Basdevant J.-L., Rich J., Spiro M. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
Подождите немного. Документ загружается.

6.9 The Oklo prehistoric nuclear reactor 323
In 100 kg of initial fuel, i.e. 3 kg of
235
U and 97 kg of
238
U, there remains,
after three years of running:
• 0.9kg of
235
U (2 kg have fissioned)
• 97 kg of
238
U (2 kg have been transformed into
239
Pu)
• 0.6kg of
239
Pu (in the above 2 kg, 1 kg fissioned)
• 1.7 kg of fission products.
It is necessary to renew the fuel elements periodically. The reactor is
stopped each year for 3 or 4 weeks. One third or one quarter of the fuel is
renewed.
The fuel re-processing . The re-processing consists of separating, in the
irradiated fuel, the uranium and the plutonium (which can be used again)
from the fission products (which are essentially useless and are very radioac-
tive, as shown in Fig. 6.15). The separation is basically a chemical process,
using various solvents.
The storage of fission products. The volume of liquid containing the fis-
sion products generated in one year by a 900 MW reactor is roughly 20 m
3
.
The liquid is first stored in special vessels refrigerated by water in order to
evacuate the residual heat. After a few years, it is possible to evaporate the
solvents and to vitrify the waste. The vitrified blocks can be stored under-
ground in ventilated areas.
After several years, the problem of long term storage of the radioactive
vitrified waste arises. Several methods are envisaged, in particular deep un-
derground storage in salt or granite.
Accelerators coupled to fissile matter could have an interesting application
in that they can be designed to destroy long lifetime radioactive waste. Un-
der neutron irradiation, plutonium, trans-plutonium elements such as Ameri-
cium and Curium, and other dangerous long-lived fission products such as
Technetium can absorb neutrons and either be transformed to short-lived or
stable isotopes or fission into lighter elements. (as a general rule, the lighter
the elements, the less dangerous they are).
6.9 The Oklo prehistoric nuclear reactor
It is interesting to note that if civilization had taken 2.5 billion years to de-
velop on Earth rather than 4.5 billion years, the use of nuclear reactors would
have been much simpler because at that time the abundance of
235
Uwassuf-
ficiently high that enrichment would have been unnecessary. In fact, on at
least one occasion, the necessary conditions for stable reactor operation were
united apparently without intelligent intervention. These conditions include
a sufficiently high concentration of uranium (> 10% by weight), a sufficiently
low concentration of nuclei with high neutron-absorption cross-sections, and
sufficient water (> 50% by weight) to serve as the moderator.
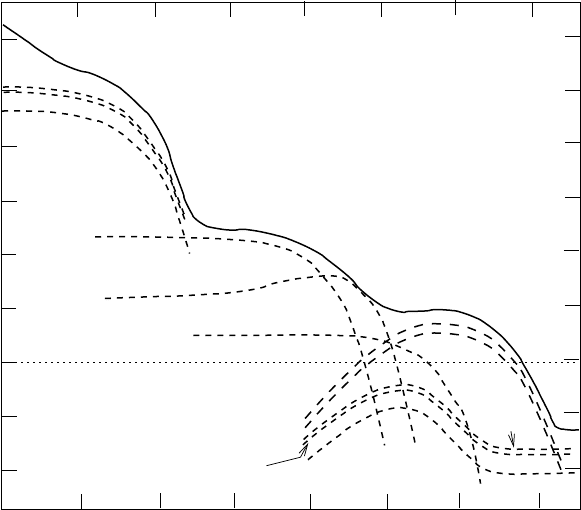
324 6. Fission
−4
−2
2
4
10
10
10 10
10
10 10
10
23
4
6
7
Years after processing
137mBa
90Y
137Cs
243Am
239Pu
99Tc
213Po
214Bi
222Rn = 210Po
218Po
229Th
1
10
10
10
5
thermal power (W)
original U
Fig. 6.15. Radioactivity of the fission products and trans-uranium elements in fuel
that has produced 100 Mw-yr of electrical energy [65]. It is assumed that 99.5%
of the uranium and plutonium was removed for reprocessing. For comparison, also
shown is the activity of the original uranium.
These conditions were present in a uranium-ore deposit in Oklo, Gabon
[67]. As illustrated in Fig. 6.16, the uranium ore in this deposit is depleted
in
235
U (as little as 0.42% instead of 0.72%) and enriched in fission products.
It is believed that the reactor operated ∼ 1.8 × 10
9
years ago for a period of
∼ 10
6
yr.
One interesting result of studies of the Oklo reactor is a very stringent
limit on the time variation of the fundamental constants [68], even stronger
than those derived from the study of
187
Re decay in Sect. 5.5.2. The limit
comes from the observation that the nuclide
149
Sm has an Oklo abundance
that is typical of reactor wastes, i.e. about 40 times less than the natural
isotopic abundance of 13.8%. This low abundance comes about because of a
resonance for thermal neutron capture (Fig. 6.17) that transforms
149
Sm to
150
Sm in the high neutron flux.
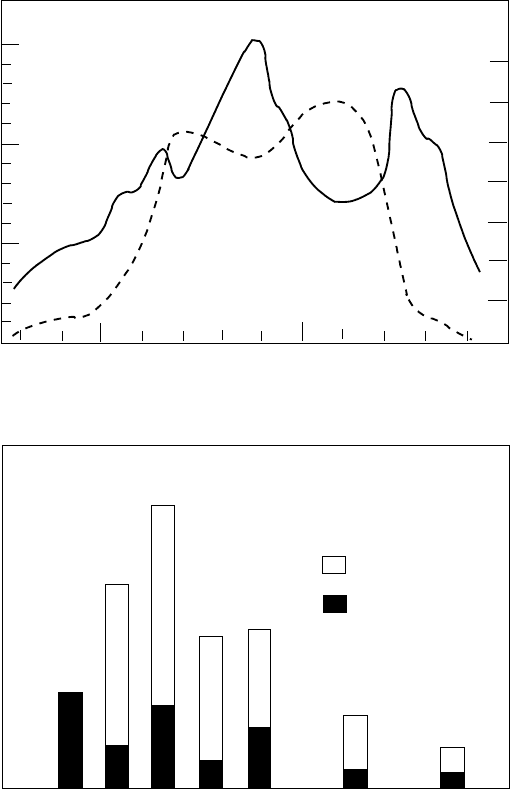
6.9 The Oklo prehistoric nuclear reactor 325
0.72
0.62
0.52
0.42
20
40
60
U−235 / U−238 (%)
U−238/ all (% by weight)
1.5 2.0
transverse postion (meters)
142
143
144
145 146 148
150
Oklo
Terrestrial
Nd
A
isotopic abundance
Fig. 6.16. The composition of a uranium deposit in Oklo, Gabon [67]. The deposit
contains several layers, each about 1 meter thick, containing very rich ore, about
50% uranium by weight. The top panel shows the uranium abundance profile across
the layer (dashed line). The solid line shows that
235
U is highly depleted in the
layer, as little as 0.42% compared to the normal 0.72%. The bottom panel shows
the abundances of Nd isotopes. The fission products
143
Nd −
150
Nd all have larger
than normal abundances compared to that of
142
Nd which cannot be produced by
fission (Exercise 6.5).
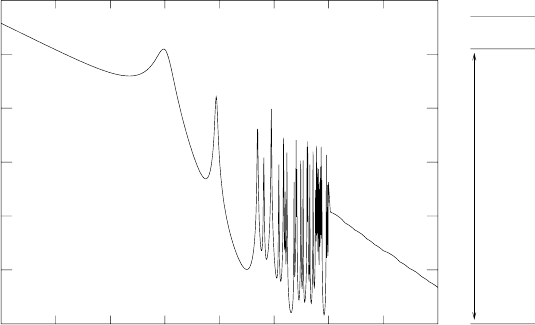
326 6. Fission
n
S
n
S
n
S
4
10
3
6
10
1
cross−section (barns)
10
neutron energy (eV)
10
110
2−4
10
−2
150
Sm
=7.987 MeV
+0.9 eV
+0.09 eV
Fig. 6.17. The radiative neutron capture cross-section on
149
Sm. The absorp-
tion resonances correspond to excited states of
150
Sm that are above the neutron-
separation energy S
n
=7.987 MeV. The diagram on the right shows the ground
state and the first two states responsible for the resonances. The first one at S
n
+0.09
can resonantly absorb thermal neutrons (3kT =0.078 eV for T = 300 K).
Thermal neutron-capture on nuclide (A, Z) are due to highly excited
states of (A +1,Z) that can decay either by photon emission or by emis-
sion of a neutron of energy E
n
∼ kT ∼ 0.02 eV. This means that, relative
to the ground state of (A +1,Z), the excited state must have an energy
E ∼ S
n
+0.02 eV where S
n
∼ 8 MeV is the neutron separation energy. The
fact that the thermal-neutron-capture resonance in
149
Sm was still operative
10
9
years ago means that the level has not changed by more than 0.02 eV
over this period. This corresponds to a relative change < 10
−8
.
The positions of nuclear levels depend on the values of the fundamental
constants, so this limit on the change in the level can be transformed into a
limit on changes in the constants. About 1% of the levels energy is electro-
static, so the limit of 10
−8
on the level change is conservatively interpreted
as a limit of 10
−6
on the change in the fine-structure constant over the last
2 billion years [68].
6.10 Bibliography
1. O. Hahn and F. Strassman, Naturwissenschaften, 27, 11 (1939).
2. L. Meitner and O.R. Frisch, Nature, 143, 239 (1939).
3. N. Bohr and J.A. Wheeler, Phys. Rev., 56, 426, (1939).
Exercises for Chapter 6 327
4. A.M. Weinberg and E.P. Wigner, The Physical Theory of Neutron Chain
Reactors, University of Chicago Press, Chicago, Ill., 1958.
5. L. Wilets, Theories of Nuclear Fission, Clarendon Press, Oxford, 1964.
6. S. Glasstone and A. Sesonke, Nuclear Reactor Engineering, Van Nos-
trand, New York, 1967.
Exercises
6.1 Consider the typical fission process induced by thermal neutrons
n
235
92
U →
140
54
Xe +
93
38
Sr + ν neutrons . (6.76)
What are the values of ν and Q
fis
for this reaction? Calculate the total fission
energy of 1 kg of
235
92
U assuming all fissions proceed via this reaction.
6.2 Assume that the average time τ between production and absorption of a
neutron in a reactor is 10
−3
s. Calculate the number of free neutrons present
at any time in the core when the reactor is operating at a power level of
1GW.
6.3 A beam of neutrons of 0.1eVisincidenton1cm
3
of natural uranium.
The beam flux is 10
12
neutrons s
−1
cm
2
. The fission cross section of
235
Uat
that energy is 250 b. The amount of
235
Uis0.72%. The density of uranium
is 19 g cm
−3
. Each fission produces 165 MeV in the material. What is the
nuclear power produced?
6.4 Consider a nuclear plant producing an electric power of 900 MW with
thermal neutrons and enriched uranium at 3.32% in
235
U. The total yield of
nuclear energy into electric energy is R =1/3 (including the thermal yield).
The total uranium mass is 70 tons.
1. How many
235
U atoms are burnt per second?
2. What mass of
235
U is used per day?
3. Assuming the plant works at constant full power, how long can it run
before changing the fuel?
6.5 Using the Table in Appendix G, explain why
142
Nd would not be ex-
pected to be abundantly produced in a nuclear reactor, unlike the other stable
Nd isotopes.
6.6 Estimate the amount of uranium needed to create 100 Mw-yr of electrical
energy assuming a thermal-to-electricity efficiency of 0.3. This is the amount
328 6. Fission
of uranium considered in Fig. 6.15. In this figure, translate the thermal power
to decay rate (in Bq) by assuming ∼ 5 MeV per decay. Discuss the origin of
the nuclides shown in the figure.
7. Fusion
Fusion reactions, taking place in the Sun, have always been the main source
of energy on Earth. Even fossil fuels like coal and petroleum were fabricated
through photosynthesis and should therefore be considered as stored solar en-
ergy. The only exception is fission energy due to the heavy elements uranium
and thorium, which were synthesized during supernova explosions.
Since the first explosive occurrence of fusion on Earth in 1952, mankind
has had the ambition to tame that form of energy. It is cleaner than fission,
creating much less long-lived radioactive waste. Its resources are unlimited
over historical time scales. In 300 liters of sea water, there is 1 g of deuterium.
Thefusionoftwo
2
H nuclei yields about 10 MeV ∼ 2 ×10
−12
J. Therefore the
water in the oceans could provide sufficient energy for human needs during
time scales of several hundred millions of years. It is particularly frustrating
to see that, unlike fission which was used industrially a few years after its
discovery, fusion is still in a prospective stage more than 50 years after its
first terrestrial use.
The apparent difficulty in using fusion reactions comes from the fact that,
unlike neutron-induced fission reactions, fusion reaction involve positively
charged nuclei that, at normal temperatures and densities, are prevented
from reacting by the Coulomb barrier. The challenge of taming fusion is to
maintain a (non-explosive) plasma that is sufficiently hot and dense to have
a useful rate of fusion.
In this chapter, we will first catalog the possible fusion reactions with the
conclusion that the most promising reaction for terrestrial energy reaction is
deuterium-tritium fusion
dt →
4
He n + 17.5MeV . (7.1)
We will then calculate fusion rates in a hot plasma as a function of tem-
perature and density. In Sect. 7.2 we derive performance criteria for fusion
reactors, in particular the Lawson criterion for effective energy generation.
The basic problem will be to maintain a plasma at a sufficiently high temper-
ature and pressure for a sufficiently long time before it cools down, mostly
through photon emission by electrons (bremsstrahlung) and atomic impu-
rities. Sections 7.3 and 7.4 will then discuss the problems of the two most
widely discussed method of maintaining the plasma, those using magnetic
confinement and those using laser induced inertial implosion.
330 7. Fusion
7.1 Fusion reactions
Because the binding energy per nucleon generally increases with A for A<50,
the fusion of two light nuclei is frequently exothermic. Some examples are :
dd →
3
He n + 3.25 MeV (7.2)
dd →
3
H p + 4 MeV (7.3)
dt →
4
He n + 17.5MeV . (7.4)
Reactions like (7.4) that produce
4
He are particularly exothermic owing to
the large binding energy of that nucleus. Other examples are reactions that
yield
4
He from the weakly bound Li-Be-B nuclei:
n
6
Li →
3
H
4
He + 4.8 MeV (7.5)
d
6
Li → 2
4
He + 22.4 MeV (7.6)
p
11
B → 3
4
He + 8.8MeV . (7.7)
The above reactions are the “terrestrial ” reactions with which we are
concerned in this chapter. The basic fusion reaction in the Sun is
pp →
2
He
+
ν
e
+0.42 MeV . (7.8)
This reaction transforms primordial hydrogen into deuterium, which sub-
sequently fuses to
4
He, and then to the heavier elements of which we are
made. Unlike the terrestrial fusion reactions, (7.8) is due to the weak inter-
actions because of the necessity for transforming a proton into a neutron.
Consequently, it has a tiny cross-section which, forgetting the Coulomb bar-
rier penetration probability, is of order ∼ (G
F
/(hc)
3
)
2
(¯hc)
2
E
2
∼ 10
−47
cm
2
,
with E =0.42 MeV. This makes it impossible to observe in a laboratory.
One would have to observe ∼ 10
20
proton–proton collisions in order to have
a chance of seeing one event of the type (7.8).
In the terrestrial reactions (7.2-7.7), the neutrons are already present in
the initial nuclei, having been produced in the primordial Universe (Chap. 9).
Therefore the reactions can proceed through the strong and electromagnetic
interactions.
The interest of fusion lies in two main facts. For civil uses, the main
advantage over fission is the large amount of available fuel, i.e. hydrogen
isotopes. For military uses, the advantage is the absence of any critical mass
constraint. The power of a fission device is limited by the fact that the pre-
ignition masses of its components cannot exceed the critical mass. This is
not the case for fusion devices since the device can explode if and only if
it is brought to sufficiently high temperatures and densities. The amount
of material is irrelevant. For the same reason, a controlled fusion installation
does not have the same risks of a nuclear accident that a critical fission system
presents.
If fusion is still not yet exploited, it is due to the fact that there are
tremendous unsolved technical problems in achieving it.
7.1 Fusion reactions 331
The best fuel for terrestrial fusion is the deuterium-tritium mixture gen-
erating energy through reaction (7.4). This is done in association with some
amount of
6
Li which absorbs neutrons and regenerates the tritium through
reaction (7.5).
Tritium itself is β-unstable,
3
H →
3
He e
−
¯
ν
e
t
1/2
=12.33 yr , (7.9)
and is consequently not present in large quantities on Earth. It is produced by
neutron irradiation of
6
Li, exploiting the exothermic reaction (7.5), or by ra-
diative neutron capture by deuterium. The radioactivity of tritium obviously
creates practical problems for its manipulation.
7.1.1 The Coulomb barrier
The physical difficulty in achieving fusion reactions is due to the fact that,
unlike neutron absorption in fission reactions, the nuclei which interact are
charged and, therefore, have a Coulomb repulsion. In order for the nuclei to
have strong interactions, they must approach one another at a distance of the
order of the nuclear forces, i.e. the radius of the nuclei. This is once again a
situation where one must cross an electrostatic potential barrier. The barrier
has a height given by
Z
1
Z
2
e
2
4π
0
a
=
Z
1
Z
2
α¯hc
a
=1.4MeV × Z
1
Z
2
1fm
a
, (7.10)
where a is the distance within which the attractive nuclear forces become
larger than the Coulomb force. For energies or temperatures less than ∼
1 MeV the barrier is operative.
If E is the energy of the particle impinging on this barrier, the probability
to cross the barrier by quantum tunneling is proportional to the Gamow
factor we have seen in Sect. 2.6:
P ∼ exp
⎡
⎣
−2
b
a
2m(V (r) − E)
¯h
2
dr
⎤
⎦
(7.11)
where m is the reduced mass m = m
1
m
2
/(m
1
+ m
2
) of the two interacting
nuclei and b is the classical turning point defined by V (b)=E where V (r)is
the repulsive Coulomb potential.
The integral in the right hand side of (7.11) can be calculated easily, since
we note that
a b =
Z
1
Z
2
e
2
4π
0
E
=
Z
1
Z
2
α¯hc
E
= 143 fm × Z
1
Z
2
10 keV
E
, (7.12)
and therefore the radius a can be taken equal to zero in good approxima-
tion. This leads to the following expression due to Gamow (in 1934) for the
tunneling probability:

332 7. Fusion
P ∼ exp
−2πZ
1
Z
2
e
2
4π
0
¯hv
=exp
−E
B
/E
. (7.13)
where v is the relative velocity and E = µv
2
/2 is the center-of-mass kinetic
energy for a reduced mass µ. The barrier is characterized by the parameter
E
B
=2π
2
Z
2
1
Z
2
2
α
2
µc
2
= 1052 keV × Z
2
1
Z
2
2
µc
2
1GeV
. (7.14)
Table 7.1. Some fusion reactions. The first three are used in terrestrial fusion
reactors. The last three make up the “PPI” cycle responsible for most of the energy
generation in the Sun. Note the tiny S(E) for the weak-reaction pp → de
+
ν
e
.It
can only be calculated using weak-interaction theory.
reaction QS(10 keV) E
B
E
G
(1 keV) E
G
(20 keV)
(MeV) (keV b) (keV) (keV) (keV)
dd → n
3
He 3.25 58.3 987. 5.1 37.5
dd → p
3
H 4. 57.3 987. 5.1 37.5
dt → n
4
He 17.5 14000. 1185 6.8 50.1
pp → de
+
ν
e
1.442 3.8 × 10
−22
526 5.1 37.5
pd →
3
He γ 5.493 2.5 × 10
−4
701 5.6 41.2
2
3
He → pp
4
He 12.859 5 × 10
3
25200. 18.5 136
We note that the argument of the exponential increases in absolute value
with the product of the charges and that it decreases as the inverse of the
velocity. The higher the energy of the nuclei is, the greater the probability to
tunnel through the barrier. Likewise, the larger the product of charges Z
1
Z
2
is, the higher the barrier, therefore at a given energy, it is the lighter nuclei
which can undergo fusion reactions. For particles of charge +1 (e.g. d + d)
we have
E =1keV⇒ P ∼ 10
−13
E =10keV ⇒ P ∼ 10
−3
.
This suggests that ∼ 10 keV is the order of magnitude of the kinetic energy
that the nuclei must have in order for fusion reactions to take place. (Much
below that energy, the cross section vanishes for all practical purposes.) The
energy of the nuclei comes from their thermal motion, therefore from the
temperature of the medium where they are contained. Hence the name ther-
monuclear reactions for fusion reactions.
We must find the factors of proportionality between the tunneling prob-
ability and the reaction cross-section. In Sect. 3.6, where we treated absorp-
tion reaction inhibited by potential barriers, we argued that the cross-section
should be of the form
