Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


class I MHC protein
human leukocyte antigen (HLA)
β
2
-microglobulin
T-cell receptor
CD8
perforin
granzymes
helper T cell
class II MHC protein
CD4
human immunodeficiency virus (HIV)
positive selection
negative selection
autoimmune disease
carcinoembryonic antigen (CEA)
IV. Responding to Environmental Changes 33. The Immune System
Problems
1.
Energetics and kinetics. Suppose that the dissociation constant of an F
ab
- hapten complex is 3 × 10
-7
M at 25°C.
See answer
(a) What is the standard free energy of binding?
(b) Immunologists often speak of affinity (K
a
), the reciprocal of the dissociation constant, in comparing antibodies.
What is the affinity of this F
ab
?
(c) The rate constant of release of hapten from the complex is 120 s
-1
. What is the rate constant for association? What
does the magnitude of this value imply about the extent of structural change in the antibody on binding hapten?

2.
Sugar niche. An antibody specific for dextran, a polysaccharide of glucose residues, was tested for its binding of
glucose oligomers. Maximal binding affinity was obtained when the oligomer contained six glucose residues. How
does the size of this site compare with that expected for the binding site on the surface of an antibody?
See answer
3.
A brilliant emitter. Certain naphthalene derivatives exhibit a weak yellow fluorescence when they are in a highly
polar environment (such as water) and an intense blue fluorescence when they are in a markedly nonpolar
environment (such as hexane). The binding of ε-dansyl-lysine to specific antibody is accompanied by a marked
increase in its fluorescence intensity and a shift in color from yellow to blue. What does this finding reveal about the
hapten-antibody complex?
See answer
4.
Avidity versus affinity. The standard free energy of binding of F
ab
derived from an antiviral IgG is -7 kcal mol
-1
(-29
kJ mol
-1
) at 25°C.
(a) Calculate the dissociation constant of this interaction.
(b) Predict the dissociation constant of the intact IgG, assuming that both combining sites of the antibody can
interact with viral epitopes and that the free-energy cost of assuming a favorable hinge angle is +3 kcal mol
-1
(12.6
kJ mol
-1
).
See answer
5.
Miniantibody. The F
ab
fragment of an antibody molecule has essentially the same affinity for a monovalent hapten as
does intact IgG.
(a) What is the smallest unit of an antibody that can retain the specificity and binding affinity of the whole protein?
(b) Design a compact single-chain protein that is likely to specifically bind antigen with high affinity.
See answer
6.
Turning on B cells. B lymphocytes, the precursors of plasma cells, are triggered to proliferate by the binding of
multivalent antigens to receptors on their surfaces. The cell-surface receptors are transmembrane immunoglobulins.
Univalent antigens, in contrast, do not activate B cells.
(a) What do these findings reveal about the mechanism of B-cell activation?
(b) How might antibodies be used to activate B cells?
See answer
7.
An ingenious cloning strategy. In the cloning of the gene for the α chain of the T-cell receptor, T-cell cDNAs were
hybridized with B-cell mRNAs. What was the purpose of this hybridization step? Can the principle be applied
generally?
See answer
8.
Instruction. Before the mechanism for generating antibody diversity had been established, a mechanism based on
protein folding around an antigen was proposed, primarily by Linus Pauling. In this model, antibodies that had
different specificities had the same amino acid sequence but were folded in different ways. Propose a test of this
model.
See answer
9.
Dealing with nonsense. Cells, including immune cells, degrade mRNA molecules in which no long open reading
frame is present. The process is called nonsense-mediated RNA decay. Suggest a role for this process in immune
cells.
See answer
10.
Crystallization. The proteolytic digestion of a population of IgG molecules isolated from human serum results in
the generation of F
ab
and F
c
fragments. Why do F
c
fragments crystallize more easily than F
ab
fragments generated
from such a population?
See answer
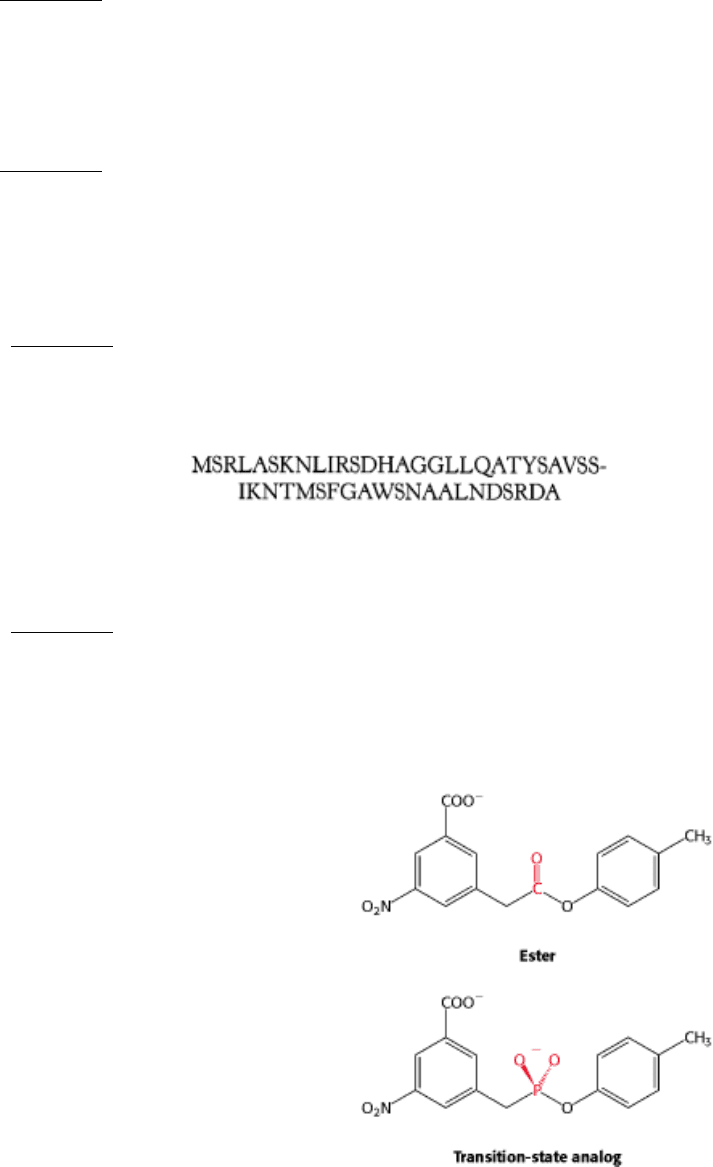
11.
Presentation. The amino acid sequence of a small protein is:
Predict the most likely peptide to be presented by the class I MHC molecule HLA-A2.
See answer
Mechanism Problem
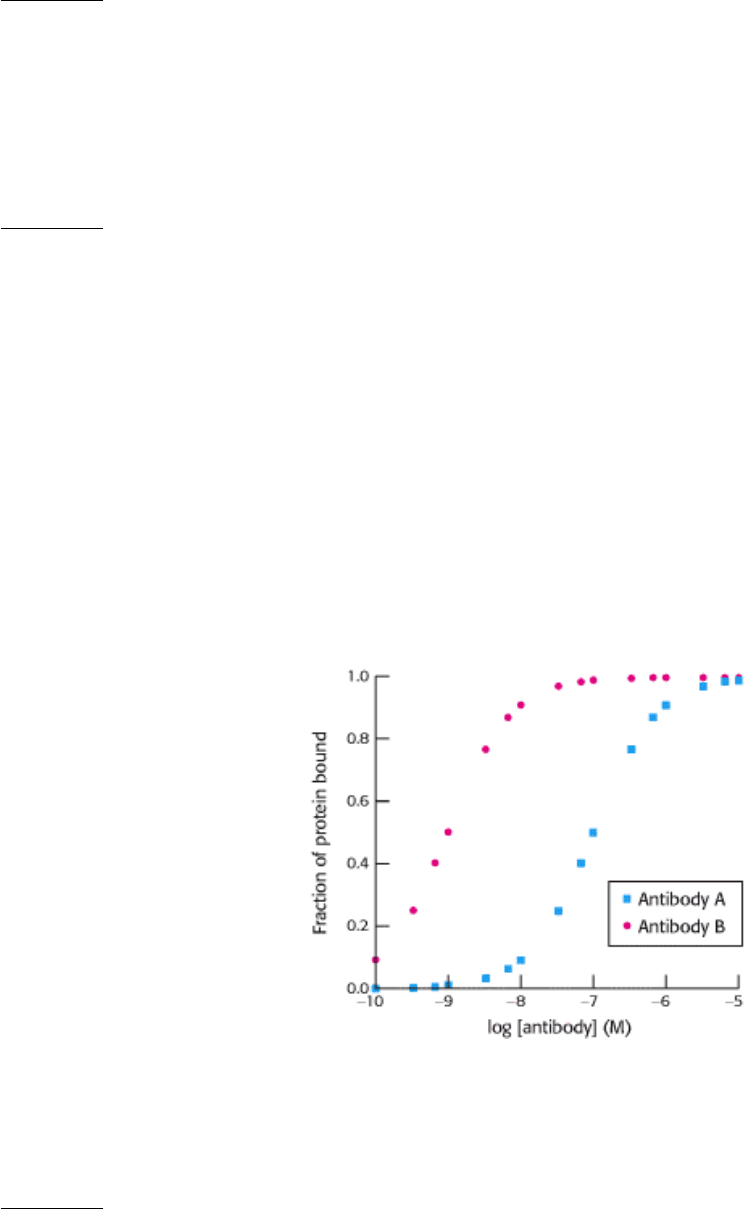
12.
Catalytic antibody. Antibody is generated against a transition state for the hydrolysis of the following ester.
Some of these antibodies catalyze the hydrolysis of the ester. What amino acid residue might you expect to find in
the binding site on the antibody?
See answer
Chapter Integration Problem
13.
Signaling. Protein tyrosine phosphatases, such as the molecule CD45 expressed in both B cells and T cells, play
important roles in activating such protein tyrosine kinases as Fyn and Lck, which are quite similar to Src. Suggest a
mechanism for the activation of such protein kinases by the removal of a phosphate from a phosphotyrosine residue.
See answer
Data Interpretation Problem
14.
Affinity maturation. A mouse is immunized with an oligomeric human protein. Shortly after immunization, a cell
line
that expresses a single type of antibody molecule (antibody A) is derived. The ability of antibody A to bind the
human protein is assayed with the results shown in the adjoining graph. After repeated immunizations with the
same protein, another cell line is derived that expresses a different antibody (antibody B). The results of analyzing
the binding of antibody B to the protein also are shown. From these data, estimate
(a) the dissociation constant (K
d
) for the complex between the protein and antibody A.
(b) the dissociation constant for the complex between the protein and antibody B.
Comparison of the amino acid sequences of antibody A and antibody B reveals them to be identical except for a
single amino acid. What does this finding suggest about the mechanism by which the gene encoding antibody B
was generated?
See answer
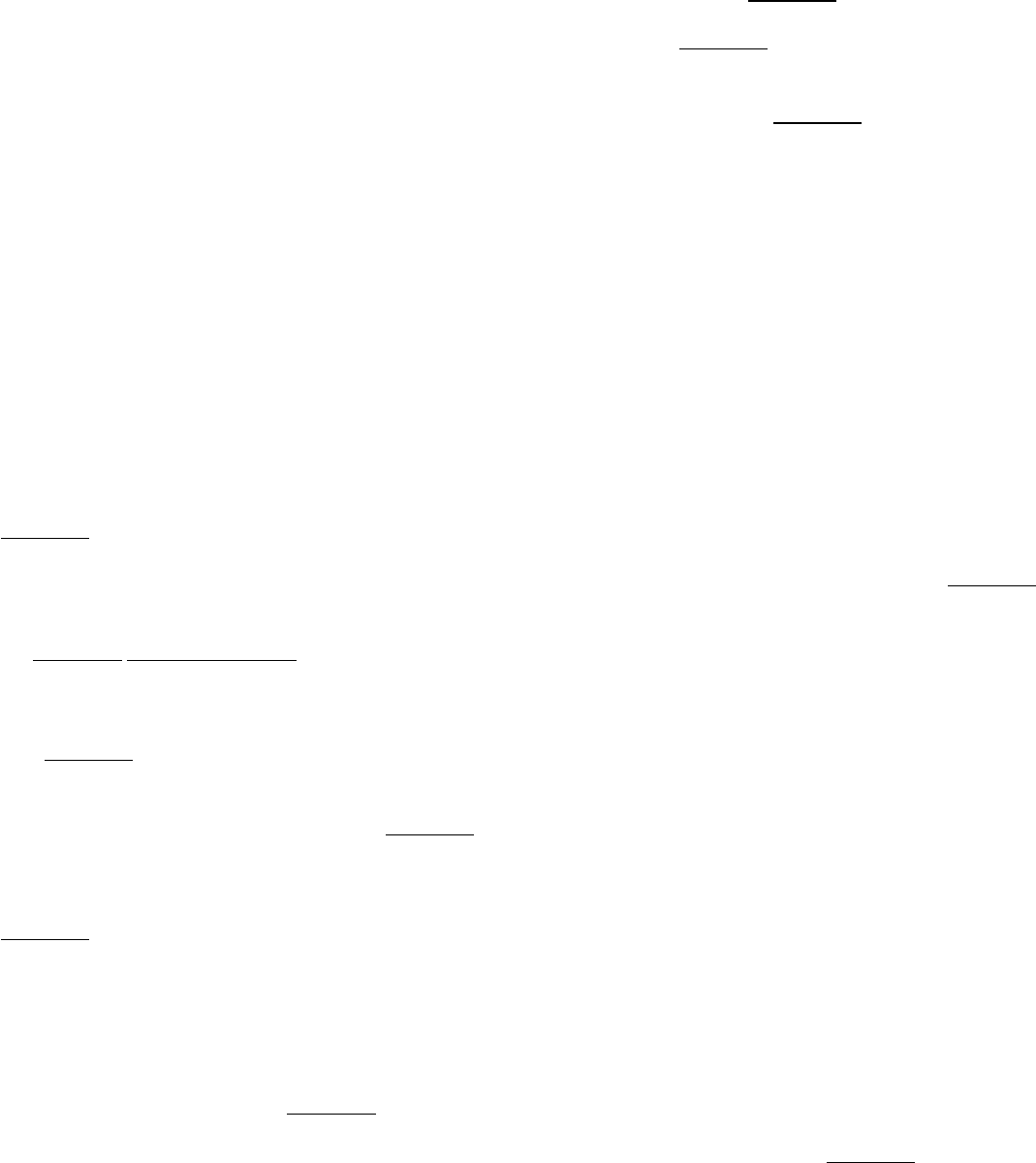
IV. Responding to Environmental Changes 33. The Immune System
Selected Readings
Where to start
G.J.V. Nossal. 1993. Life, death, and the immune system Sci. Am. 269: (3) 53-62.
S. Tonegawa. 1985. The molecules of the immune system Sci. Am. 253: (4) 122-131. (PubMed)
P. Leder. 1982. The genetics of antibody diversity Sci. Am. 246: (5) 102-115. (PubMed)
S.K. Bromley, W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen,
and M.L. Dustin. 2001. The immunological synapse Annu. Rev. Immunol. 19:: 375-396. (PubMed)
Books
Goldsby R. A. Kindt T. J. Osborne B. A. 2000. Kuby Immunology (4th ed.). W. H. Freeman and Company.
Abbas, A. K., Lichtman, A. H., and Pober, J. S., 1992. Cellular and Molecular Immunology (2d ed). Saunders.
Cold Spring Harbor Symposia on Quantitative Biology, 1989. Volume 54. Immunological Recognition.
Nisinoff, A., 1985. Introduction to Molecular Immunology (2d ed.). Sinauer.
Weir, D. M. (Ed.), 1986. Handbook of Experimental Immunology . Oxford University Press.
Structure of antibodies and antibody-antigen complexes
D.R. Davies, E.A. Padlan, and S. Sheriff. 1990. Antibody-antigen complexes Annu. Rev. Biochem. 59:: 439-473.
(PubMed)
R.J. Poljak. 1991. Structure of antibodies and their complexes with antigens Mol. Immunol. 28:: 1341-1345. (PubMed)
D.R. Davies and G.H. Cohen. 1996. Interactions of protein antigens with antibodies Proc. Natl. Acad. Sci. USA 93:: 7-
12. (PubMed) (Full Text in PMC)
M. Marquart, J. Deisenhofer, R. Huber, and W. Palm. 1980. Crystallographic refinement and atomic models of the intact
immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 Å and 1.9 Å resolution J. Mol. Biol. 141:: 369-
391. (PubMed)
E.W. Silverton, M.A. Navia, and D.R. Davies. 1977. Threedimensional structure of an intact human immunoglobulin
Proc. Natl. Acad. Sci. USA 74:: 5140-5144. (PubMed)
E.A. Padlan, E.W. Silverton, S. Sheriff, G.H. Cohen, G.S. Smith, and D.R. Davies. 1989. Structure of an antibody-
antigen complex: Crystal structure of the HyHEL-10 F
ab
lysozyme complex Proc. Natl. Acad. Sci. USA 86:: 5938-5942.
(PubMed)
J. Rini, U. Schultze-Gahmen, and I.A. Wilson. 1992. Structural evidence for induced fit as a mechanism for antibody-
antigen recognition Nature 255:: 959-965.
T.O. Fischmann, G.A. Bentley, T.N. Bhat, G. Boulot, R.A. Mariuzza, S.E. Phillips, D. Tello, and R.J. Poljak. 1991.
Crystallographic refinement of the three-dimensional structure of the FabD1.3-lysozyme complex at 2.5-Å resolution J.
Biol. Chem. 266:: 12915-12920. (PubMed)
D.R. Burton. 1990. Antibody: The flexible adaptor molecule Trends Biochem. Sci. 15:: 64-69. (PubMed)
Generation of diversity
S. Tonegawa. 1988. Somatic generation of immune diversity Biosci. Rep. 8:: 3-26. (PubMed)
T. Honjo and S. Habu. 1985. Origin of immune diversity: Genetic variation and selection Annu. Rev. Biochem. 54:: 803-
830. (PubMed)
M. Gellert and J.F. McBlane. 1995. Steps along the pathway of VDJ recombination Philos. Trans. R. Soc. Lond. B Biol.
Sci. 347:: 43-47. (PubMed)
R.S. Harris, Q. Kong, and N. Maizels. 1999. Somatic hypermutation and the three R's: Repair, replication and
recombination Mutat. Res. 436:: 157-178. (PubMed)
S.M. Lewis and G.E. Wu. 1997. The origins of V(D)J recombination Cell 88:: 159-162. (PubMed)
D.A. Ramsden, D.C. van Gent, and M. Gellert. 1997. Specificity in V(D)J recombination: New lessons from
biochemistry and genetics Curr. Opin. Immunol. 9:: 114-120. (PubMed)
D.B. Roth and N.L. Craig. 1998. VDJ recombination: A transposase goes to work Cell 94:: 411-414. (PubMed)
M.J. Sadofsky. 2001. The RAG proteins in V(D)J recombination: More than just a nuclease Nucleic Acids Res. 29:: 1399-
1409. (PubMed) (Full Text in PMC)
MHC proteins and antigen processing
P.J. Bjorkman and P. Parham. 1990. Structure, function, and diversity of class I major histocompatibility complex
molecules Annu. Rev. Biochem. 59:: 253-288. (PubMed)
A.L. Goldberg and K.L. Rock. 1992. Proteolysis, proteasomes, and antigen presentation Nature 357:: 375-379.
(PubMed)
D.R. Madden, J.C. Gorga, J.L. Strominger, and D.C. Wiley. 1992. The three-dimensional structure of HLA-B27 at 2.1 Å
resolution suggests a general mechanism for tight binding to MHC Cell 70:: 1035-1048. (PubMed)
J.H. Brown, T.S. Jardetzky, J.C. Gorga, L.J. Stern, R.G. Urban, J.L. Strominger, and D.C. Wiley. 1993. Three-
dimensional structure of the human class II histocompatibility antigen HLA-DR1 Nature 364:: 33-39. (PubMed)
M.A. Saper, P.J. Bjorkman, and D.C. Wiley. 1991. Refined structure of the human histocompatibility antigen HLA-A2
at 2.6 Å resolution J. Mol. Biol. 219:: 277-319. (PubMed)
D.R. Madden, J.C. Gorga, J.L. Strominger, and D.C. Wiley. 1991. The structure of HLA-B27 reveals nonamer self-
peptides bound in an extended conformation Nature. 353:: 321-325. (PubMed)
P. Cresswell, N. Bangia, T. Dick, and G. Diedrich. 1999. The nature of the MHC class I peptide loading complex
Immunol. Rev. 172:: 21-28. (PubMed)
D.R. Madden, D.N. Garboczi, and D.C. Wiley. 1993. The antigenic identity of peptide-MHC complexes: A comparison
of the conformations of five viral peptides presented by HLA-A2 Cell 75:: 693-708. (PubMed)
T-cell receptors and signaling complexes
J. Hennecke and D.C. Wiley. 2001. T cell receptor-MHC interactions up close Cell 104:: 1-4. (PubMed)
Y.H. Ding, K.J. Smith, D.N. Garboczi, U. Utz, W.E. Biddison, and D.C. Wiley. 1998. Two human T cell receptors bind
in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids Immunity 8:: 403-411.
(PubMed)
E.L. Reinherz, K. Tan, L. Tang, P. Kern, J. Liu, Y. Xiong, R.E. Hussey, A. Smolyar, B. Hare, R. Zhang, A. Joachimiak,
H.C. Chang, G. Wagner, and J. Wang. 1999. The crystal structure of a T cell receptor in complex with peptide and MHC
class II Science 286:: 1913-1921. (PubMed)
J.R. Cochran, T.O. Cameron, and L.J. Stern. 2000. The relationship of MHC-peptide binding and T cell activation
probed using chemically defined MHC class II oligomers Immunity 12:: 241-250. (PubMed)
J.R. Cochran, T.O. Cameron, J.D. Stone, J.B. Lubetsky, and L.J. Stern. 2001. Receptor proximity, not intermolecular
orientation, is critical for triggering T-cell activation J. Biol. Chem. 276:: 28068-28074. (PubMed)
K.C. Garcia, L. Teyton, and I.A. Wilson. 1999. Structural basis of T cell recognition Annu. Rev. Immunol. 17:: 369-397.
(PubMed)
B.S. Gaul, M.L. Harrison, R.L. Geahlen, R.A. Burton, and C.B. Post. 2000. Substrate recognition by the Lyn protein-
tyrosine kinase: NMR structure of the immunoreceptor tyrosine-based activation motif signaling region of the B cell
antigen receptor J. Biol. Chem. 275:: 16174-16182. (PubMed)
P.S. Kern, M.K. Teng, A. Smolyar, J.H. Liu, J. Liu, R.E. Hussey, R. Spoerl, H.C. Chang, E.L. Reinherz, and J.H. Wang.
1998. Structural basis of CD8 coreceptor function revealed by crystallographic analysis of a murine CD8alphaalpha
ectodomain fragment in complex with H-2Kb Immunity 9:: 519-530. (PubMed)
R. Konig, S. Fleury, and R.N. Germain. 1996. The structural basis of CD4-MHC class II interactions: Coreceptor
contributions to T cell receptor antigen recognition and oligomerization-dependent signal transduction Curr. Top.
Microbiol. Immunol. 205:: 19-46. (PubMed)
M. Krummel, C. Wulfing, C. Sumen, and M.M. Davis. 2000. Thirty-six views of T-cell recognition Philos. Trans. R.
Soc. Lond. B Biol. Sci. 355:: 1071-1076. (PubMed)
C.J. Janeway. 1992. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T
cell activation Annu. Rev. Immunol. 10:: 645-674. (PubMed)
E.R. Podack and A. Kupfer. 1991. T-cell effector functions: Mechanisms for delivery of cytotoxicity and help Annu.
Rev. Cell Biol. 7:: 479-504. (PubMed)
M.M. Davis. 1990. T cell receptor gene diversity and selection Annu. Rev. Biochem. 59:: 475-496. (PubMed)
D.J. Leahy, R. Axel, and W.A. Hendrickson. 1992. Crystal structure of a soluble form of the human T cell coreceptor
CD8 at 2.6 Å resolution Cell 68:: 1145-1162. (PubMed)
B. Lowin, M. Hahne, C. Mattmann, and J. Tschopp. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and
Fas lytic pathways Nature 370:: 650-652. (PubMed)
HIV and AIDS
A.S. Fauci. 1988. The human immunodeficiency virus: Infectivity and mechanisms of pathogenesis Science 239:: 617-
622. (PubMed)
R.C. Gallo and L. Montagnier. 1988. AIDS in 1988 Sci. Am. 259: (4) 41-48. (PubMed)
P.D. Kwong, R. Wyatt, J. Robinson, R.W. Sweet, J. Sodroski, and W.A. Hendrickson. 1998. Structure of an HIV gp120
envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody Nature 393:: 648-659.
(PubMed)
Discovery of major concepts
G.L. Ada and G. Nossal. 1987. The clonal selection theory Sci. Am. 257: (2) 62-69. (PubMed)

R.R. Porter. 1973. Structural studies of immunoglobulins Science 180:: 713-716. (PubMed)
G.M. Edelman. 1973. Antibody structure and molecular immunology Science 180:: 830-840. (PubMed)
G. Kohler. 1986. Derivation and diversification of monoclonal antibodies Science 233:: 1281-1286. (PubMed)
C. Milstein. 1986. From antibody structure to immunological diversification of immune response Science 231:: 1261-
1268. (PubMed)
C.A. Janeway Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology Cold Spring Harbor
Symp. Quant. Biol. 54:: 1-13. (PubMed)
IV. Responding to Environmental Changes
34. Molecular Motors
Organisms, from human beings to bacteria, move to adapt to changes in their environments, navigating toward food and
away from danger. Cells, themselves, are not static but are bustling assemblies of moving proteins, nucleic acids, and
organelles (Figure 34.1). Remarkably, the fundamental biochemical mechanisms that produce contractions in our
muscles are the same as those that propel organelles along defined paths inside cells. In fact, many of the proteins that
play key roles in converting chemical energy in the form of ATP into kinetic energy, the energy of motion, are members
of the same protein family, the P-loop NTPases. These molecular motors are homologous to proteins that we have
encountered in other contexts, including the G proteins in protein synthesis, signaling, and other processes. Once again
we see the economy of evolution in adapting an existing protein to perform new functions.
Molecular motors operate by small increments, converting changes in protein conformation into directed motion.
Orderly motion across distances requires a track that steers the motion of the motor assembly. Indeed, we have
previously encountered a class of molecular motors that utilize mechanisms that we will examine here
namely, the
helicases that move along DNA and RNA tracks (Section 28.1.7). The proteins on which we will focus in this chapter
move along actin and microtubules protein filaments composed of repeating identical subunits. The motor proteins
cycle between forms having high or low affinity for the filament tracks in response to ATP binding and hydrolysis,
enabling a bind, pull, and release mechanism that generates motion.
We will also consider a completely different strategy for generating motion, one used by bacteria such as E. coli. A set of
flagella act as propellers, rotated by a motor in the bacterial cell membrane. This rotary motor is driven by a proton
gradient across the membrane, rather than by ATP hydrolysis. The mechanism for coupling the proton gradient to
rotatory motion is analogous to that used by the F
0
subunit of ATP synthase (Section 18.4.2). Thus, both of the major
modes for storing biochemical energy
namely, ATP and ion gradients have been harnessed by evolution to drive
organized molecular motion.
IV. Responding to Environmental Changes 34. Molecular Motors
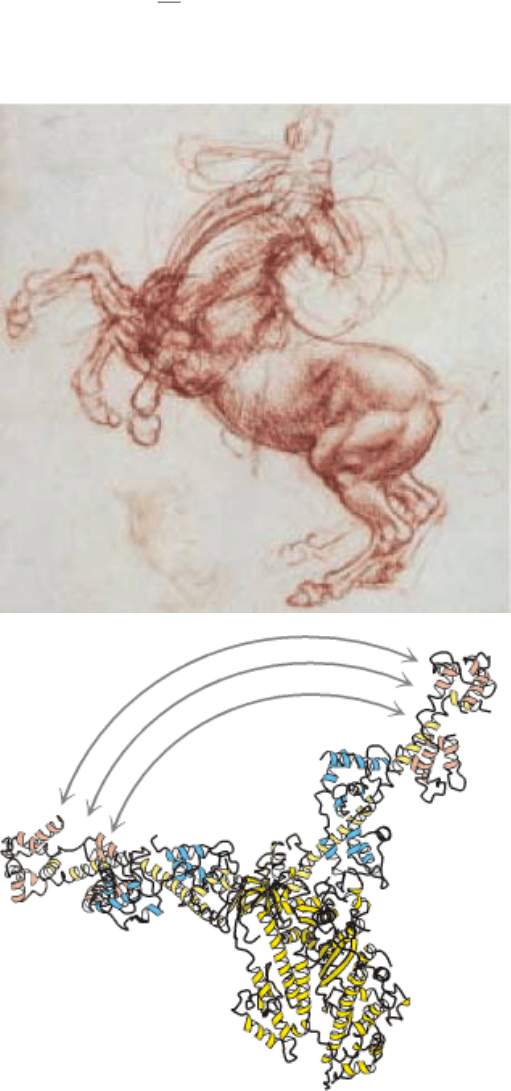
Figure 34.1. Motion Within Cells. This high-voltage electron micrograph shows the mitotic apparatus in a metaphase
mammalian cell. The large cylindrical objects are chromosomes, and the threadlike structures stretched across the center
are microtubules tracks for the molecular motors that move chromosomes. Many processes, including chromosome
segregation in mitosis, depend on the action of molecular-motor proteins. [Courtesy of J. R. McIntosh.]
IV. Responding to Environmental Changes 34. Molecular Motors
The horse, like all animals, is powered by the molecular motor protein, myosin. A portion of myosin moves
dramatically (as shown above) in response to ATP binding, hydrolysis, and release, propelling myosin along an actin
filament. This molecular movement is translated into movement of the entire animal, excitingly depicted in da Vinci's
rearing horse. [(Left) Leonardo da Vinci's "Study of a rearing horse" for the battle of Anghiari (c. 1504) from The Royal
Collection © Her Royal Majesty Queen Elizabeth II.]
IV. Responding to Environmental Changes 34. Molecular Motors
34.1. Most Molecular-Motor Proteins Are Members of the P-Loop NTPase
Superfamily
Eukaryotic cells contain three major families of motor proteins: myosins, kinesins, and dyneins. At first glance, these
protein families appear to be quite different from one another. Myosin, first characterized on the basis of its role in
muscle (Section 34.2.1), moves along filaments of the protein actin. Muscle myosin consists of two copies each of a
heavy chain with a molecular mass of 87 kd, an essential light chain, and a regulatory light chain. The human genome
appears to encode more than 40 distinct myosins; some function in muscle contraction and others participate in a variety
of other processes. Kinesins, which have roles in protein, vesicle, and organelle transport along microtubules, including
chromosome segregation, often consist of two copies each of a heavy chain and a light chain. The heavy chain is
approximately one-half the size of that for myosin. The human genome encodes more than 40 kinesins. Dynein powers
the motion of cilia and flagella in some eukaroytic cells, among other roles. Dyneins are enormous, with heavy chains of
molecular mass greater than 500 kd. The human genome appears to encode approximately 10 dyneins.
Comparison of the amino acid sequences of myosins, kinesins, and dyneins did not reveal significant relationships
between these protein families but, after their three-dimensional structures were determined, members of the myosin and
kinesin families were found to have remarkable similarities. In particular, both myosin and kinesin contain P-loop
NTPase cores homologous to those found in G proteins. Sequence analysis of the dynein heavy chain reveals it to be a
member of the AAA subfamily of P-loop NTPases that we encountered previously in the context of the 19S proteasome
(Section 23.2.2). Dynein has six sequences encoding such P-loop NTPase domains arrayed along its length. Thus, we
can draw on our knowledge of G proteins and other P-loop NTPases as we analyze the mechanisms of action of these
motor proteins.
34.1.1. A Motor Protein Consists of an ATPase Core and an Extended Structure
Let us first consider the structure of myosin. The results of electron microscopic studies of skeletal muscle myosin show
it to be a two-headed structure linked to a long stalk (Figure 34.2). As we saw in Chapter 33, limited proteolysis can be a
powerful tool in probing the activity of large proteins. Treatment of myosin with trypsin and papain results in the
formation of four fragments: two S1 fragments, an S2 fragment, also called heavy meromyosin (HMM), and a fragment
called light meromyosin (LMM; Figure 34.3). Each S1 fragment corresponds to one of the heads from the intact structure
and includes 850 amino-terminal amino acids from one of the two heavy chains as well as one copy of each of the light
chains. Examination of the structure of an S1 fragment at high resolution reveals the presence of a P-loop NTPase-
domain core that is the site of ATP binding and hydrolysis (Figure 34.4).
Extending away from this structure is a long α helix from the heavy chain. This helix is the binding site for the two light
chains. The light chains are members of the EF-hand family, similar to calmodulin, although most of the EF hands in
light chains do not bind metal ions (Figure 34.5). Like calmodulin, these proteins wrap around an α helix, serving to
thicken and stiffen it. The remaining fragments of myosin S2 and light meromyosin are largely α helical, forming
two-stranded coiled coils created by the remaining lengths of the two heavy chains wrapping around each other (Figure
34.6). These structures, together extending approximately 1700 Å, link the myosin heads to other structures. In muscle
myosin, several LMM domains come together to form higher-order bundles.
Conventional kinesin, the first kinesin discovered, has a structure having several features in common with myosin
(Figure 34.7). The dimeric protein has two heads, linked by an extended structure. The size of the head domain is
approximately one-third of that of myosin. Determination of the three-dimensional structure of a kinesin fragment
revealed that this motor protein also is built around a P-loop NTPase core (Figure 34.8). The myosin domain is so much
larger than that of kinesin because of two large insertions in the myosin domain. For conventional kinesin, a region of
approximately 500 amino acids follows the head domain. Like the corresponding region in myosin, the extended part of
kinesin forms an α-helical coiled coil. Unlike myosin, the α-helical region directly adjacent to the head domain is not the
binding site for kinesin light chains. Instead, kinesin light chains, if present, bind near the carboxyl terminus.
