Basdevant J.-L., Rich J., Spiro M. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
Подождите немного. Документ загружается.

8.3 Stellar nucleosynthesis 373
8.3 Stellar nucleosynthesis
One of the great triumphs of nuclear physics has been its ability to provide
semiquantitative understanding of the observed abundances of the elements
and their isotopes. Most attention has focused on “solar-system abundances”
that mostly reflect the initial composition of the pre-solar cloud that con-
densed 4.5 million years ago to form the Sun, the planets, and meteorites.
About 98% of the solar system mass consists of
1
Hand
4
He and most of
these two nuclei were produced in the primordial Universe when the cosmo-
logical temperature was kT ∼ 60keV. The processes leading to the formation
of these two elements will be discussed in Chap. 9. The remaining 2% of the
solar system mass consists of heavier elements that are believed to have been
produced in stars. Prior to the formation of the solar system, these elements
were dispersed into the interstellar medium either by continuous mass loss or
by supernovae-like events. This pollution of the primordial mixture of helium
and hydrogen was essential for the formation of Earth-like planets and the
emergence of life.
8.3.1 Solar-system abundances
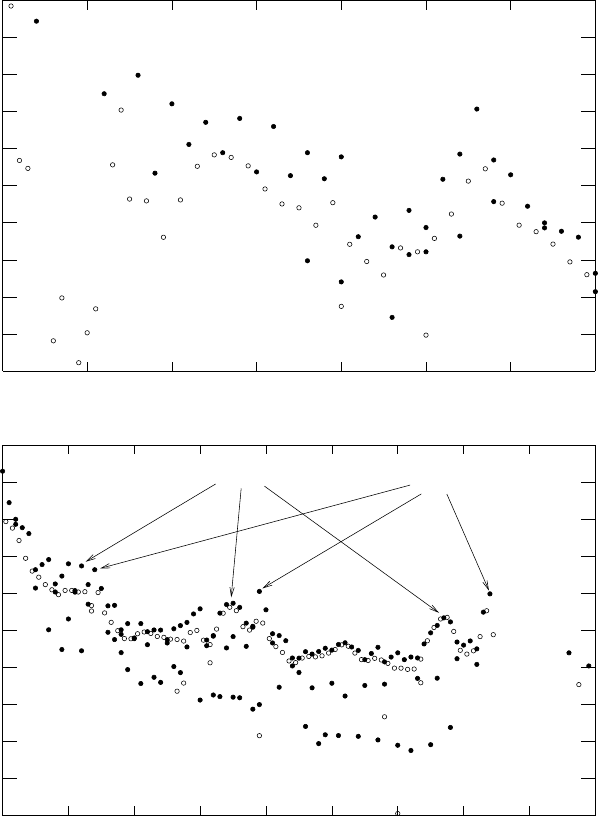
The estimated solar-system abundances are shown in Fig. 8.9 [79]. The dis-
tribution falls with increasing A with peaks at the α nuclei
4
He,
12
C,
16
O,
20
Ne,
24
Mg,
28
Si,
32
S,
36
Ar and
40
Ca that all consist of an integer number of
4
He nuclei. A prominent peak is also seen at
56
Fe which is believed to be the
result of the decay of the α nuclei
56
Ni produced in the last stage of stellar
nuclear burning.
Beyond the iron peak, the distribution continues to fall with increasing A
but shows peaks at A =80, 87, 130, 138, 195, 208. We will see that these peaks
are due to the systematics of neutron captures responsible for the production
of heavy elements.
The solar-system abundances are derived from a variety of sources. Only
the Sun and the giant planets could be expected to have started with an en-
tirely representative sample of material since gravitational attraction was the
dominant factor in the formation of these bodies. The small planets and me-
teorites condensed out via processes that depended on chemistry and caution
must therefore be exercised in using elemental abundances derived from these
bodies. While chemical separation is important, the formation of small bod-
ies would not have been expected to result in isotopic separation (with a few
exceptions). Therefore, isotopic abundance ratios for the Earth or meteorites
can generally be taken as representative of the Solar System.
Since most of the mass of the solar system is in the Sun, it would seem
best to use “photospheric abundances” from the absorption lines that appear
in the Sun’s continuous spectrum. Reliable elemental abundances can be de-
rived for most elements. It should be emphasized that photosphere estimates
depend on detailed models of the Solar atmosphere since the importance of
374 8. Nuclear Astrophysics
Ta
180
Pb
208
58
Ni
Fe
54
36
Ar
S
32
O
16
N
14
C
12
11
B
B
10
Be
9
Li
7
Li
6
He
4
He
3
H
2
H
1
Rb
88
Ba
138
Th
232
U
238
U
235
Log
ρ
Log
ρ
Fe
56
40
Ca
Mg
24
nuclei
α−
Si
28
Ne
20
10
−10
0
20 30 40 50 60 70
16080 100 120 140 180 200 220 24
0
60
−14
−4
−12
−10
−8
−6
iron peak
s peaks
r peaks
p−nuclei
Te,Xe
Ir,Pt
−8
−4
−2
0
A
A
−6
Fig. 8.9. The solar system abundances ρ(A, Z)/ρ
tot
[79]. The filled circles corre-
spond to even-even nuclei. For A<70, the distribution is visually dominated by
cosmogenic
1
H and
4
He and by “iron-peak” elements near A = 56 Between these
two features, the distribution is dominated by “α-nuclei” comprised of an integer
number of
4
He nuclei. For A>60, the distribution has peaks corresponding to
neutron magic numbers that are produced by the s-process. The r-process produces
peaks shifted to lower A after neutron-rich magic-N nuclei β-decay to the bottom
of the valley of stability. Rare elements are produced by the p-process.
8.3 Stellar nucleosynthesis 375
a particular line is highly dependent on the photosphere temperature and
density which determines the populations of atomic levels. In this regard, it
should be noted that one of the most prominent lines in the solar spectrum is
due to calcium, a rather rare element. This caused a great deal of confusion
before the development of quantum mechanics allowed one to understand the
physics behind the creation of absorption lines. A more fundamental prob-
lem with the use of photosphere abundances is that they give only elemental
abundances since isotopic splittings of lines are generally narrower than the
thermally determined line widths. Exceptions are the isotopic abundances
of carbon and oxygen where the vibrational and rotational lines of the CO
molecule are directly determined by the atomic weights of the constituent
atoms.
The most accurate elemental abundances for most elements come from the
analysis of “carbonaceous chondritic meteorites” that are thought to have a
representative sample of elements with the important exceptions of hydro-
gen, carbon, nitrogen, oxygen and the noble gases. While the formation of
meteorites was a complicated process involving chemical separation, this type
contains three representative phases (silicate, sulfide, and metallic) that give
consistent results. With a few exceptions, agreement with photospheric abun-
dances is to within ±10%.
The most important elemental abundance that is accurately determined
neither by photosphere nor meteor abundances is that of
4
He. This nuclide
was not retained in the formation of small bodies. In fact, the majority of
terrestrial
4
He is believed to be due to α decay of heavy elements after the
formation of the Earth. In the Sun, helium lines are seen only in the chromo-
sphere where its abundance may not be entirely representative. In fact, the
most reliable estimation of the helium abundance appears to be that derived
from solar models where the initial helium abundance is a free parameter that
is adjusted so as to predict that correct solar radius and luminosity [73, 74].
The derived helium abundance is confirmed by measurements of the helio-
seismological oscillation frequencies [73]. These frequencies depend directly
on the sound speed v
s
∼ kT/µ where µ is the mean atomic weight. The
temperature profile is well determined in solar models so the sound speed
determines µ. Since the Sun is essentially made of hydrogen and helium, this
then determines the abundance of helium.
Once the elemental abundances are determined, the nuclear abundances
are generally found by multiplying elemental abundances by the terrestrial
isotopic abundance. Once again, this does not work for the noble gases. An
extreme example is argon where the atomic weight listed in the periodic table
is 39.948 reflecting the fact that
40
Ar is the dominant terrestrial isotope. In
reality, most terrestrial argon comes from
40
K decay (Fig. 5.1) while the α
nucleus
36
Ar dominates in the Sun. The isotopic abundance of this element
is therefore best determined from the solar wind.
376 8. Nuclear Astrophysics
8.3.2 Production of A<60 nuclei
In previous sections we have seen how a succession of stellar burning stages
produces elements from
4
He up to the iron-peak. This process favors α-nuclei
since their high binding energy involves them in many exothermic reactions.
The distribution shown in Fig. 8.9 has peaks at these elements so it is rea-
sonable to suppose that they were produced in stars and then later dispersed
into the interstellar medium.
One possible dispersion mechanism would be core-collapse supernovae. As
illustrated in Figs. 8.7, the iron core that will collapse to a neutron star is
surrounded by concentric shells of the ashes of the different stages of nuclear
burning. These shells will be blown off after the core-collapse.
To see whether this mechanism can account for the observed abundances,
we note that about 2M
of
16
O would be dispersed per supernova. The rate
for core-collapse supernova explosions in Milky Way-type galaxies is about
2 per century so there have been about 10
8
core-collapse supernova over the
∼ 10
10
yr life of our galaxy, about half of which occurred before the formation
of the solar system. This gives about 2 × 10
8
M
of
16
O or about 1% of
the total mass of the Milky Way. This nicely matches the observed solar-
system abundance of 0.9% ! While this comparison can hardly be considered
quantitative, it seems reasonable to say that a significant fraction of the
observed intermediate mass nuclei were dispersed in supernovae.
Core-collapse supernovae are not very efficient in dispersing iron-peak nu-
clei since it is these nuclei that collapsed to form a neutron star. On the other
hand, we saw in Sect. 8.2.3 that type Ia supernovae lead to the destruction
of a ∼ 1.4M
carbon–oxygen white dwarf after its explosive burning to
56
Ni.
After β-decay to
56
Fe there would be about 0.7 M
of
56
Fe dispersed into
the interstellar medium. The rate for type Ia supernovae is a about 1/4 the
core-collapse rate so the total amount of
56
Fe produced is not far from the
0.1% observed in the solar system.
8.3.3 A>60: the s-, r- and p-processes
The production of elements with A>56 is paradoxical in the sense that
thermal equilibrium requires the dominance of either iron-peak elements (at
low temperatures) or of
4
He and nucleons (at high temperatures). In spite of
this, captures of single protons or neutrons by nuclei near the stability line
are always exothermic, so it is possible to create heavy nuclei from a non-
equilibrium mixture of nucleons and iron-peak nuclei. Thermal equilibrium
could then be approached by later fissioning of the heavy nuclei, though the
time scale for spontaneous fission is so long that equilibrium is reached only
after time scales that are enormous even for astrophysics.
The modern theory of the nucleosynthesis of elements beyond the iron
peak was spelled out in a classic paper by Burbidge, Burbidge, Fowler and
Hoyle [80]. These authors realized the importance of neutron captures in
8.3 Stellar nucleosynthesis 377
the production of heavy elements. While neutrons are present in only tiny
numbers in most stars, neutron capture has the advantage not having the
Coulomb barrier associated with proton captures. Its importance is imme-
diately suggested by the fact that for values of A>56 with more than one
β-stable isobar, the most neutron-rich isobar is the most abundant.
Of course the problem with neutron captures is that normally very few
neutrons are present in stars. Important neutron-producing exothermic reac-
tions are (α,n) reactions on the relatively rare nuclei
13
Cand
22
Ne
4
He
13
C → n
16
O Q=3.00 MeV (8.69)
4
He
22
Ne → n
25
Mn Q=0.30 MeV . (8.70)
The slow build-up of heavy nuclei by capture of such neutrons is called the
“s-process” for “slow” neutron capture.
Neutrons would also be expected to be present in large numbers in explo-
sive events like supernovae or neutron-star collisions. This type of nucleosyn-
thesis is called the “r-process” for “rapid” neutron capture. In fact, we will
see that the existence of both the s- and r- process is necessary to explain
the observed abundances.
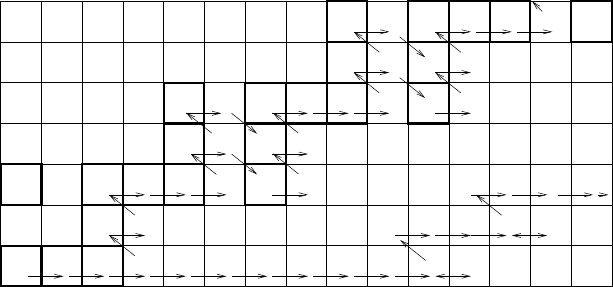
The workings of the s and r processes are illustrated in Fig. 8.10 which
shows how heavy nuclei can be constructed by neutron captures on
56
Fe.
After first reaching the stable nuclei
57
26
Fe
31
and
58
26
Fe
32
,theβ-unstable nu-
cleus
59
26
Fe
33
(t
1/2
=44.5 day) is produced. If the rate of neutron captures
is slow compared to the β-decay rate, the
59
26
Fe
33
decays “immediately” to
59
27
Co
32
. The next neutron capture produces
60
27
Co
33
which immediately de-
cays to
60
28
Ni
32
and so on. This is the s-process where neutron capture is
slow compared to β-decay. The s-process produces nuclei by following a well-
defined path along the bottom of the valley of stability. The path sometimes
bifurcates (at
64
29
Cu
35
in Fig. 8.10) only to quickly merge (at
65
29
Cu
36
).
On the other hand, if the rate of neutron capture is higher than typical
decay rates, it is possible to produce nuclei far from the bottom of the valley
of stability. Referring to Fig. 8.10, starting with
56
26
Fe
30
, the r-process can
sequentially produce
57
Fe,
58
Fe,
59
Fe
60
Fe and so on until the addition of a
further neutron yields an unstable nucleus that decays by neutron emission,
A
Fe → n
A−1
Fe. In fact, the r-process is expected to take place in at high
temperature in the presence of many photons so the limiting reaction is most
likely photon dissociation γ
A
Fe → n
A−1
Fe. In Fig. 8.10 we show this happen-
ing at
66
40
Fe
26
, though this is uncertain because nuclei so far from the bottom
of the stability valley have not been studied in the laboratory.
66
Fe has a
very short half-life, 0.4s, for β-decay producing
66
Co. This nucleus can then
captures neutrons continuing the r-process.
Events producing the r-process are expected to last a very short time
(about 10 sec for a type II supernova) and at the end of the event nuclei
would have been produced along the southern slope of the stability valley
378 8. Nuclear Astrophysics
Fe
26
Co
27
Ni
28
Cu
29
Zn
30
Ge
32
Ga
31
6.6 13.7 2.6 1.8 0.8 0.3 −0.4 −0.4 −0.3 −1.0 −0.8
7.4 6.8 8.2 3.8 2.0 1.4 −0.5 0.1 −0.6 −0.4 −0.7 −0.6 −0.8 −0.7
12.4 9.5 4.0 5.3 1.3 1.3 1.1 0.3 0.3
1.9 3.2 4.1 2.8 4.7 2.5 5.3 1.5 2.2 0.7 1.3 0.8 0.6
2.2 1.9 4.5 3.4 7.3 3.5 2.2 5.2 1.4 2.0
−0.8 −0.9 1.5 2.2 3.0 4.5 5.5 3.6 3.1 4.7 4.2 2.7 2.1
−1.0 1.8 1.5 3.9 3.1 7.4 5.1 6.0 3.7
N
=
30 3
1
3
2
33 3
4
35 36 37 38 39
4
0
41 42 4
3
44
Fig. 8.10. Nucleosynthesis by neutron capture starting at
56
Fe. The decimal loga-
rithm of the half-life in seconds is shown for β-unstable nuclei. If the neutron flux is
small, β-decay occurs “immediately” after neutron absorption and the path follows
the nuclei at the bottom of the stability valley indicated by the arrows. This is the
s-process. On the other hand, if the neutron flux is sufficiently high, the r-process
is operative where nuclei can absorb many neutrons before β-decaying so the path
may ascend the sides of the valley until the nuclei are either photo-dissociated or β
decay. Along the Fe-line, this is shown as happening at
66
40
Fe
26
. After the neutron
flux is turned off, the nuclei on the slopes of the valley β-decay down to the bottom.
Note that the neutron capture path in a nuclear reactor (Fig. 6.12) is intermediate
between the astrophysical s- and r- processes since the time for neutron absorption
is typically a month or so.
as shown in Fig. 8.11. After the neutron flux is turned off, the nuclei then
β-decay to the bottom of the valley.
The systematics of the s- and r-process explain the peaks in the abun-
dances of A>80 nuclei shown in Fig. 8.9. The s-process produces an ac-
cumulation of nuclei with magic N where subsequent neutron captures are
difficult because of the low cross-section. The s-process peaks would occur at
N =50, 82, 126 corresponding to A = 90, 138, and 208.
On the other hand, the r-process path in Fig. 8.11 follows the lower edge of
the valley but takes a northern direction at the neutron magic numbers 50, 82
and 126. This is because the addition of a neutron to a closed shell is inhibited
both because of a small cross-section for neutron capture and because, once
captured, the extra neutron is weakly bound and easily ejected by a photon.
We can therefore expect that at the end of the r-process event there is an
accumulation of nuclei along the segments of the path following the magic
N lines. After the subsequent β-decays, this will lead to an accumulation
of nuclei at A ∼ 80, 130 and 195. Such r-process peaks are observed in the
solar-system abundances (Fig. 8.9).
We note that the details of the r-process peaks depend on the extent
to which the shell structure and their magic numbers is maintained for very
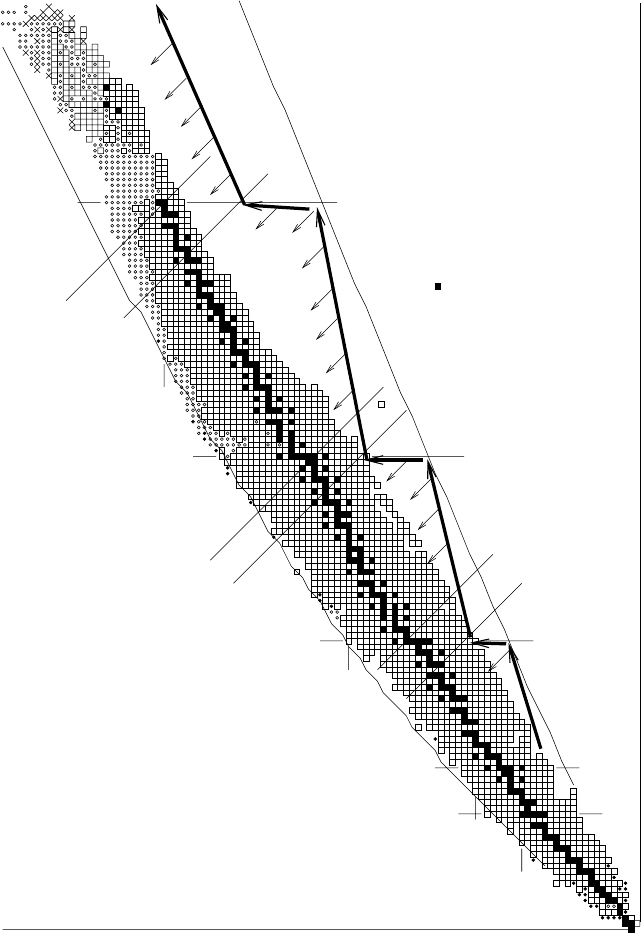
8.3 Stellar nucleosynthesis 379
Z=20
Z=28
Z=50
Z=82
N=20
N=28
N=50N=50
N=82
N=126
Z
A=208
A=195
N=126
A=138
A=130
N=82
A=90
A=80
N
stable
Fig. 8.11. The paths for nucleosynthesis by slow and fast neutron capture. In the
s-process, the prompt β-decays insure that all produced nuclei are near the bottom
the of the stability valley. In the r-process, rapid neutron absorption during an
intense pulse of neutrons leads to nuclei distributed along the thick arrow on the
southern slope of the valley. After the pulse is turned off, the nuclei β-decay down
to the bottom of the valley, as indicated by the thin arrows.
380 8. Nuclear Astrophysics
neutron-rich nuclei. This is an important area of investigation in experimental
nuclear physics.
Ba
56
La
57
Ce
58
Pr
59
Nd
60
Pm
61
Sm
62
Pd
46
Ag
47
Cd
48
In
49
Sb
51
Sn
50
26.5 11.7
48.2
12.5 12.8 24.1 12.2 28.7 7.5
4.3 95.7
1.0 0.6 0.3 14.5 7.7 24.2 8.6 32.6 4.6 5.8
57.4 42.6
2.4 6.6 7.9 11.2 71.7
0.1 99.9
0.2 0.2 88.5 11.1
100.
27.1 12.2 23.8 8.3 17.2 5.8 5.6
3.1 15.0 11.3 13.8 7.4 26.7 22.7
N=61
N=78
62 63 64 65 66 67 68 69 70 71 72 73 74 75
79 80 81 82 83 84 85 86 87 88 89 90 91 92
s−only
r−only
p−only
r−only
r−only
s−only
s−only
p−only
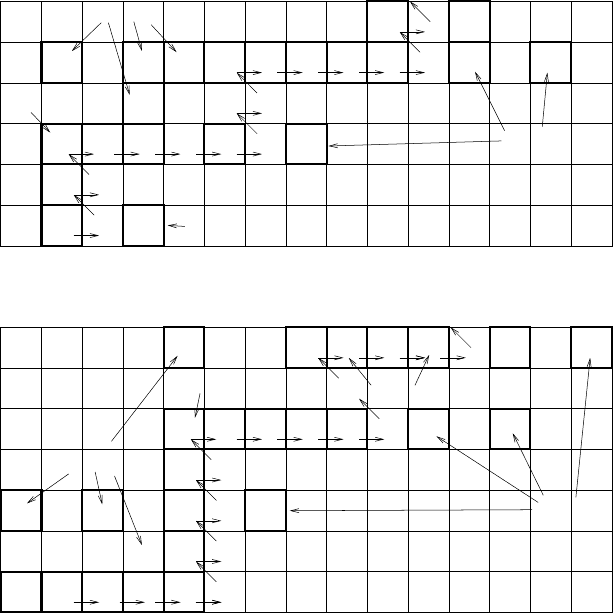
Fig. 8.12. Isotopic abundances and the s-process path for 46 ≤ Z ≤ 51 and for
56 ≤ Z ≤ 62. Nuclei below the s-process path are labeled “r-only” and can only
be produced by rapid neutron capture in, for instance, supernovae. Nuclei that are
shielded from the r-process by a r-only nucleus are labeled “s-only.” Nuclei above
the s-process path have low isotopic abundances. The can only be produced by the
“p-process,” due, for instance, to (p, γ)or(γ, n) reactions.
Further indication that both s- and r-processes are needed to explain the
solar-system abundances comes from the fact that there are nuclei that can
only be produced by the s-process and also nuclei that can only be produced
by the r-process. The s-process is clearly incapable of producing elements
heavier than
209
Bi since the β-stable elements for A = 210, 211 are the short-
lived α-emitters
210
Po (t
1/2
= 138.376 day) and
211
Bi (t
1/2
=2.14 m). The
existence of natural Uranium and Thorium therefore necessitates the exis-
tence of the r-process.
8.4 Nuclear astronomy 381
Selected r-only and s-only nuclei are shown in Fig. 8.12. This figure show
the solar-system isotopic abundances of the stable nuclei and the s-process
path. We can see in Fig. 8.12 that
110
Pd,
122
Sn, and
124
Sn cannot be produced
bythes-process.Thesameistrueof
148
Nd,
150
Nd,
152
Sm, and
154
Sm. On the
other hand,
110
Cd can only be produced by the s-process since it is “shielded”
by the β-stable nucleus
110
Pd from the β-decay of neutron-rich nuclei at the
end of an r-process event. In Fig. 8.12, we see that
148
Sm and
150
Sm are
shielded from the r-process by
148
Nd and
150
Nd.
Comparing the abundances of the s-only Sm isotopes with those of the
r-only isotopes, we see that the two processes produce comparable quantities
of nuclei. This is surprising in view of the quite different nature of the two
processes.
In Fig. 8.12 we also see a number of proton-rich nuclei that can be
produced neither by the s- nor the r-process. By definition, these nuclei
are created by the “p-process.” These nuclei all have very low solar-system
abundances indicating that the p-process is less important than the s- and
r-processes. Originally, it was thought that these nuclei would be created
by proton capture, but more recent work has indicated that (γ,n) reactions
(photo-ejection of a neutron) in explosive environments may be the dominant
process.
8.4 Nuclear astronomy
While the nuclear processes discussed in this chapter explain much about en-
ergy production in stars and nuclear abundances, direct evidence for nuclear
reactions in astrophysical conditions is extremely difficult to obtain. Most
reactions take place deep inside stars so the only products that escape are
neutrinos produced in hydrogen burning and in stellar collapse. The cross-
sections for reactions that can be used to to detect these neutrinos are, unfor-
tunately, so small that astronomical neutrinos have only been observed from
the Sun and from the nearby (∼ 50 kpc) core-collapse supernova SN1987A in
the Large Magellanic Cloud.
The other observable reaction product are γ-rays from regions of space
that are sufficiently transparent to MeV-photons. One source is long-lived
radioactive nuclei that have been dispersed into the interstellar medium by
supernovae. Much more abundant are continuous spectra of γ-rays associated
with a variety of mechanisms, often related to the acceleration and interac-
tions of cosmic-rays. All these photons have a small probability of penetrating
the Earth’s atmosphere so are better observed by γ-telescopes in orbit about
the Earth.

382 8. Nuclear Astrophysics
8.4.1 Solar Neutrinos
Neutrinos are necessarily produced in hydrogen burning stars by the weak-
interaction processes that transform protons to neutrons. Most of the 27 MeV
liberated in the hydrogen-helium transformation is thermalized in the Sun
and eventually escapes from the surface as photons. (On average, only about
500 keV immediately escapes with the neutrinos.) Therefore, the total neu-
trino production rate is simply determined by the observed solar luminosity,
L
:
dN
ν
dt
∼
2L
27 MeV
=2×10
38
Bq. (8.71)
(This formula assumes that the sun is in a steady state, an assumption that
is justified by detailed solar models.) At Earth, this gives a solar neutrino
flux
φ
ν
=6×10
6
m
−2
s
−1
. (8.72)
While the total neutrino flux is easy to calculate, the energy spectrum
is not since it depends on the nature of the nuclear reactions that create
them. As discussed in Sect. 8.2.1, energy production in the Sun is believed to
be dominated by the PPI, PPII, and PPIII cycles, which produce neutrinos
through the reactions:
1
H
1
H →
2
He
+
ν
e
Q = 420 keV PPI, II, III (8.73)
e
− 7
Be →
7
Li ν
e
Q = 862 keV PPII (8.74)
8
B →
8
Be e
+
ν
e
Q=16MeV PPIII (8.75)
The Q’s determine the energies of the produced neutrinos. Neutrinos from
reaction (8.73) share the Q with the final-state positron, so they have a
continuous spectrum with an endpoint of 420 keV. Neutrinos produced in
this reaction are often called “ν
pp
.” In PPI, two ν
pp
are produced.
In PPII, one ν
pp
is produced along with one “ν
Be
” in the electron capture
(8.74). Being produced in a two body reaction, the ν
Be
is monochromatic
with an energy of 861 keV (branching ratio 90 percent) or 383 keV (branching
ratio 10 percent with the production of an excited state of
7
Li). In PPIII,
one ν
pp
is produced along with one “ν
B
”intheβ-decay (8.75). The ν
B
have
a β-spectrum with an endpoint of 16 MeV.
Additional neutrinos come from the CNO decays
13
N →
13
Ce
+
ν
e
Q=1.20 MeV , (8.76)
15
O →
15
Ne
+
ν
e
Q=1.73 MeV , (8.77)
17
F →
17
Oe
+
ν
e
Q=1.74 MeV , (8.78)
and to the “pep” reaction
