Bhadeshia H.K.D.H., Honeycombe R. Steels: Microstructure and Properties
Подождите немного. Документ загружается.


218 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
stuck at about 1 µm. The reason is recalescence, which is the rise in tempera-
ture of the steel caused by release of the latent heat of transformation at a rate
which is so high that it cannot easily be dissipated by diffusion. It causes the
temperature of the steel to rise, thus reducing G
γα
V
and preventing the achieve-
ment of ultrafine grain structures. Large-scale thermomechanical processing is
therefore limited by recalescence and is unlikely to lead to grain sizes which are
uniformly less than about 1 µm.
10.2.4 Dispersion strengthening during controlled rolling
The solubility data imply that, in a micro-alloyed steel, carbides and carbo-
nitrides of Nb, Ti and V will precipitate progressively during controlled rolling
as the temperature falls. While the primary effect of these fine dispersions is
to control grain size, dispersion strengthening will take place. The strength-
ening arising from this cause will depend both on the particle size r, and the
interparticle spacing which is determined by the volume fraction of precipi-
tate (Equation (2.10)). These parameters will depend primarily on the type of
compound which is precipitating, and that is determined by the micro-alloying
content of the steel. However the maximum solution temperature reached and
the detailed schedule of the controlling rolling operation are also important
variables.
It is now known, not only that precipitation takes place in the austenite,
but that further precipitation occurs during the transformation to ferrite. The
precipitation of niobium, titanium and vanadium carbides has been shown to
take place progressively as the interphase boundaries move through the steel.
This is the interphase precipitation discussed in Section 4.4.3. As this precipi-
tation is normally on an extremely fine scale occurring between 850
◦
C and
650
◦
C, it is likely to be the major contribution to the dispersion strengthening.
In view of the higher solubility of vanadium carbide in austenite, the effect
will be most pronounced in the presence of this element, with titanium and
niobium in decreasing order of effectiveness. If the rate of cooling through
the transformation is high, leading to the formation of supersaturated plates
of ferrite, the carbides will tend to precipitate within the grains, usually on the
dislocations which are numerous in this type of ferrite.
In arriving at optimum compositions of micro-alloyed steels, it should be
borne in mind that the maximum volume fraction of precipitate which can be
put into solid solution in austenite at high temperatures is achieved by use
of stoichiometric compositions. For example, if titanium (atomic weight 47.9)
is used, it will combine with approximately one quarter its weight of carbon
(atomic weight 12), so that for a 0.025 wt% C steel, 0.10 wt% of Ti will provide
carbide of the stoichiometric composition. In Fig. 10.9 the stoichiometric line
for TiC is shown superimposed on the solubility curves for titanium carbide at
1100
◦
C,1200
◦
C and 1300
◦
C. If the precipitation in steels with 0.10 wt% titanium
cooled from 1200
◦
C is considered, at low carbon contents, i.e. to the left of the
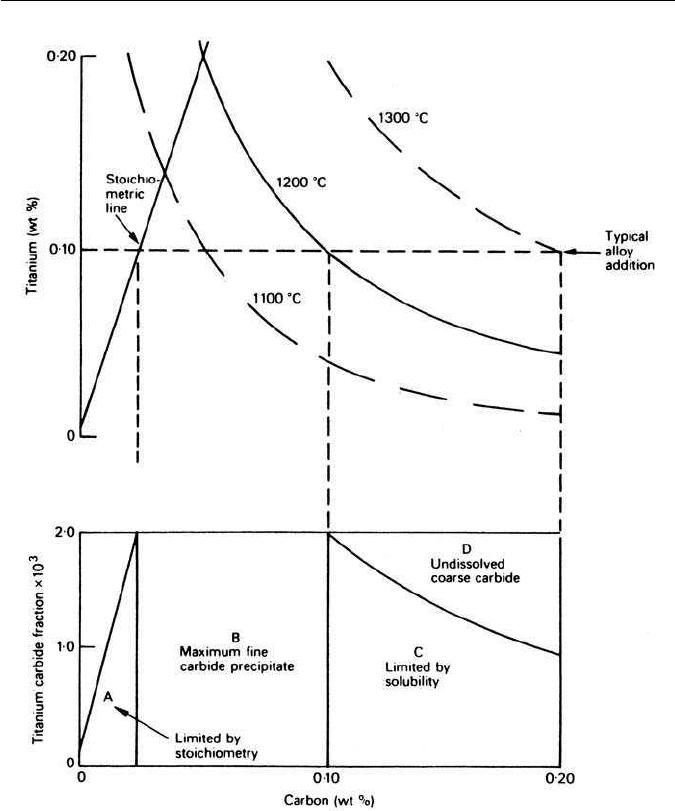
10.2 CONTROLLED ROLLING OF LOW-ALLOY STEELS 219
Fig. 10.9 Effect of stoichiometry on the precipitation ofTiC in a micro-alloyed steel (Gladman
et al., Micro-alloying 75, Union Carbide corporation, New York, USA, 1975).
stoichiometric line, the carbide fraction is limited by the carbon content, i.e.
zone A, lower diagram. For carbon contents between the stoichiometric line
and the solubility line at 1200
◦
C, the full potential volume fraction of fine TiC
will form on cooling (zone B). When the carbon content exceeds the solubility
limit (>0.10 wt%),the titanium is progressively precipitated at 1200
◦
C as coarse
carbide, thus reducing the amount of titanium available to combine with carbon
to form fine TiC during cooling. As coarse carbide particles are ineffective in

220 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
controlling grain growth, it is highly desirable to have steel compositions which
avoid their formations. It also follows from Fig. 10.9 that high austenitizing
temperatures are essential to obtain full benefit from the precipitation of finely
divided carbide phases.
10.2.5 Strength of micro-alloyed steels: an overall view
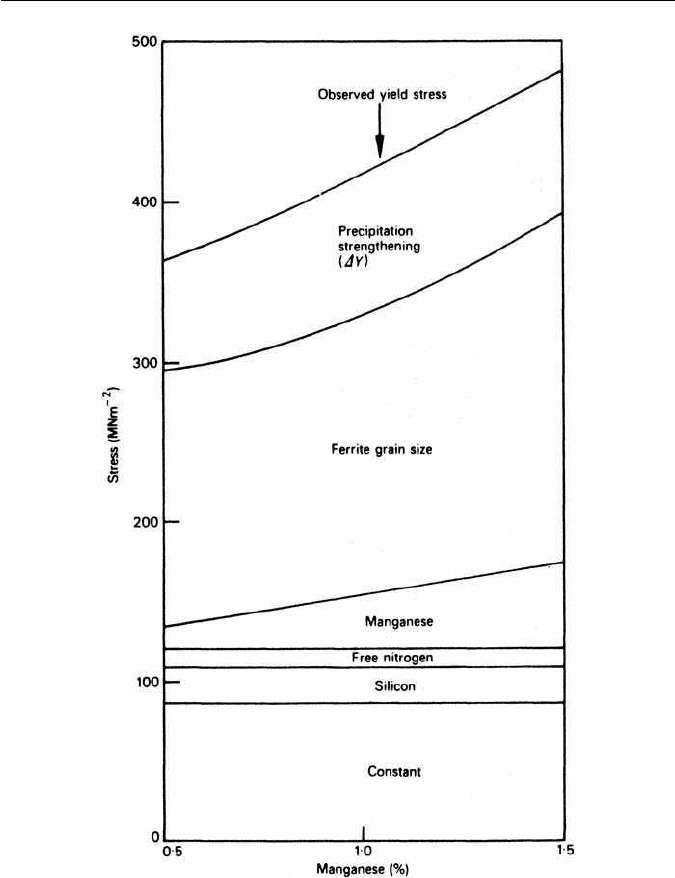
In modern control-rolled micro-alloyed steels, there are at least three strength-
ening mechanisms which contribute to the final strength achieved. The relative
contribution from each is determined by the composition of the steel and,
equally important, the details of the thermomechanical treatment to which
the steel is subjected. The several strengthening contributions for steels with
0.2 wt% carbon, 0.2 wt% silicon, 0.15 wt% vanadium and 0.015 wt% nitrogen
as a function of increasing manganese content are shown schematically in
Fig. 10.10. Firstly, there are the solid solution strengthening increments from
manganese, silicon and uncombined nitrogen. Secondly, the grain size contribu-
tion to the yield stress is shown as a very substantial component, the magnitude
of which is very sensitive to the detailed thermomechanical history. Finally, a
typical increment for dispersion strengthening is shown. The total result is a
range of yield strengths between about 350 and 500 MN m
−2
. In this particular
example, the steel was normalized (air cooled) from 900
◦
C,but had it been con-
trol rolled down to 800
◦
C or even lower, the strength levels would have been
substantially raised.
The effect of the finishing temperature for rolling is important in determin-
ing the grain size and, therefore, strength level reached for a particular steel.
It is now becoming common to roll through the transformation into the com-
pletely ferritic condition, and so obtain fine subgrain structures in the ferrite,
which provide an additional contribution to strength. Alternatively, the rolling
is finished above the γ/α transformation, and the nature of the transformation is
altered by increasing the cooling rate. Slow rates of cooling obtained by coiling
at a particular temperature will give lower strengths than rapid rates imposed
by water spray cooling following rolling. The latter route can change the ferrite
from equi-axed to Widmanstätten with a much higher dislocation density. The
result is a steel with improved mechanical properties and, in many cases, the
sharp yield point can be suppressed. This has practical advantages in fabrica-
tion of sheet steel, e.g. pipe manufacture, where a continuous stress–strain curve
is preferred.
10.3 DUAL-PHASE STEELS
The HSLA steels described in Section 10.2 give improved strength to weight
ratios over ordinary standard steels. However, they are not readily formed,
e.g. by cold pressing and related techniques. The worldwide demand for safety
10.3 DUAL-PHASE STEELS 221
Fig. 10.10 The contributions to strength in a 0.2C–0.15V wt% steel as a function of Mn
content (Gladman et al., Micro-alloying 75, Union Carbide Corporation, 1975).
and fuel economy in automobiles has led to the development of a number of
steel types which are not only strong, but at the same time have the formability
required for mass production of car bodies and components. A measure of
formability is the product of strength and uniform elongation.
222 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
(a)
(b)
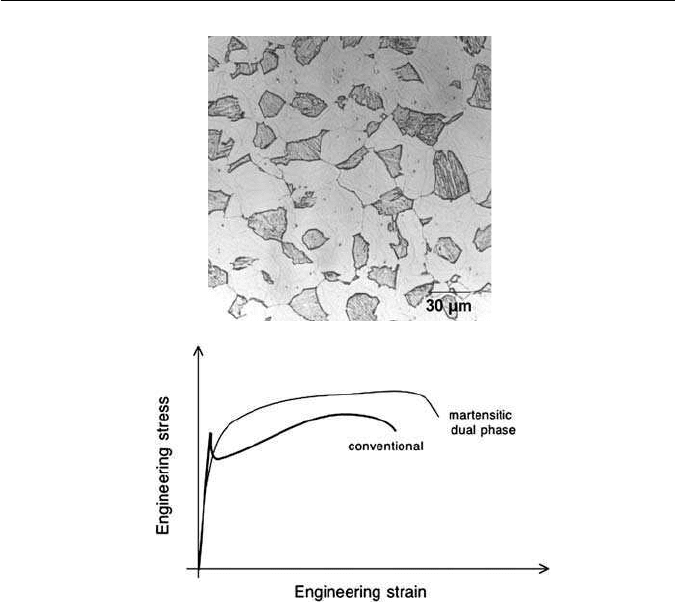
Fig. 10.11 (a)Typical microstructure of dual-phase steel, consisting of a mixture of martensite
(dark) and ferrite. (b) Schematic stress–strain curves comparing the behaviour of a conventional
automobile steel with that of a dual-phase steel.
The dual-phase steels are low-alloy steels which satisfy these requirements
by exploiting microstructures in which there are two major phases (Fig. 10.11),
one of which is soft and the other significantly harder. The ferrite–martensite
dual-phase steels typically contain manganese and silicon, and are strong and
yet are formable. They exhibit continuous yielding, i.e. no sharp yield point,
and a relatively low proof strength (300–350 MN m
−2
). The simplest steels in
this category contain 0.08–0.2C, 0.5–1.5Mn wt%, but steels micro-alloyed with
vanadium are also suitable, while small additions of Cr (0.5 wt%) and Mo (0.2–
0.4 wt%) are frequently used to control the development of microstructure.
The simplest way of achievinga duplex structure is to use intercritical anneal-
ing in which the steel is heated into the (α +γ) region between the Ae
1
and Ae
3
and held, typically at 790
◦
C for several minutes to allow small regions of austen-
ite to form in the ferrite. As it is essential to transform these regions of austenite
into martensite, cooling to ambient temperature must be sufficiently rapid to

10.4 TRIP-ASSISTED STEELS 223
avoid other intervening transformations. Alternatively, the hardenability of the
austenite must be enhanced by adding between 0.2 and 0.4 wt% Mo to a steel
already containing 1.5 wt% manganese. The required microstructure can then
be obtained by air cooling after intercritical annealing.
To eliminate an extra heat treatment step, dual-phase steels have now been
developed which can be given the required structure during cooling after con-
trolled rolling. Typically, these steels have additions of 0.5Cr and 0.4Mo wt%.
After completion of hot rolling around 870
◦
C, the steel forms approximately
80% ferrite on the water cooled run-out table from the mill. The material is
then coiled in the metastable region (510–620
◦
C) below the pearlite/ferrite
transformation and, on subsequent cooling, the austenite regions transform to
martensite.
10.4 TRIP-ASSISTED STEELS
The steels developed to exploit the properties obtained when the martensite
reaction occurs duringplastic deformation areknown astransformation-induced
plasticity (TRIP) steels. They are strong and exhibit considerable uniform elon-
gation before failure. There are several varieties of such steels. Those which are
made fully austenitic by using large quantities of austenite-stabilizing solutes,
but transform to martensite when stressed, are simply called the TRIP steels
(discussed in Chapter 12). When the austenite is a minor phase in the overall
microstructure, but undergoes martensitic transformation during straining, the
steels are said to be TRIP assisted and are usually low alloy steels.
Martensitic transformation induced by local stress has the effect of relieving
stress concentrations,increasing the work-hardening rate, and promoting homo-
geneous deformation, with consequent improvements in the strength, ductility
and toughness of steels. TRIP-assisted steels are mass produced, made using a
complex heat treatment which is often completed within a short time during the
processing of steel strip. Their microstructure consists of allotriomorphic fer-
rite as the major phase together with a total of 30–40% of harder regions. The
latter consist of mixtures of bainite, martensite and carbon-enriched retained
austenite. The chemical composition is typically Fe–0.12C–1.5Si–1.5Mn wt%.
Some austenite is retained in spite of the low overall solute content because
when the bainite forms, the silicon prevents cementite precipitation, thereby
enriching the residual austenite with carbon (Chapter 6). The major application
of TRIP-assisted steels is in the automobile industries, both for painted surfaces
and for enhancing the safety of the passenger compartment in the event of a
crash.
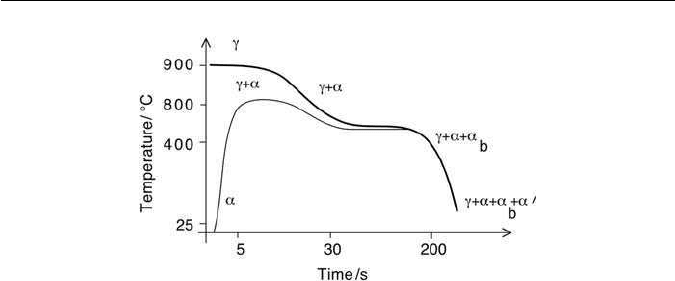
There are two kinds of TRIP-assisted steels. In the first case a cold-rolled
strip is heated rapidly from ambient temperature for intercritical treatment in
the α +γ phase field between the Ac
1
and Ac
3
temperatures (Fig. 10.12). The
intercritical annealing induces partial transformation to austenite and at the
224 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
Fig. 10.12 The two kinds of heat treatment used to generate the microstructures of
TRIP-assisted steels. The terms γ, α, α
b
and α
′
represent austenite, allotriomorphic ferrite,
bainite and martensite, respectively.
same time recrystallizes the residual ferrite. The strip is then cooled at a con-
trolled rate during which some of the austenite transforms into allotriomorphic
ferrite and at lower temperatures into bainitic ferrite. This latter reaction causes
the austenite to become enriched in carbon, allowing it to be retained to ambient
temperature (Fig. 10.13).
The details ofthe microstructureand mechanical propertiescan be alteredby
manipulating the cooling condition. For example, it is common practice to allow
more time in the bainite transformation range than at the higher temperatures
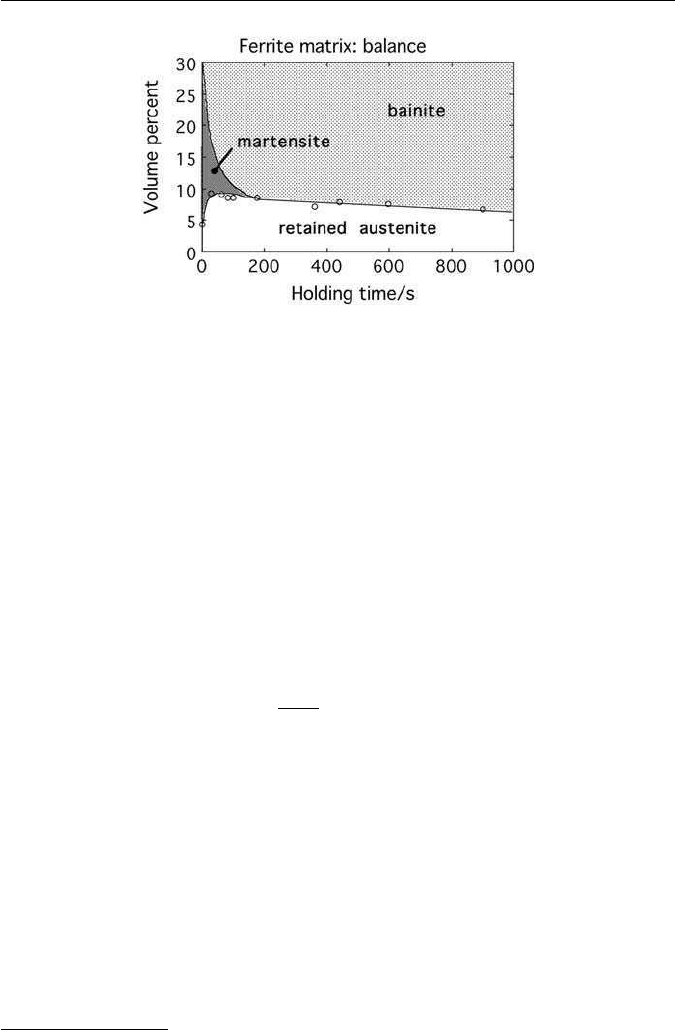
where allotriomorphic ferrite grows. Figure 10.14 shows the effect of holding in
the bainite transformation temperature range on the final microstructure. An
inadequate amount of bainite leaves the austenite susceptible to martensitic
transformation. Similarly, because the carbon concentration in the austenite is
limited by the T
0
curve (Chapter 6), transformation to bainite at too high a
temperature also renders the austenite unstable.
The second kind of heat treatment starts from a hot-rolled strip which is
fully austenitic (Fig. 10.12) and forms both allotriomorphic ferrite and bainite
during the cooling part of the thermal cycle. This has the advantage that the
microstructure can be produced directly from the hot strip which has been rolled
to its final dimensions. The process is cheap since the strip does not have to be
heated to the intercritical annealing temperature. However, hot-rolling mills
are restricted by rolling loads to strips thicker than about 3 mm, although there
are modern mills which can cope with 1.4 mm thickness. Cold-rolled strips can,
on the other hand, be made routinely into thinner gauges. Hot-rolled strips are
preferred for automobile applications where cost is a prime factor in the choice
of materials.
The transformation strain due to the formation of martensite does not
account for the observed uniform tensile elongation of some 15–30%. The
shape deformation due to martensite (Chapter 5) is at most equivalent to a
2% tensile strain because the amount of austenite available for transformation
10.4 TRIP-ASSISTED STEELS 225
(a)
(b)
(c)
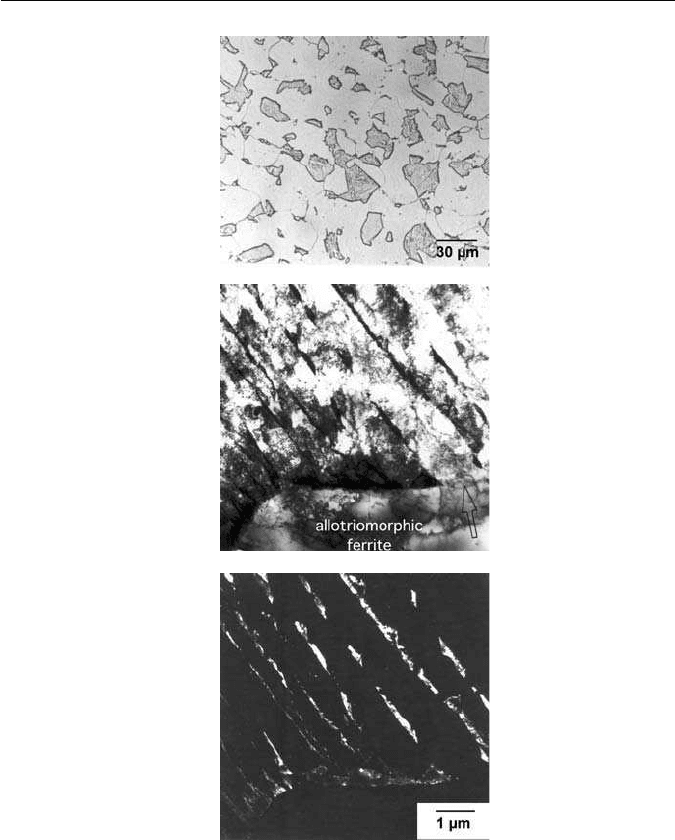
Fig. 10.13 TRIP-assisted steel showing a mixed microstructure of allotriomorphic ferrite,
bainitic ferrite and retained austenite films. (a) Optical micrograph. (b) Bright field transmission
electron micrograph. (c) Dark field image of retained austenite.
is quite small in TRIP-assisted steels. The major contributions to uniform elon-
gation arise partly from the enhanced work-hardeningcoefficient ofthe material
due to the progressive formation of hard martensite during deformation. There
is a further significant contribution from dislocations induced into the ferrite
226 CHAPTER 10 THERMOMECHANICAL TREATMENT OF STEELS
Fig. 10.14 Evolution of room temperature microstructure as a function of the time dur-
ing isothermal transformation to bainite (Girault, E., Mertens, A., Jacques, P., Houbaert, Y.,
Verlinden, B. and van Humbeek, J., Scripta Materialia 44, 885, 2001).
by the strains associated with martensitic transformation.
3
These dislocations
strengthen the ferrite and are visible in Fig. 10.13.
The austenite also delays the necking process during a tensile test by trans-
forming to martensite at stress concentrations. It is therefore important to delay
the transformation of retained austenite to the late stages of deformation when
significant damage accumulates in the steel. It is at this point that the TRIP
effect can be most beneficial. It is useful therefore to examine further the
transformation of austenite as a function of plastic strain.
It is reasonable to assume that the change in the fraction ofmartensite (dV
α
′
)
obtained for a given increment of plastic strain (dǫ) should be proportional to
the fraction of remaining austenite:
dV
α
′
dǫ
= k
γ
V
γ
, (10.1)
where k
γ
is a function of the steel composition and test temperature and V
γ
is
the fraction of austenite remaining untransformed. If the fraction of austenite at
zero strain is V
γ
0
, then V
α
′
=V
γ
0
−V
γ
, and integration of Equation (10.1) gives:
ln{V
γ
0
}−ln{V
γ
}=k
γ
ǫ.
The form of this equation is illustrated in Fig. 10.15.
10.4.1 Low- or zero-silicon TRIP-assisted steels
The substantial silicon addition to TRIP-assisted steels leads to the formation
of hard, adherent oxide (Fe
2
SiO
4
) which is difficult to remove prior to hot
3
Jacques, P., Furnemont, Q., Mertens, A. and Delannay, F. Philosophical Magazine A 81,
1789, 2001.
10.4 TRIP-ASSISTED STEELS 227
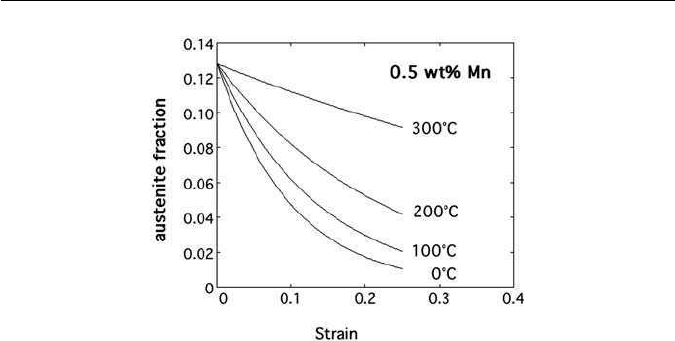
Fig. 10.15 Martensitic transformation of retained austenite in a TRIP-assisted steel as a func-
tion of deformation temperature and plastic strain (Sherif, M., Garcia-Mateo, C., Sourmail, T.
and Bhadeshia, H. K. D. H. Materials Science and Technology 20, 319, 2004).
rolling, resulting in a poor surface finish. This restricts automobile applications
to components which are hidden from view. Low-silicon TRIP steels do exist,
as illustrated in Fig. 10.16. The austenite in such alloys is more difficult to retain
because of the tendency to precipitate cementite, but this can be minimized with
careful heat treatment. When this is done, strengths in excess of 600 MPa with
a uniform ductility of 15% have been achieved.
10.4.2 Galvanizing of TRIP-assisted steels
There are two basic methods of galvanizing, by dipping the steel in liquid zinc
or by electrolytically depositing the zinc. The typical concentrations of silicon
and manganese in TRIP-assisted steels lead to a stable Mn
2
SiO
4
oxide film on
the surface during the heat treatment that leads to the desired microstructure.
This makes it difficult for the zinc to wet the steel surface, making it necessary
to electrolytically galvanize such alloys.
The problem can be alleviated by increasing the humidity in the annealing
furnace. The oxide coverage of the surface is then reduced, giving better wet-
ting by zinc. The higher humidity causes the internal oxidation of Mn and Si
below the steel surface, thus reducing their availability to form Mn
2
SiO
4
at the
surface.
An alternative approach is to eliminate the silicon and add aluminium to
retain the cementite-free microstructure. Aluminium oxidizes easily to form
alumina by internal oxidation near the surface, again limiting the amount of
FeAl
2
O
4
that can form at the free surface when the humidity in the annealing
furnace is low. Such a steel can easily be hot-dip galvanized. At high humidity,
