Bhadeshia H.K.D.H., Honeycombe R. Steels: Microstructure and Properties
Подождите немного. Документ загружается.

228 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
(a)
(b)
(c)
(d)
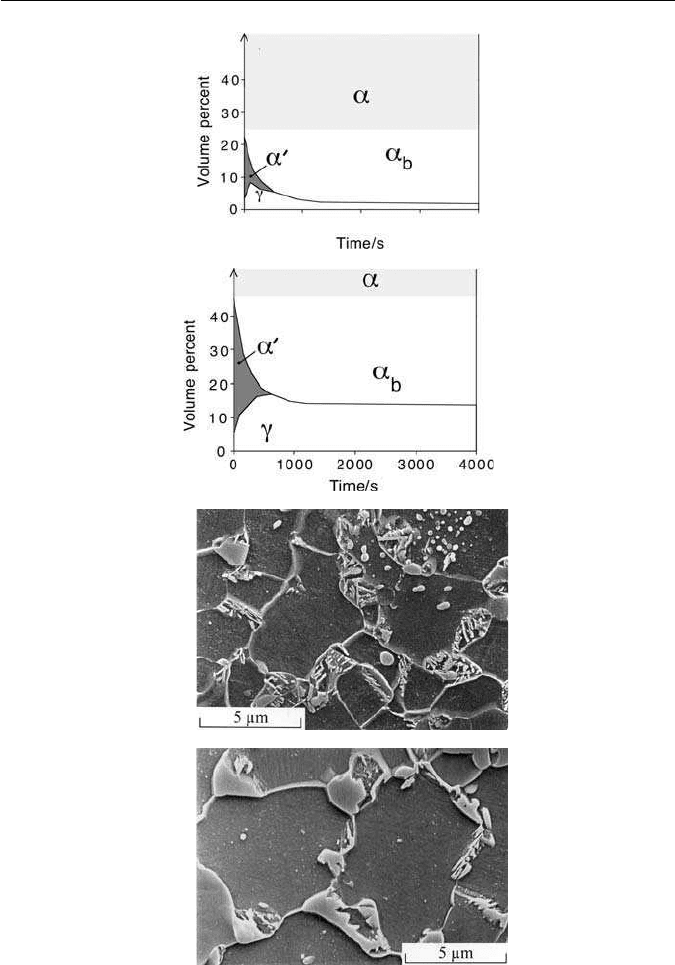
Fig. 10.16 Evolution of room-temperature microstructure as a function of time during
isothermal transformation to bainite. (a) Low-silicon steel Fe–0.16C–0.38Si–1.3Mn wt%.
(b) High-silicon steel Fe–0.29C–1.41Si–1.42Mn wt%. (c) Scanning electron micrograph of
low-silicon alloy isothermally transformed to bainite for 1800 s. Much of the austenite has
decomposed to bainitic ferrite and cementite. (d) Corresponding micrograph for high-silicon
steel transformed to bainite for 900 s with plenty of austenite evident (courtesy of Pascal
Jacques).

10.5 TWIP STEELS 229
MnO begins to cover the surface leading to a deterioration in the ability of the
liquid zinc to wet the surface. The aluminium-containing steels are therefore
better suited to continuous galvanizing lines.
It is clear that silicon and manganese must diffuse to the surface to form
oxides. Some of the diffusion flux is via grain boundaries. When phosphorus
is present, its segregation to the grain boundaries reduces the boundary
diffusion-flux, thereby reducing the extent to which oxides form. Such steels
are more amenable to wetting by molten zinc (in contrast, the same effect
makes it more difficult to form iron–zinc compounds during the galvannealing
process).
10.5 TWIP STEELS
There are three essential modes by which steels can be permanently deformed
at ambient temperature, without recourse to diffusion. Individual dislocations
whose Burgers vectors correspond to lattice vectors can glide, leading to a
change in shape without altering the crystal structure or volume. In contrast, a
displacive transformation (e.g. martensite or bainite) results not only in a plastic
strain, but also a change of crystal structure and density; this is the phenomenon
exploited in the TRIP steels.
The third mode of deformation is mechanical twinning, in which the crystal
structure of the steel is preserved but the twinned region is reoriented in the
process. Mechanical twinning results in a much larger shear strain s =1/
√
2,
compared with displacive transformations where s is typically 0.25. There is a
particular class of extraordinarily ductile alloys of iron, known as the TWIP
steels, which exploit mechanical twinning to achieve their properties.
TWIP stands for twinning-induced plasticity. The alloys are austenitic and
remain so during mechanical deformation, but the material is able to accommo-
date strain via both the glide of individual dislocations and through mechanical
twinning on the {111}
γ
< 11
2 >
γ
system. The alloys typically contain a
large amount of manganese, some aluminium and silicon (e.g. Fe–25Mn–3Si–
3Al wt%) with carbon and nitrogen present essentially as impurities. Larger
concentrations of carbon may be added to enhance strength. At high man-
ganese concentrations, there is a tendency for the austenite to transform into
ǫ-martensite (hexagonal close packed) during deformation. ǫ-martensite can
form by the dissociation of a perfect a/2 < 011>
γ
dislocation into Shockley
partials on a close packed {11
1}
γ
plane, with a fault between the partials. This
faulted regionrepresents a three layer thick plate of ǫ-martensite.A reduction in
the fault energy therefore favours the formation of this kind of martensite. The
addition of aluminium counters this because it raises the stacking fault energy
of the austenite. Silicon has the opposite effect of reducing the stacking fault
energy, but like aluminium, it leads to a reduction in the density of the steel;
the combination of Al and Si at the concentrations used typically reduces the
overall density from some 7.8 g cm
−3
to about 7.3 g cm
−3
.
230 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
(a)
(b)
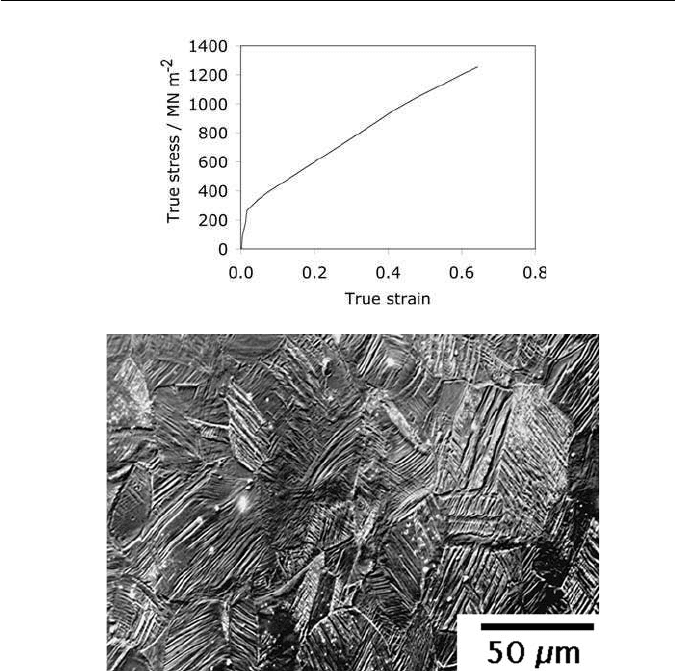
Fig. 10.17 (a) Typical stress–strain curve for a TWIP steel. (b) Optical microstructure of
a TWIP steel following deformation, showing profuse twinning (image and data courtesy of
Frommeyer, G., Brüx, U. and Neumann, P).
The alloys have a rather low yield strength at 200–300MN m
−2
but the
ultimate tensile strength can be much higher, in excess of 1100 MN m
−2
. This
is because the strain-hardening coefficient is large, resulting in a great deal
of uniform elongation, and a total elongation of some 60–95%. The effect
of mechanical twinning is two-fold. The twins add to plasticity, but they also
have a powerful effect in increasing the work-hardening rate by subdividing the
untwinned austenite into finer regions (Fig. 10.17).
One major advantage of TWIP steels is that they are austenitic and they
maintain attractive properties at cryogenic temperatures (−150
◦
C) and high
strain rates, e.g., 10
3
s
−1
. They therefore have great potential in enhancing the
safety of automobiles by absorbing energy during crashes.

10.6 STEELS SUBJECTED TO THERMOMECHANICAL TREATMENTS 231
10.6 INDUSTRIAL STEELS SUBJECTED TO THERMOMECHANICAL
TREATMENTS
Micro-alloyed steels produced by controlled rolling are a most attractive prop-
osition in many engineering applications because of their relatively low cost,
moderate strength, and very good toughness and fatigue strength, together with
theirability to bereadilywelded.They have,to a considerabledegree,eliminated
quenched and tempered steels in many applications.
These steels are most frequently available in control-rolled sheet, which is
then coiled over a range of temperatures between 750
◦
C and 550
◦
C.The coiling
temperature has an important influence as it represents the final transformation
temperature, and this influences the microstructure. The lower this temperature,
under the same conditions, the higher the strength achieved. The normal range
of yield strength obtained in these steels varies from about 350 to 550 MN m
−2
(50–80 ksi). The strength is controlled both by the detailed thermomechanical
treatment, by varying the manganese content from 0.5 to 1.5 wt%, and by using
the micro-alloying additions in the range 0.03 to above 0.1 wt%. Niobium is used
alone, or with vanadium, while titanium can be used in combination with the
other two carbide-forming elements. The interactions between these elements
are complex,but in general terms niobium precipitates more readily in austenite
than does vanadium as carbide or carbo-nitride, so it is relatively more effective
as a grain refiner. The greater solubility of vanadium carbide in austenite under-
lines the superior dispersion strengthening potential of this element shared to a
lesser degree with titanium. Titanium also interacts with sulphur and can have
a beneficial effect on the shape of sulphide inclusions. Bearing in mind that the
total effect of these elements used in conjunction is not a simple sum of their
individual influence, the detailed metallurgy of these steels becomes extremely
complex.
One of the most extensive applications is in pipelines for the conveyance
of natural gas and oil, where the improved weldability due to the overall lower
alloying content (lower hardenability) and, particularly, the lower carbon levels
is a great advantage. Furthermore, as the need for larger diameter pipes has
grown, steels of higher yield stress have to be used to avoid excessive wall
thicknesses. In practice, wall thicknesses of 10–12.5 mm have been found to be
the most convenient. Typical compositions (wt%) to achieve a yield stress of
around 410 MN m
−2
(60 ksi):
C 0.12 S 0.012 Mn 1.35 Nb 0.03
C 0.12 S 0.006 Mn 1.33 Nb 0.02 V 0.04
for higher yield strengths (450 MN m
−2
):
C 0.06 S 0.006 Mn 1.55 Nb 0.05 V 0.10
However, it should be emphasized that often higher yield stresses are
achieved by control of the fabrication variables such as the temperature at which

232 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
Table 10.1 Typical compositions of micro-alloyed vanadium steels
Element Typical composition (%)
Yield strength 550 (MN m
−2
) (80 ksi)
345 (MN m
−2
) (50 ksi)
Carbon 0.08–0.12 0.12–0.17
Manganese 0.75–1.10 1.20–1.55
Phosphorus 0.008–0.013 0.008–0.013
Sulphur 0.007–0.020 0.007–0.020
Silicon 0.05–0.15 0.30–0.55
Aluminium 0.03–0.06 0.03–0.06
Vanadium 0.03–0.07 0.10–0.14
Nitrogen 0.006–0.012 0.015–0.022
Cerium 0.02–0.06 0.02–0.06
rolling is finished and the temperature used for coiling the sheet. Nitrogen is
often deliberately used as an alloying element. One successful range of steels
relies on vanadium to form carbo-nitride precipitates for grain size control and
dispersion strengthening. In some steels, rare earth additions are made to con-
trol the inclusion shape.Typical compositions at lower and higher strength levels
are given in Table 10.1.
At the higher strength levels, micro-alloyed steels are used for heavy duty
truck frames, tractor components, crane booms and lighting standards, etc. The
control of sulphide inclusions gives the steels a high degree of formability in cold
fabrication processes. This recent development has allowed the use of HSLA
steels for many applications involving substantial cold forming which previously
led to cracking in the absence of rare earth additions.
A steel is said to have been ausformed when martensite is produced from
plastically deformed austenite Ausforming has provided some of the strongest,
toughest steels so far produced, with the added advantage of very good fatigue
resistance. However, they usually have high concentrations of expensive alloy-
ing elements and must be subjected to large deformations which impose heavy
work loads on rolling mills. Nevertheless, these steels are particularly useful
where a high strength to weight ratio is required and where cost is a secondary
factor. Typical applications have included parts for undercarriages of aircraft,
special springs and bolts.
The 12 wt% Cr transformable steels respond readily to ausforming to the
extent that tensile strengths of over 3000 MN m
−2
(>200 tsi) can be obtained in
appropriate compositions.A 0.4C–6Mn–3Cr–1.5Si steelhas beenausformed to a
tensile strength of 3400 MN m
−2
, with an improvement in ductility over the con-
ventional heat treatment. Similar high strength levels with good ductility have
been reported for0.4C–5Cr–1.3Mo–1.0Si–0.5V wt% steel (H11)(Fig. 10.18).All
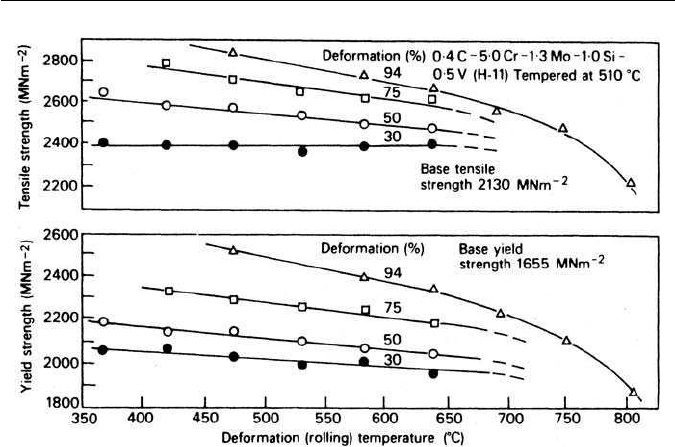
FURTHER READING 233
Fig. 10.18 Effect of amount and temperature of deformation on the yield and tensile strength
of 0.4C–5.0Cr–1.3Mo–1.0Si–0.5V wt% steel (H11) (Zackay, in National Physical Laboratory
Symposium, No. 15, HMSO, London, UK, 1963).
of these steels are sufficiently highly alloyed to allow adequate time for substan-
tial deformation in the austenitic bay of the time–temperature–transformation
curve prior to transformation.
FURTHER READING
Bhadeshia, H. K. D. H., TRIP-Assisted Steels? ISIJ International, 42, 1059, 2002.
Cooman, B. C. de, Structure–properties relationship in TRIP steels containing carbide-free
bainite, Current Opinion in Solid State and Materials Science 8, 285, 2004.
Davenport, A. T. (ed.), Formable HSLA and Dual-phase Steels, The Metallurgical Society of
AIME, USA, 1979.
Davies, G., Materials for Automobile Bodies, Elsevier, London, 2003.
Frommeyer, G., Brüx, U. and Neumann, P., Supra-ductile and high-strength manganese-
TRIP/TWIP steels for high energy absorption purposes, ISIJ International 43, 438, 2003.
Gladman,T., Physical Metallurgy of Microalloyed Steels, Institute of Materials, London, 1997.
HSLA Steels – Metallurgy and Applications. Proceedings of an International Conference,
Beijing, 1985, Chinese Society of Metals, ASM International.
International Conference on Processing, Microstructure and Properties of Microalloyed and
Other Modern Low Alloy Steels, Pittsburgh, 1991.
Jacques, P. J., Transformation-induced plasticity for high strength formable steels, Current
Opinion in Solid State and Materials Science 8, 259, 2004.

234 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
Kozasu, I., Processing – thermomechanical controlled processing in Materials Science and
Technology (eds Cahn, R. W., Haasen, P. and Kramer, E. J.), Vol. 7, Constitution and
Properties of Steels (ed. Pickering, F. B.), VCH,Weinheim, Germany, 1992.
Krauss, G. (ed.), Deformation, Processing and Structure, American Society for Metals, Ohio,
USA, 1984.
Maki, J., Mahieu, J., de Cooman, B. C. and Claessens, S., Galvanisability of silicon free CMnAl
TRIP steels, Materials Science and Technology 19, 125, 2003.
Physical Metallurgy of Thermo-mechanical Processing of Steels and other Metals (Thermec
88), Iron and Steel Institute of Japan, 1988.
Yokota, T., Garcia-Mateo, C., and Bhadeshia, H. K. D. H., Transformation-induced plasticity
for high strength formable steels, Scripta Materialia 51, 767, 2004.

11
THE EMBRITTLEMENT AND FRACTURE
OF STEELS
11.1 INTRODUCTION
Most groups of alloys can exhibit failure by cracking in circumstances where
the apparent applied stress is well below that at which failure would normally
be expected. Steels are no exception to this, and probably exhibit a wider var-
iety of failure mechanisms than any other category of material. While ultimate
failure under excessive stress must occur and can be reasonably predicted by
appropriate mechanical tests, premature failure is always dangerous, involving
a considerable element of unpredictability. However, a detailed knowledge of
structure and of the distribution of impurities in steels is gradually leading to a
much betterunderstanding of the origins and mechanisms of the various types of
cracks encountered. Furthermore, the now well-established science of fracture
mechanics allows the quantitative assessment of growth of cracks in various
stress situations, to an extent that it is now frequently possible to predict the
stress level to which steel structures can be confidently subjected without the
risk of sudden failure.
11.2 CLEAVAGE FRACTURE IN IRON AND STEEL
Cleavage fracture is familiar in many minerals and inorganic crystalline solids
as a crack propagation frequently associated with very little plastic deformation
and occurring in a crystallographic fashion along planes of low indices, i.e. high
atomic density. A low temperatures zinc cleaves along the basal plane, while
body-centred cubic (bcc) iron cleaves along {100} planes (Fig. 11.1), as do all
bcc metals. This behaviour would appear to be an intrinsic characteristic of iron,
but it has been shown that iron, highly purified by zone refining and containing
minimal concentrations of carbon, oxygen and nitrogen, is very ductile even at
235
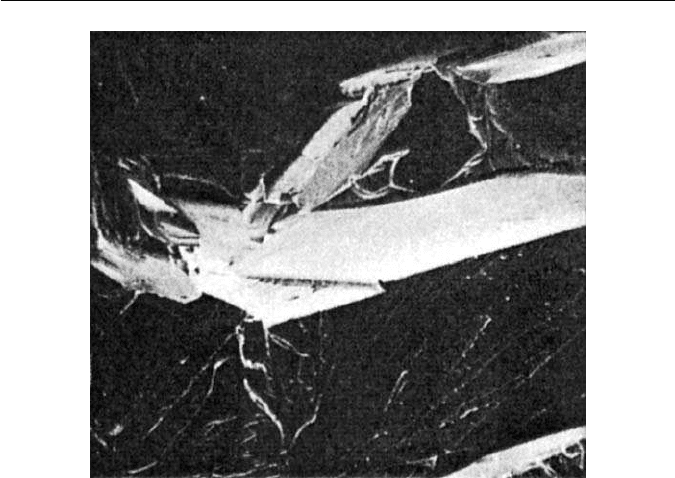
236 CHAPTER 11 THE EMBRITTLEMENT AND FRACTURE OF STEELS
Fig. 11.1 Cleavage fracture in pure iron–0.04 P at 55
◦
C (courtesy of Shell) ×60.
extremely low temperatures. For example, at 4.2 K reductions in area in ten-
sile tests of up to 90% have been observed with iron specimens of the highest
available purity. As the carbon and nitrogen content of the iron is increased, the
transition from ductile to brittle cleavage behaviour takes place at increasing
temperatures, until in some steels this can occur at ambient and above-ambient
temperatures. Clearly, the significant variables in such a transition are of great
basic and practical importance.
The propagation of a cleavage crack in iron and steel requires much less
energy than that associated with the growth of a ductile crack. This is easily
shown by carrying out impact tests in a pendulum apparatus (Charpy, Izod and
Housfield type tests) over a range of temperature. The energy absorbed by the
specimen from the pendulum when plotted as a function of temperature usually
exhibits a sharp change in slope (Fig. 11.2) as the mode of fracture changes from
ductile to brittle. These impact transition curves are a simple way of defining
the effect of metallurgical variables, e.g. heat treatment (Fig. 11.2) on the frac-
ture behaviour of a steel from which a fairly precise transition temperature, T
c
,
can be readily obtained for a particular heat treatment. However, it should be
emphasized that T
c
is not an absolute value and it is likely to change appre-
ciably as the mode of testing is altered. It nevertheless provides a simple way of
comparing the effects of metallurgical variables on the fracture behaviour.
More sophisticated tests have been developed in which it is recognized that
the propagation of the fracture is the important stage. These fracture toughness
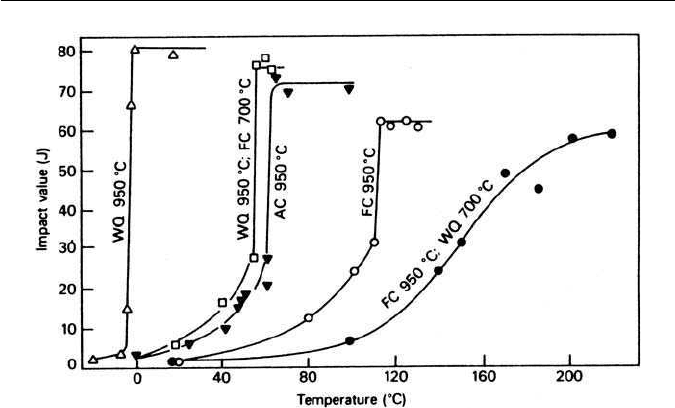
11.3 FACTORS INFLUENCING THE ONSET OF CLEAVAGE FRACTURE 237
Fig. 11.2 Effect of heat treatments on the impact transition temperature of a pure
iron–0.12C wt% alloy (after Allen et al., Journal of Iron and Steel Institute 174, 108, 1953). AC, FC
and WQ represent air-cooling, furnace-cooling and water-quenching, respectively.
tests used notched and pre-cracked specimens, the cracks being initiated by
fatigue. The stress intensity factor, K, at the root of the crack is defined in terms
of the applied stress σ and the crack size c:
K = σ(πc)
1/2
.
When a critical stress intensity K
c
is reached, the transition to rapid fracture
takes place.
11.3 FACTORS INFLUENCING THE ONSET OF CLEAVAGE
FRACTURE
There are several factors, some interrelated, which play an important part in the
initiation of cleavage fracture:
1. The temperature dependence of the yield stress.
2. The development of a sharp yield point.
3. Nucleation of cracks at twins.
4. Nucleation of cracks at carbide particles.
5. Grain size.
All bcc metals including iron shown a marked temperature dependence of
the yield stress, even when the interstitial impurity content is very low, i.e. the
stress necessary to move dislocations, the Peierls–Nabarro stress, is strongly
