Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

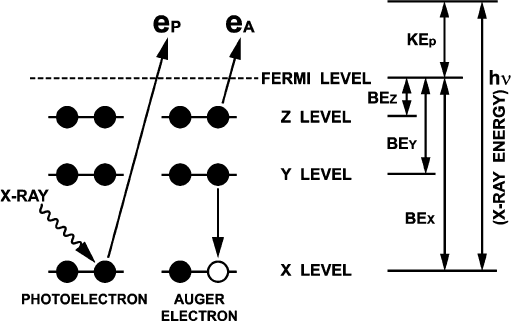
12.4.1. PRINCIPLES OF X-RAY PHOTOELECTRON SPECTROSCOPY
XPS measures the kinetic-energy distribution of electrons (photoelectrons) emit-
ted from core levels of the elements constituting a solid when the sample is irradiated
by X-rays (Fig. 12.4.1). The measured core electron binding energy (BE
X
), defi ned as
the difference between the energy of the primary photon (hn) and the kinetic energy
of the photoelectron (KE
P
), allows the element in question to be identified:
BE
x
¼ hn KE
P
ð1Þ
In practice, Eq. (1) must be corrected for some experimental factors, as described
below.
When an atom emits a photoelectron, it is left with a vacancy in its inner orbital,
and becomes unstable. Auger transition is one of the processes by which the un-
stable, excited atom undergoes relaxation (Fig. 12.4.1). Here, an outer-orbital elec-
tron is transferred to the vacanc y, and the excess energy is released through the
emission of another outer-orbital electron (XYZ Auger electron). The Auger electron
kinetic energy (KE
A
), given approximately by Eq. (2), is a characteristic of an Auger
transition for a specific atom:
KE
A
¼ BE
x
BE
Y
BE
Z
ð2Þ
XPS measures both photoelectrons and Auger electrons.
The X-ray beam penetrates the sample to a depth of 1–100 mm, causing the ejec-
tion of photoelectrons and Auger electrons. Electrons that escape to the surface from
deeper parts of the sample may lose kinetic energy through inelastic scattering. It is
such inelastically scattered electrons that give rise to the background in an XPS
spectrum. The inelastic mean free-path of an electron in the solid depends on its
Fig. 12.4.1. Photoelectron (e
p
) and Auger electron (e
A
) emission processes induced by X-ray.
Chapter 12.4: X-ray Photoelectron Spectroscopy866
kinetic energy. Both photoelectron and Auger electron measured by conventional
XPS have kinetic energy less than 1500 eV and their inelastic free-path is very short,
typically of the order of nm. Therefore XPS is a surface-sensitive technique. All
elements, other than H and He, are detectable by XPS when their concentrations
exceed 0.1–1%. For samples with a flat surface, the spectral background can be
reduced by using a total-reflection X-ray or a grazing-incidence X-ray because of its
shallow depth of penetration (Kawai et al., 1995). Since XPS is non-destructive, the
characteristic features of photoelectron and Auger electron spectra also provide
information on the state of chemical bonding of the elements concerned.
Recent developments in XPS inst rumentation enable photoelectron images with a
lateral resolution in the micrometer to submicrometer range to be obtained through
scanning or direct imaging techniques (Garwood, 1995). In particular, photoelectron
emission microscopy (PEEM) is a promising technique for observing direct photo-
electron images of surfa ce chemical composition (Kordesch, 1995; De Stasio et al.,
1998). A lateral resolution down to 20 nm was achieved using X-ray PEEM
(X-PEEM) with a synchrotron-radiation photon source (De Stasio et al., 1999). The
application of X-PEEM to the analysis of geological samples was reported by De
Stasio et al. (2001).
12.4.2. EXPERIMENTAL TECHNIQUES
A. Instrumentation and Sample Handling
XPS measurements are conducted under ultra-high vacuum (o10
–8
Torr) so as to
avoid collision between photoelectrons and gas molecules in the spectrometer, while
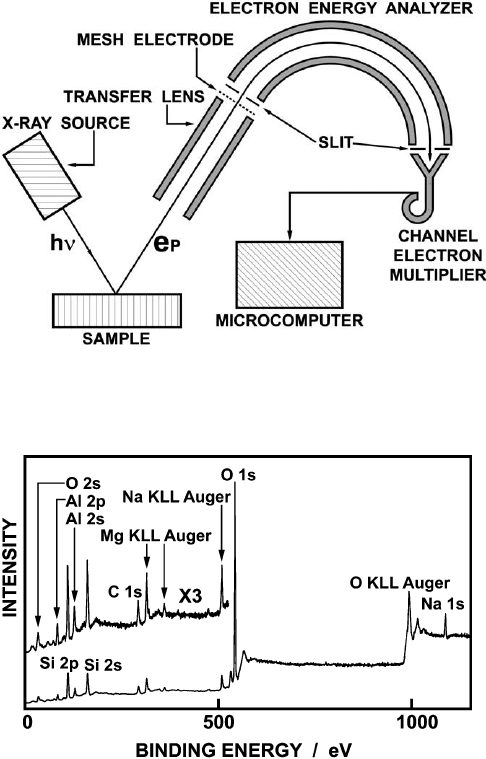
surface contamination from residual gases is minimized. Fig. 12.4.2 shows a sche-
matic diagram of a typic al X-ray photoelectron spectrometer, consisting of an X-ray
source, an electron energy analyzer, and a photoelectron detector. Common X-ray
sources are Al Ka (1486.6 eV) and Mg Ka (1253.6 eV). By using monochromatized
X-rays with a narrow line width, satellite spectra exited by Ka
3,4
and Kb lines can be
eliminated, and the energy resolution of photoelectrons improved. The ejected pho-
toelectrons are transferred to an electron energy analyzer, and separat ed according
to their kinetic energy. Among the various types of analyzers (Briggs and Seah,
1990), the concentric hemispherical analyzer (CHA) and cylindrical mirror analyzer
(CMA) are the most commonly employed. Following energy analysis, the photo-
electrons are detected by electron multipliers.
Fig. 12.4.3 shows a wide-scan spectrum of Na
+
-montmorillonite excited by Al
Ka, demonstrating the multi-element detection capability of XPS. In this respect, it
is comparable with X-ray fluorescence (XRF) spectrometry for bulk elemental anal-
ysis. The wide-scan XPS spectrum shows the photoelectron and Auger electron
lines of the constituent elemen ts, notably Si (2s, 2p), Al (2s, 2p), Mg (KLL Auger),
Na (1s, KLL Auger), and O (2s, 1s, KLL Auger). The set of core photoelectron lines,
12.4.2. Experimental Techniques 867
together with the X-ray-induced Auger electron lines, are useful for element iden-
tification. At the same time, however, the probability of overlap with lines of dif-
ferent atoms increases. This can sometimes cause difficulties in identifying and
quantifying minor constituents.
Because clays are electrical insulators, positive electrostatic charges can build up at
the sample surface due to electron emission during measurement. In practice, the
measured kinetic energy (KE
m
) differs from the ideal value (KE
i
), identifiable with
Fig. 12.4.2. Schematic diagram of XPS measurement system equipped with a concentric
hemispherical analyzer (CHA).
Fig. 12.4.3. Wide-scan X-ray photoelectron spectrum of Na-montmorillonite excited by Al
Ka radiation.
Chapter 12.4: X-ray Photoelectron Spectroscopy868

KE
P
in Eq. (1) or KE
A
in Eq. (2). The relationship between KE
m
and KE
i
is given by:
KE
m
¼ KE
i
F
sp
E
c
ð3Þ
where F
sp
is the work function of the spectrometer; that is, the energy required to
bring the electron from its zero binding energy (or Fermi) level to that of the spec-
trometer. E
c
is the additional retarding energy due to sample charging. In order to
determine the photoelectron binding energy and Auger electron kinetic energy, the
measured electron energy should be corrected for F
sp
and E
c
.
The C 1s or Au 4f
7/2
line is widely used as a primary standard for the calibration of
electron energies. It is often convenient to use the C 1s line (of hydrocarbons) for this
purpose since this peak usually arises from adventitious surface contamination by
vacuum pump oil. However, the C 1s binding energy depends on the nature of the
carbon source, and hence is not an accurate binding energy standard. Further, dif-
ferential charging of the sample and hydrocarbons (due to differences in electric non-
conductance) may cause an unexpected error in binding energy determination. On the
other hand, the Au 4f
7/2
line of a gold film, evaporated onto the sample, is a reliable
standard since its binding energy (84.0 eV) was precisely measured (Ebel et al., 1983;
Anthony and Seah, 1984). Thus, the electron binding energy of the constituent elements
in a given sample is frequently determined relative to that of Au 4f
7/2
on the assumption
that the Fermi level of the sample coincides with that of the evaporated gold.
Mineral samples for analysis by XPS usually come in the form of a powder or a
thin section. Before being inserted into the apparatus, the sample is dried and at-
tached to a metal sample holder by a double-sided sticky tape, an electrically con-
ductive paste, or by other means. Samples may also be mounted by placing a
suspension of the powder on the sample holder, and by allowing the dispersant to
evaporate. Alternat ively, the sample may be pressed into a soft metal, such as in-
dium. Whatever method is adopted, due care should be taken to avoid or, at least,
minimize surface contamination.
B. Quantitative Analysis
The area under a given peak in a photoelectron spectrum is directly proportional to
the conc entration of the element in question, assuming that the element is homo-
geneously distribut ed in the analyzed volume. Since peak intensity increases as the
effective surface area of the sample increases, it is difficult to estimate the absolute
concentration of a given element. In practice, one derives the concentration of el-
ement A relative to that of a reference element B (atomic ratio, N
A
/N
B
) from the
measured photoelectron intensities (I
A
for element A and I
B
for element B):
N
A
N
B
¼
1
S
AB
I
A
I
B
ð4Þ
12.4.2. Experimental Techniques 869
where S
AB
is the relative atomic sensitivity factor. Included in this factor are such
parameters as the photoionization cross-section, anisotropy of photoelectron emission,
detection efficiency of the spectrometer, and mean free-paths of photoelectrons. Rela-
tive atomic sensitivity factors are derived either theoretically (from calculated
photoionization cross-sections and other parameters), or experimentally (from XPS
measurements of reference compounds of known homogeneous chemical composi-
tion). Commercial XPS instruments are equipped with a microcomputer for instrument
control, data acquisition, and data handling. Relative surface concentrations (elemen-
tal compositions) can be calculated by the computer using appropriate software
(smoothing, background subtraction, peak deconvolution, peak area calculation, etc.).
The uncertainty in elemental quantification by XPS mainly arises from the
application of the atomic sensitivity factor to samples with different matrices (Seah,
1980). In our experience, the relative surface concentration of an element can be
obtained within an error of 10–20%, using experimentally determined relative
atomic sensitivity factors. However, for photoelectrons with a low kinetic energy
(i.e., a high binding energy) the values derived are less certain. This is because the
mean free-path of the corresponding photoelectrons is shorter, and their intensity is
more susceptible to surface contamination, than their high-energy counterparts.
The reproducibility in deriving relative surface concentrations from XPS spectra
was checked by Seyama and Soma (1984, 1988). From measurement of the area
intensity ratios of Si 2s to Al 2p of montmorillonite samples having different ex-
changeable cations, these workers concluded that the values are reproducible within
710%. On the surfaces of freshly prepared silicates (clay and related minerals), the
relative abundances of major cati ons (Si, Al, Fe, Mg, Na, K, etc.) usually correspond
to their bulk chemical compositions. However, the surface concentration of oxygen
tends to exceed the bulk value because of the presence of OH
ions and/or water
molecules at the surface (Seyama and Soma, 1985, 1988).
C. Chemical Information
Characteristic features of photoelectron and Auger electron spectra, such as peak
position (electron bind ing or kinetic energy), satellite structure, and multiplet split-
ting, reflect the bonding state of elements. For some elements, it is possible to
measure both photoelectron and Auger electron spectra. In such cases, it is especially
informative to obtain a two-dimensional plot of both electron energies (Wagner
et al., 1979). Such a ‘chemical-state plot’ was used to characterize the bonding state
of some elements in clay minerals (Seyama and Soma, 1984, 1988; Dutta et al., 1999).
Wagner et al. (1979) also measured the modified Auger pa rameter (a
0
), defined as
the sum of the photoelectron binding energy and Auger electron kinetic energy of an
element, and proposed that the value of a
0
is unique to each chemical-bonding state.
For bonding state characterization it is customary to compare the photoelectron
binding energy and/or Auger electron kinetic energy of an element in the sample
Chapter 12.4: X-ray Photoelectron Spectroscopy870
with that of a reference compound. The shift in peak (electron energy) position
relative to that of the reference compound is called the chemical shift. The chemical
shift in the photoelectron binding energy (DBE
X
) is dependent on the state (chemical
environment) of the atom, in particular its electron density (oxidation number).
The lower the electron density, the higher the photoelectron binding energy. The
Fe 2p photoelectron spectrum is a case in point. The Fe 2p
3/2
binding energy of
Fe
3+
in silicate minerals (about 712 eV) is higher than that of Fe
2+
(710–711 eV).
Further, the Fe 2p spectra of silicate minerals have satellite peaks characteristic of
the oxidation state of the iron (Seyama and Soma, 1987). The nature of atoms
surrounding the host atom, that is of nearest neighbours (and, in some cases, non-
nearest neighbours), also influences the chemical shift. For example, for silicate
minerals there is a positive correlation between the photoelectron binding energies of
Si 2s (2p) and O 1s (Seyama and Soma, 1985, 1988). These values refer to the silicate
framework (structure), and decrease as the negative charge on the silicate structure
increases.
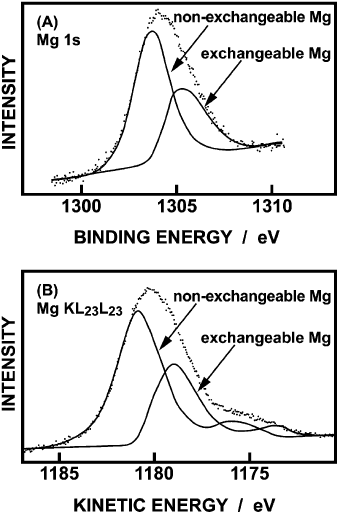
Fig. 12.4.4, showing the Mg 1s and Mg KL
23
L
23
Auger electron spectra of
Mg
2+
-montmorillonite excited by Al Ka radiation, gives another example of
Fig. 12.4.4. (A) Mg 1s and (B) KL
23
L
23
Auger spectra of Mg-montmorillonite (Reproduced,
by permission of The Royal Society of Chemistry, from Seyama and Soma, 1984).
12.4.2. Experimental Techniques 871
deriving chemical information from XPS analysis (Seyama and Soma, 1984). For
both electrons the spectra are broad, and each can be deconvoluted into two
components corresponding to: (i) non-exchangeable Mg
2+
ions occupying octahe-
dral sites within the layer structure; and (ii) exchangeable Mg
2+
ions occupying
interlayer sites.
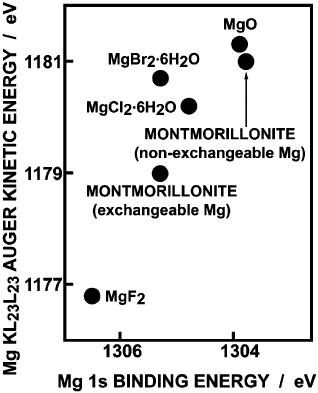
Fig. 12.4.5 shows a chemical-state plot for magnesium, comparing the relation-
ship between Mg 1s binding energy and Mg KL
23
L
23
Auger kinetic energy for
Mg
2+
-montmorillonite with that of magnesium halides and magnesium oxide. The
position of exchangeable (interlayer) Mg
2+
ion falls between that of MgCl
2
.6H
2
O
and MgF
2
. In contrast, the point for non-exchangeable (structural) Mg
2+
ion is
close to that for MgO. The Mg 1s binding energy for non-exchangeable Mg
2+
ions is
1.5 eV lower, while the Mg KL
23
L
23
Auger kinetic energy is 2.0 eV higher, than the
corresponding values for exchangeable Mg
2+
ions. These large differences may be
attributed to the effect of neighbouring atoms. Apparently, the flow of electronic
charge to Mg
2+
as well as the extra-atomic relaxation (a screening of the final-state
ion in the Auger transition by electrons from neighbouring atoms) are larger for
non-exchangeable Mg
2+
ions because of its stronger interaction with O
2–
and OH
–
ions within the aluminosilicate layers.
Although XPS is essentially non-destructive, irradiation by X-rays can occasion-
ally cause chemical damage (alteration) to the sample. For example, during XPS
measurement Cu
2+
ions (structural, adsorbed, or interlayer) in clay minerals can be
converted into Cu
+
through photoreduction (Mosser et al., 1992).
Fig. 12.4.5. Chemical-state plot for Mg (Reproduced, by permission of The Royal Society of
Chemistry, from Seyama and Soma, 1984).
Chapter 12.4: X-ray Photoelectron Spectroscopy872
12.4.3. APPLICATIONS
Despite its limited sensitivity in terms of spatial and binding energy (chem ical
shift) resolution, XPS can provide valuabl e information on the surface composition
of clays and clay minerals, and the chemical bonding of the constituent elements.
The examples below illustrate this capability.
The particles of naturally occ urring clay minerals (phyllosilicates) are often coated
by ferric (hydr)oxides which, therefore, may significantly influence surface proper-
ties. Conventionally this (external) coating of iron can be extracted by treatment with
sodium citrate–dithionite–bicarbonate (CDB), leaving the structural iron more or
less intact. Using this approach, Soma et al. (1992) were able to characteriz e the
surface chemical composition of five New Zealand halloysites with different particle
morphologies by XPS. Except for the sample from Hamilton, most of the iron in the
halloysites were structural, and hence not extractable with CDB. The Hamilton
halloysite contained the highest amoun t of iron, of which about 80% was external
and extractable by CDB. The Fe 2p
3/2
photoelectron binding energy (711.6 eV) of
this sample was consistent with that of ferric (hydr)oxides, such as goethite (McIn-
tyre and Zetaruk, 1977). Its iron-to-silicon (Fe/Si) atomic ratio, determined by XPS,
was also significantly larger than the bulk value determined by X-ray fluorescence
analysis. On the other hand, the Fe 2p
3/2
binding energy of the other halloysites
(about 712.8 eV) was characteristic of ferric ion in silicate structures (Seyama and
Soma, 1987). Their surface Fe/Si atomic ratio (by XPS) was close to, or less than, the
bulk value, indicating that the surface layers of some halloysites were depleted in
(structural) iron. This observation would have important implications for determin-
ing the surface charge characteristics and chemi cal reactivity. After CDB extraction,
the surface Fe/Si atomic ratio of Hamilton halloysite was comparable with the
corresponding bulk ratio, while the Fe 2p
3/2
binding energy shifted to 712.6 eV, close
to the value for structural iron. This example clearly demonstrates the capability of
XPS in providing infor mation on the radial distribution and chemical-bonding state
of an element (here iron) in minerals with a heterogeneous microstructure.
XPS analysis of siliceous ferrihydrites provides insight into the bonding state and
localization of silicon (Soma et al., 1996). The surface Si/Fe atomic ratios of five
natural siliceous ferrihydrites (determined by XPS) were close to, or slightly smaller
than, their respective bulk values (0.18–0.43). This indicated that Si was well dis-
persed throughout the ferrihydrite matrix at the scale of the photoelectron mean
free-path (of the order of several nm), with a tendency for depletion in the outer
(surface) layers. The Si 2s binding energy of 152.9 7 0.2 eV for all five natural
samples was close to that for olivine (152.9–153.0 eV) (Seyama et al., 1996), a neso-
silicate with isolated SiO
4
tetrahedra. However, it was markedly lower than the value
for quartz (154.4 eV) (Seyama et al., 1996), indicating the absence of a three-
dimensional network of SiO
4
tetrahedra. Thus, both the surface Si/Fe atomic ratio
and the Si 2s photoelectron binding energy of natural siliceous ferrihydrites were
consistent with the model of Parfitt et al. (1992). These workers suggested that
12.4.3. Applications 873
silicate is bonded to, or forms a bridge between, the surfaces of ‘micro-crystalline
domains’ making up a primary ferrihydrite particle of several nm in diameter.
The XPS results for a synthetic ferrihydrite with an Si/Fe atomic ratio of about
0.1 and a low Si 2s binding energy also suggest that Si is well dispersed in the matrix
and, as such, would inhibit the conversion (of ferrihydrite) into hematite (Glasauer
et al., 2000). On the other hand, for a synthetic precipitate of ferrihydrite with a
relatively high-silicon content, the surface Si/Fe atomic ratio (0.48) was much larger
than the bulk value (0.18), while the Si 2s binding energy (154. 1 eV) was close to that
of quartz (Soma et al., 1996). These observations indicate that silica layers with
three-dimensionally polymerized SiO
4
units covered the outer particle surfaces of the
precipitated ferrihydrite. In line with this postulate, the O 1s photoelectron spectrum
showed two peaks of which the one with a higher binding energy was assigned to
oxygen in a three-dimensional SiO
4
network (Seyama and Soma, 1985).
An important aspect of the surface chemistry of phyllosilicates (clay minerals) is
related to their anisotropic layer structure, exposing basal-plane, and crystal-edge
surfaces. The spatial resolution of conventional XPS is not sufficient to differentiate
between these two surfaces of micrometer-size clay mineral particles. However, an-
isotropic surface reactivity may be assessed using macrocrystalline micas with a
specimen surface area comparable to that of an X-ray beam. For example, Ilton
et al. (1997) used XPS to investigate the interaction of chromate ions (CrO
4
2
) with
mica (biotite and phlogopite) crystals in aqueous solutions containing an alkaline
salt. The study was undertaken because hexavalent chromium is toxic, and its fate in
the environment is of great concern. The photoelectron spectra of the treated biotite
showed the presence of chromi um on the edge surface but none on the basal plane.
In the case of phlogopite, however, no chromium was detected on either the edge or
basal surface. The Cr 2p
3/2
binding energy indica ted that chromium was bound as a
trivalent species to the edge surface of biotite. This finding was explained in terms of
the reduction of hexavalent chromate by ferrous cations, present in the layer struc-
ture, followed by adsorption (at the biotite edge surface) of the resultant trivalent
chromium cation. The overall process was promoted by sodium and lithium salts
whereas the presence of RbCl and CsCl in solution had an inhibiting effect. It is
suggested that hydrated Na
+
and Li
+
ions could replace interlayer K
+
ions in
biotite, and so facilitate the heterogeneous reduction of chromate at the edge/
solution interface.
Both biotite and phlogopite were able to adsorb Cr
3+
cations from solutions
containing NaCl and KCl (Ilton et al., 2000). However, adsorption was larger, and
the photoelectron binding ene rgies of Cr 2p
3/2
and 3p were higher for the NaCl than
the KCl system. The adsorption of Cr
3+
ions from KCl solution was apparently
confined to crystal-edge surfaces. In NaCl solution, on the other hand, Cr
3+
was
adsorbed at both edge and interlayer sites since Cr
3+
and Na
+
could replace in-
terlayer K
+
ions in mica.
The interacti ons of organic matter with clay minerals have important technical
and environmental applications . As already remarked, the C 1s photoelectron line is
Chapter 12.4: X-ray Photoelectron Spectroscopy874
unsuitable for characterizing organic compounds adsorbed on mineral surfaces since
this peak is affected by hydrocarbons from vacuum pump oil. On the other hand, the
N 1s photoelectron line can often serve as a convenient marker of nitrogen-
containing organic molecules, such as amines. The adsorption from aqueous solu-
tions of partially hy drolyzed polyacrylamide (HPAM) by kaolinite, feldspar, and
quartz illustrates this point (Graveling et al., 1997; Allen et al., 1998). Measurement
of the N 1s and Si 2p lines in the XPS spectrum following adsorption (of HPAM)
allowed the surface concentration of N to be normalized for surface Si sites. Among
the three minerals investigated, kaolinite showed the highest adsorption of HPAM
(pH 2–10), probably involving hydrogen bonding between basal oxygens of the clay
mineral and amide groups of the polymer. The high reactivity of kaolinite was also
demonstrated by imaging XPS of mixed kaolinite/quartz crystals after adsorption of
HPAM (Allen et al., 1998). Using Si 2p, Al 2p, and N 1s photoelectron images with
o10 mm spatial resolution it was possible to differentiate between domains in the
kaolinite matrix containing more HPAM and quartz grains with fewer polymers. We
would expect that further developments in XPS-associated microscopy techniques
will extend the application of XPS to complex systems containing clays and clay
minerals.
The magnitude and distribution of surface charge have a great influence on
the charge characteristics of clay minerals. The application of XPS to assessing
the surface charge of some micas (margarite, muscovite, and sericite) was reported by
Gier and Johns (2000) and Johns and Gier (2001). After the replacement of surface
cations by Ba
2+
these workers determined the atomic ratios of surface Ba
2+
to
interlayer Ca
2+
(for margarite), K
+
(for muscovite and sericite), and Na
+
(for
margarite, muscovite, and sericite). These measured ratios were indicative of the
relative layer charge on external surface and the uppermost interlayer. Assuming that
the interlayer charge was equally divided between the two external cleavage surfaces,
the Ba
2+
/interlayer cation atomic ratio would indicate the occurrence of layer charge
asymmetry. The O/Ba atomic ratio of mica, determined by XPS, could also be used to
assess the magnitude and distribution of surface charge (Johns and Gier, 2001).
Photoelectron intensity from a single crystal surface as a function of electron take-
off angle shows a characteristic fluctuating pattern. This phenomenon, known as
‘photoelectron diffraction’, is due to the elastic scattering of photoelectron s by
neighbouring atoms. Since the X-ray photoelectron diffraction (XPD) pattern
depends on crystal structure, it can yield information about the location of atoms
within the crysta l. Evans and co-wo rkers (Evans and Raftery, 1980, 1982; Ash et al.,
1987) measured the XPD patterns of mica single crystals, rotating at an angle about
a specific crystallographic axis, and examined the location (sites) of minor elements
in the minerals. They were able to show that in biotite Ti preferentially occupied
octahedral sites whereas in lepidolite Rb and Mn, respectively, occupied sites that
were essent ially identical with those of K (interlayer) and Li (octahedral).
Aluminium in silicate minerals can occupy both tetrahedral and octahedral sites.
The distribution of Al
3+
ions between the two sites is important in determining the
12.4.3. Applications 875
