951. Pages 1-91 discuss pulp-
ing of nonwood fibers.
Silica and
kraft
liquor recovery
23.
Grubshein, B.D., Recovery of lime in the
alkaline pulping of straw and reed, Bumazh.
Prom.
36(8):
12-13(1961).
Russian.
24.
Ibrahim, H., Silica is no longer a problem in
the recovery of heat and chemicals from
nonwood plant fibers black liquor, 1988
International
Non-wood Fiber Pulping and
Papermaking
Conference,
Ghina,
pp
877-889.
25.
Lengyel, P., Silica removal from black
liquors, Zellstojfu. Pfl/?/^r 9(3):89-94(1960).
26.
Sawheny, R.S., Desilicanization of black
liquor-a
glance,
1988
International Non-wood
Fiber
Pulping
and
Papermaking
Conference,
Ghina, pp 933-942.
27.
Schwalbe, G.G., Process for desalination of
alkaline silicious spent liquors, German
Patent 522,730 (1929).
30
HYDRAULICS
30.1 FLOW OF LIQUIDS
Introduction
The material in this section is examined in
more detail in many introductory works such as
McGill (1980). Related concepts have been
presented elsewhere, such as the static head of a
headbox (page 213).
Reynolds number
The flow of liquids in pipes can be difficult
to describe mathematically. In order to simplify
the mathematical development, flow is often
considered in circular—cross—section pipe (as is
done here). The Reynolds number is an important
parameter used to predict the flow pattern in a
pipe.
The Reynolds number is defined as:
Re^
Du.p
where
Re
D
= Reynolds number
= inside diameter of pipe
= bulk fluid velocity
= fluid viscosity
If the Reynolds number is below 2000, the
flow tends to be laminar or streamline; that is,
the pressure and flow velocity at any point remains
constant with time. Each point on a concentric
circle has the same pressure and flow velocity.
The flow velocity is highest at the center point.
If the Reynolds number is above 2000, the
flow tends to be unstable or
turbulent;
that is, the
flow velocity at a given point is not constant with
time,
although there may be a layer of laminar
flow near the pipe wall. At high Reynolds num-
bers the flow becomes quite turbulent.
The addition of a small amount of fiber to
water (on the order of
0.3%
consistency although
it can be higher at higher flow velocities) may
actually reduce friction compared to water alone.
When this is true, the plug of fibers tends to flow
in the center of the pipe, while a clear water
annulus flows near the edge of the pipe.
Flow
in
pipes
For laminar flow in smooth—walled, straight,
circular
pipes,
the Hagen—Poiseuille law gives the
flow rate j2 as a function of the pressure gradient
across the length of the pipe (AP), the pipe radius
(r),
the pipe length (L), and the fluid viscosity (jx):
Q =
irAPr^/iSiiL)
The Darcey equation gives frictional losses
(h) as a function of frictional factor (f), pipe
length (L), average velocity (v), and acceleration
due to gravity (g) for flow in circular pipes of
diameter/) as:
h=fLvV(2Dg)
For Reynolds number below 2000, / has been
empirically shown to be equal to 64/Re.
Water hammer
Water hammer is the sudden change in
pressure caused by a column of water that sudden-
ly changes velocity (as when a valve is closed).
30.2 PUMP BASICS
Introduction
Pumps may be considered as positive—dis-
placement or dynamic types. Dynamic pumps
usually rely on centrifugal force. Centrifugal
pumps are the most common type of dynamic
pump and the most common type of pump used in
pulp and paper mills.
Cavitation
and related problems
The inlet of any pump operates at a lower
pressure than the source of the liquid; it is called
the suction side of the pump. This is what causes
the liquid to flow into the pump. If the pressure
at the suction side of the pump drops below the
vapor pressure of the liquid, gas bubbles (cavities)
will form that interfere with the proper operation
of the pump. The gas bubbles collapse on the
output side of the pump where the pressure is
high, making much noise and causing undue wear.
This condition is known as cavitation.
641

642 30. HYDRAULICS
Cavitation is aggravated by using liquids at
high temperatures where their vapor pressure is
high, by using filters with high pressure drops, or
by using inlet lines that have high pressure drops
at the operating flow rates.
Cavitation can be alleviated by having a
generous geodetic height for the liquid entering the
pump; this is accomplished by lowering the pump
or raising the reservoir. Inlet pipes and filters
with larger inside diameters will decrease the
frictional and velocity pressure drops. If the flow
rate is pulsating with a period less than a few
seconds, the use of a dampening system can level
the flow rate at the inlet. In some cases the liquid
in the reservoir can be padded with a pressurized
inert gas, although this can lead to bubble forma-
tion downstream (much like the bends that may
occur with scuba divers). Booster pumps may be
used to solve difficult cavitation problems.
The use of an adequate NPSH will avoid
cavitation. Much difficulty and premature wear
encountered in the use of pumps are the result
of an inadequate NPSH.
NPSH,
NPSHA, NPSHR
NPSH, the net positive suction
head,
is the
absolute suction head minus the vapor pressure of
the liquid being pumped. NPSHA is the net
positive suction head available and must be greater
than NPSHR, the net positive suction head re-
quired. NPSH increases as the square of the
pump flow rate near and above the design flow
rate of a pump. It does not increase this fast at
flow rates much below the rated flow rate.
Good design practices on the inlet piping help
insure an adequate NPSH. Suction piping should
be at least twice the diameter of the pump inlet
nozzle. Pump inlet velocities should be below 10
ft/s. Place pipe elbows at least 5—10 pipe diame-
ters from the pump inlet, with the lower ratio
applicable to large pipe diameters. When this is
impossible, the use of rotating vanes before the
elbow can solve problems. Inlet screening devic-
es,
if used, must not have high pressure drops.
High elevation reduces atmospheric pressure
and NPSHA. Every 1000 feet of elevation reduc-
es NPSHA the equivalent of 1.1 feet of water
head.
Pump selection
One must consider the properties of the fluid
to be controlled (abrasiveness, viscosity, corro-
siveness, and specific gravity), the flow rates in-
volved, the pressure of the system, the tempera-
ture range and gradient to which the pump will be
exposed, and the length and frequency of cycling.
The suction conditions are also very important. If
a pump is being replaced, consider the reason the
old pump is no longer suited to the job.
30.3 POSITIVE DISPLACEMENT PUMPS
Positive displacement pumps actually enclose
the fluid to be moved through the system. The
pressure and volume at which the liquid can be
discharged depend only upon the mechanical
strength and size of the properly operating pump
and the energy supplied to the pump (neglecting
leakage). Pressure relief valves are used to
prevent damage to the system or buildup of high,
dangerous pressures. Priming of positive displace-
ment pumps may or may not be required.
Reciprocating and
diaphragm
pumps
Reciprocating pumps use a piston moving
back and forth in a cylinder (see Fig. 30-16).
Two check valves for the input and output at one
end of the cylinder allow one—way flow; this
gives a pulsed output. Because rotational motions
of engines are not easily converted to reciprocating
motions, this type of pulp is seldom used on
mobile equipment. Two cylinders can be used to
give a continuous output as in pumps for high-
performance liquid chromatography, or one cylin-
der can be made to deliver with either stroke. The
diaphragm pump (Fig. 30-1) is similar in princi-
ple,
except that the piston assembly is replaced by
a diaphragm and housing; diaphragm pumps are
often powered by compressed air and occur as
double—diaphragm pumps.
Rotary
pumps
(gear
and lobe types)
Rotary pumps use a variety of means to
generate flow. A pair of gears with many teeth
(external to the gear and called an external gear
pump) in gear pumps or a few lobes in
lobe
pumps
is demonstrated in Fig. 30-2. Internal gear pumps
use a gear within a gear design; internal means
the teeth of one gear are on the inside, projecting
inward. Gear pumps are useful for pumping
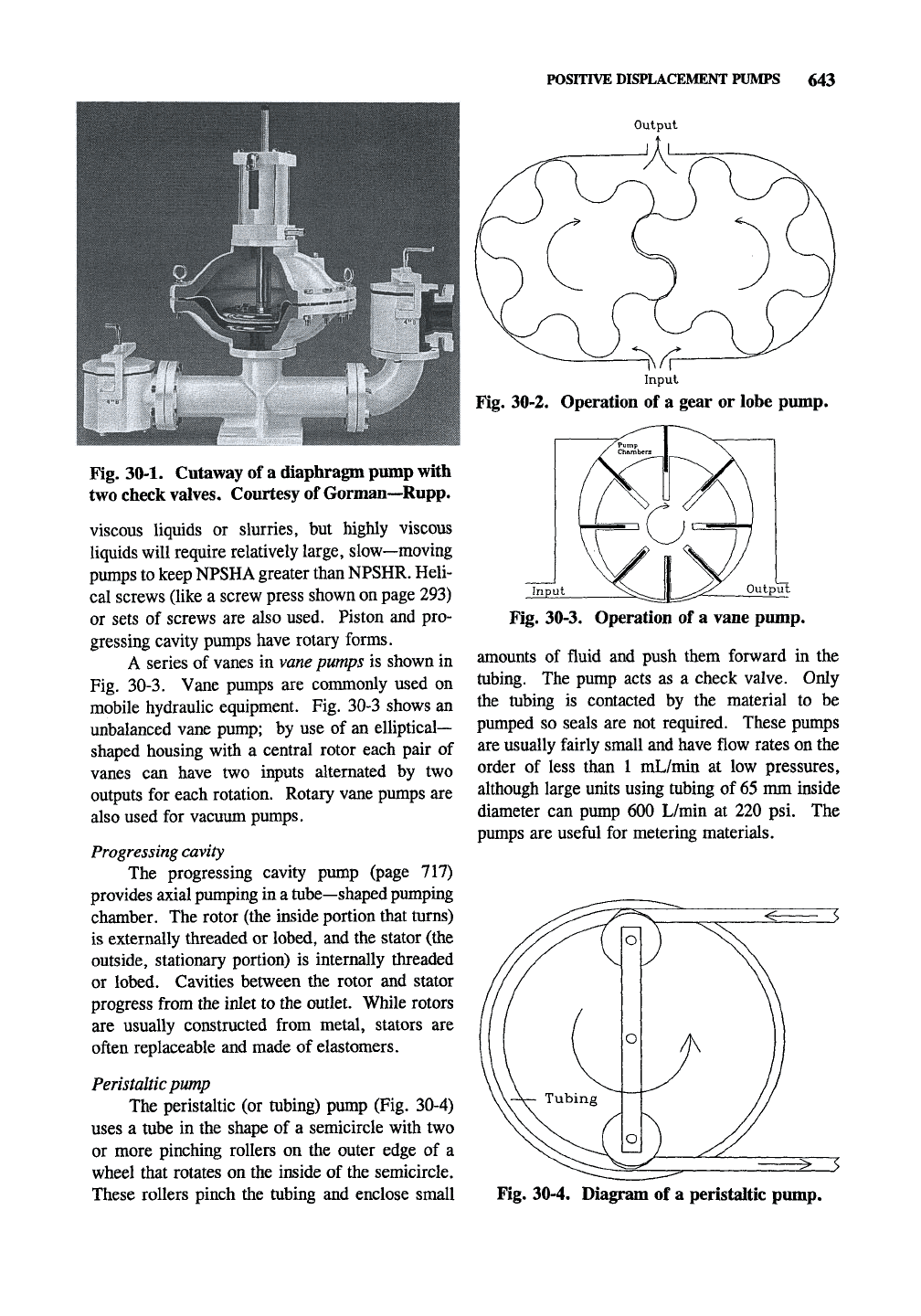
POSITIVE DISPLACEMENT PUMPS 643
Output
Fig. 30-2. Operation of a gear or lobe pump.
Fig. 30-1. Cutaway of a diaphragm pump with
two check valves. Courtesy of Gorman—Rupp.
viscous liquids or slurries, but highly viscous
liquids will require relatively large, slow—moving
pumps to keep NPSHA greater than NPSHR. Heli-
cal screws (like a screw press shown on page 293)
or sets of screws are also used. Piston and pro-
gressing cavity pumps have rotary forms.
A series of vanes in
vane
pumps is shown in
Fig. 30-3. Vane pumps are commonly used on
mobile hydraulic equipment. Fig. 30-3 shows an
unbalanced vane pump; by use of an elliptical-
shaped housing with a central rotor each pair of
vanes can have two inputs alternated by two
outputs for each rotation. Rotary vane pumps are
also used for vacuum pumps.
Progressing cavity
The progressing cavity pump (page 717)
provides axial pumping in a tube—shaped pumping
chamber. The rotor (the inside portion that turns)
is externally threaded or lobed, and the stator (the
outside, stationary portion) is internally threaded
or lobed. Cavities between the rotor and stator
progress from the inlet to the outlet. While rotors
are usually constructed from metal, stators are
often replaceable and made of elastomers.
Peristaltic pump
The peristaltic (or tubing) pump (Fig. 30-4)
uses a tube in the shape of a semicircle with two
or more pinching rollers on the outer edge of a
wheel that rotates on the inside of the semicircle.
These rollers pinch the tubing and enclose small
Fig. 30-3. Operation of a vane pump.
amounts of fluid and push them forward in the
tubing. The pump acts as a check valve. Only
the tubing is contacted by the material to be
pumped so seals are not required. These pumps
are usually fairly small and have flow rates on the
order of less than 1 mL/min at low pressures,
although large units using tubing of
65
mm inside
diameter can pump 600 L/min at 220 psi. The
pumps are useful for metering materials.
Fig. 30-4. Diagram of a peristaltic pump.
