Basdevant J.-L., Rich J., Spiro M. Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology
Подождите немного. Документ загружается.

252 5. Radioactivity and all that
5.2.2 Cosmogenic radioactivity
Most interstellar matter is in the form of a thermalized gas (mostly hydrogen
and helium). There exists, however a non-thermal component of cosmic-rays
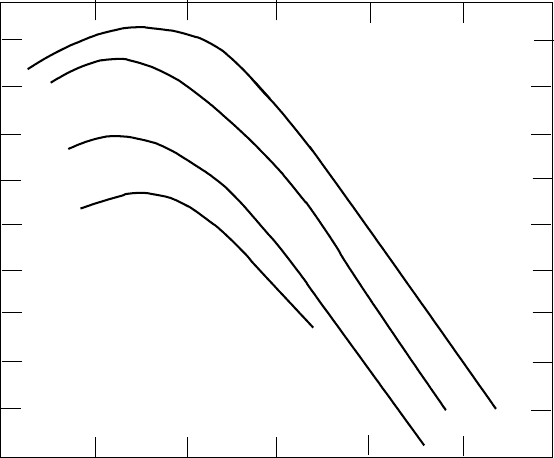
of similar chemical composition but with an energy spectrum (Fig. 5.3) peak-
ing at a kinetic energy of ∼ 300 MeV and then falling like ∼ E
−3
. While the
origin of this component is not entirely established, it is believed that it re-
sults from acceleration of particles by time-varying magnetic fields produced
by pulsars (neutron stars) and supernova remnants.
1
10
kinetic energy (MeV/nucleon)
sr s MeV/nucleon)
He
2345
6
10
10
10 10
10
C
Fe
H
flux (m
2
−1
−2
10
−4
10
10
10
−8
−6
Fig. 5.3. The flux of cosmic radiation outside the Earth’s atmosphere [54]. Most
particles are protons or
4
He nuclei with smaller numbers of heavy nuclei. Carbon
and Iron are important examples.
When cosmic-rays enter the Earth’s atmosphere, they lose energy through
ionization and nuclear reactions. The Earth’s atmosphere is sufficiently thick
that most of the primary cosmic-radiation stops in the atmosphere. Most
cosmic radiation that reaches the Earth’s surface consists of muons and neu-
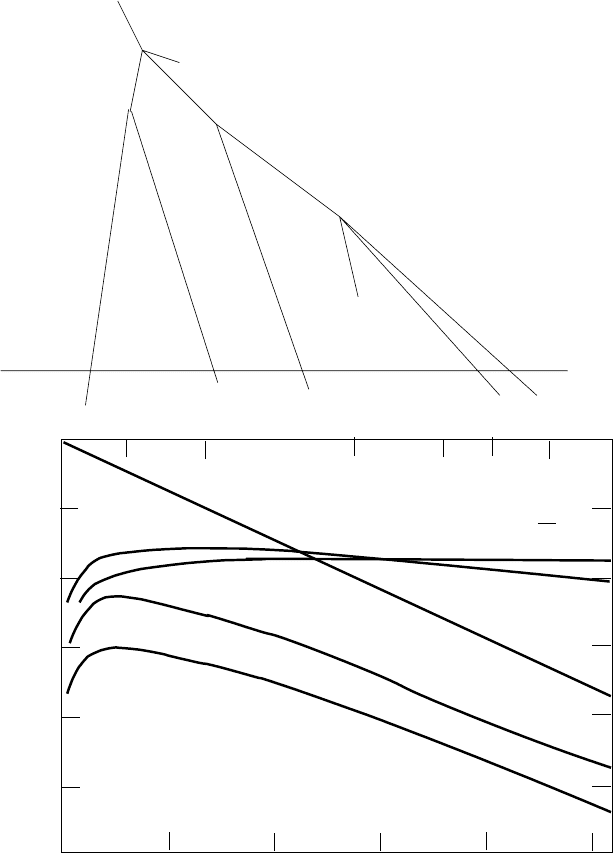
trinos from the decays of pions produced in these collisions (Fig. 5.4). A small
nuclear component consisting mostly of neutrons reaches the surface but is
quickly absorbed in the first few meters of the Earth’s crust.
5.2 Sources of radioactivity 253
0
200
400
600 800 1000
Atmospheric depth ( g cm
15
10
5
321
0
Altitude (km)
2
−2
10
−1
10
1
10
10
10
3
4
10
sr
svertical flux ( m
−2 −1 −1
)
nuclear fragment
π
µ
ν
µ
e
π
Earth surface
ν
ν
cosmic−ray proton
ν
µµ
,ν
µ
+−
p, n
e
+−
+−
π
−2
)
Fig. 5.4. An example of a “shower” induced by a cosmic-ray proton in the upper
atmosphere. Two pions and a nuclear fragment are produced when the proton
strikes a nucleus. The two pions decay π → µν. and one of the muons decay µ →
eν
¯
ν The undecayed muon reaches the Earth’s surface where it stops because of
ionization energy loss. The lower panel shows the fluxes as a function of depth in
the atmosphere [54].
254 5. Radioactivity and all that
Cosmic-rays produce radioactive nuclei via their interactions with nuclei
in the atmosphere and in the Earth’s crust. In the atmosphere, the most
common radioactivity produced is that of
14
C. This nucleus is a secondary
product of neutrons who are themselves produced by high-energy cosmic-ray
protons that breakup nuclei in the atmosphere. The neutrons then either de-
cay or are absorbed. The most common absorption process is the exothermic
(n,p) reaction on abundant atmospheric nitrogen
n
14
N →
14
Cp t
1/2
(
14
C) = 5730 yr . (5.12)
The produced
14
C is mixed throughout the atmosphere and enters the food
chain through CO
2
ingesting plants. This results in a
14
C abundance in live
organic material of about 10
−12
relative to non-radioactive carbon. The re-
sulting activity is about 250 Bq kg
−1
. As we will see in Sect. 5.5.2, this allows
the estimation of ages of dead organic material.
High energy cosmic rays also produce radioactive nuclei through “spalla-
tion” reactions where one or more nucleons are removed from a nucleus (Sect.
3.1.5). For example a neutron with kinetic energy greater than ∼ 20 MeV can
remove two nucleons for a germanium nucleus, e.g.
n
70
Ge → 3n
68
Ge t
1/2
(
68
Ge) = 270.7day . (5.13)
This reaction results in a radioactivity of 0.3 mBq kg
−1
in germanium crystals
produced at the Earth’s surface [53]. In high-purity germanium crystals used
for detection of low-level radioactivity, it is the most important source of
intrinsic radioactivity. If the crystals are placed underground, the production
of
68
Ge ceases and the activity decays away.
In rare circumstances, nucleons removed in spallation reactions can com-
bine to form nuclei. For example the radioactive tritium isotope of hydrogen
can be produced by cosmic rays by (for example) the reaction
n
16
O →
3
H
14
N t
1/2
(
3
H) = 12.35 yr . (5.14)
The atmospheric tritium combines with oxygen to form water that rains
down on the Earth. Prior to the atmospheric testing of nuclear weapons
and the Chernobyl reactor accident, this was the primary source of naturally
occurring tritium. Because of its short half-life, tritium is absent in water
from deep water reserves and also in crude oil.
5.2.3 Artificial radioactivity
Radioactive nuclei can be created in the laboratory by the same reactions
that are induced by cosmic-rays, though in the laboratory we can choose
beams and targets that maximize production rates. Two general techniques
are used, those based on charged particle beams, and those using (thermal)
neutrons produced by nuclear reactors.
To produce a radioactive nucleus, it is generally necessary to take a stable
nucleus and add or subtract nucleons, or to transmute protons to neutrons
5.2 Sources of radioactivity 255
ion source
ion trap/source
post accelerator
mass,charge separator
thin target
thick target
primary beam primary beam
secondary target
storage−
cooler ring
ISOL In−Flight
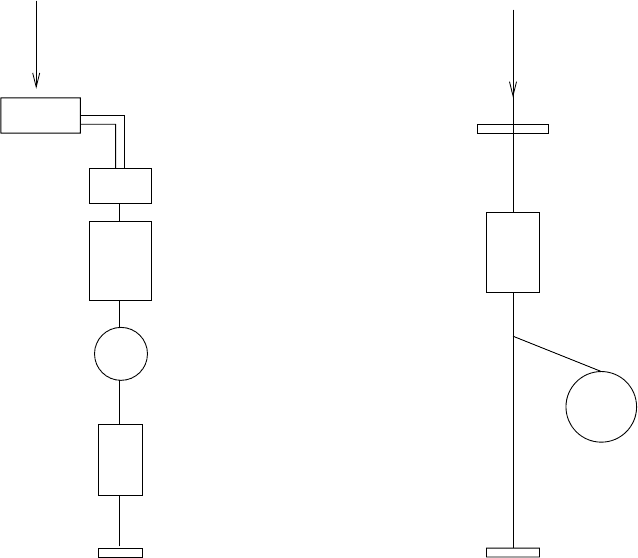
Fig. 5.5. The two primary methods of producing beams of radioactive nuclei [31].
In the ISOL method (“Isotope Separator On-Line”) a primary ion beam is incident
upon a thick target. Nuclei produced in inelastic reactions are stopped in the target
but eventually diffuse into a system that collects, purifies, and accelerates ions.
In the “In-Flight” technique, the primary ions are incident upon a target that is
sufficiently thin that produced nuclei are not stopped. Nuclei emerging from the
target can then be mass selected.
or vice versa. High energy ion collisions (Sect. 3.1.5) can be used to produce
a wide variety of nuclides by fragmentation or fusion reactions. If a specific
nuclide is desired, it is more efficient to use low-energy ions. For example, in
order to produce the radioactive germanium isotope
71
Ge (t
1/2
=11.44 day),
two simple reactions come immediately to mind. The first uses an accelerated
proton beam:
p
71
Ga → n
71
Ge . (5.15)
The proton kinetic should be about 10 MeV so that the Coulomb barrier
between the proton and gallium nucleus is insufficient to prevent the reaction,
while the energy is too small to have appreciable cross-sections for other
256 5. Radioactivity and all that
inelastic reactions involving the ejection of nucleons. A target of enriched
71
Ga is exposed to the proton beam and then dissolved and chemically treated
to extract any germanium. If the gallium is isotopically pure, the germanium
is nearly pure
71
Ge.
A second possibility uses the (n, γ) reaction
n
70
Ge → γ
71
Ge . (5.16)
A sample of germanium is placed in a neutron beam. After irradiation, the
sample contains a mixture of
70
Ge,
71
Ge and
71
Ga from the decay of
71
Ge.
Chemical treatment can then yield a mixture of stable
70
Ge and radioactive
71
Ge.
Both charged-particle- and neutron-activation are currently used to pro-
duce radioactive nuclei. Charged particles have the disadvantage in that ions
lose their energy through ionization when they enter the sample, so that they
quickly become unable to induce nuclear reactions because of the Coulomb
barrier. Thermal neutrons, on the other hand, perform a random walk until
they are absorbed. One then gets more activity per incident particle with
neutrons than with ions. For this reason, large activity sources are generally
produced in intense neutron fluxes available at nuclear reactors. For example,
cobalt sources used for medical purposes and food sterilization are produced
through the reaction
n
59
Co → γ
60
Co . (5.17)
Sources of activity > 10
16
Bq can be made by placing the sample of cobalt in
a reactor for a period of weeks (Exercise 5.2).
In nuclear research, it is often useful to produce beams of radioactive
nuclei that can be accelerated to energies necessary to study their reactions.
In recent years, much progress has been made in the production of radioactive
beams. The two generic methods of production are illustrated in Fig. 5.5.
Examples of experiments using radioactive beams are illustrated in Figs. 1.4,
1.5 and 2.18.
5.3 Passage of particles through matter
Particles produced in nuclear reactions or decays interact with matter in ways
that depend on their nature. We can distinguish the following cases:
• Charged nuclei and particles. These particles lose their energy by ionizing
the atoms in the medium and eventually come to rest. This process is
described in Sect. 5.3.1 for particles with masses m
e
. The special case
of electrons and positrons is studied in Sect. 5.3.3.
• Photons. γ-rays generally lose energy in material through Compton scat-
tering on atomic electrons
γ e
−
→ γ e
−
. (5.18)
5.3 Passage of particles through matter 257
The secondary electrons then deposit their energy in the medium through
ionization. The photon continues to Compton scatter until it is photoelec-
trically absorbed,
γ atom → e
−
atom
+
. (5.19)
Photons with energies greater than 2m
e
can be directly absorbed by pro-
duction of electron–positron pairs
γ (A, Z) → e
+
e
−
(A, Z) . (5.20)
These processes are studied in Sect. 5.3.4.
• Neutrons. Neutrons lose energy by elastic scattering on nuclei until they
thermalize. They are eventually absorbed, generally by the (n, γ) reaction
n(A, Z) → γ(A + 1, Z). These processes are described in Sect. 5.3.5.
In this section, we describe these physical process. Their biological effects
will be briefly described in Sect. 5.4.
5.3.1 Heavy charged particles
When a charged particle traverses a medium, it progressively loses its energy
by transferring it to the electrons of the atoms of the medium. The rate of
energy loss can be estimated by considering an ion of mass m
ion
and charge
z
ion
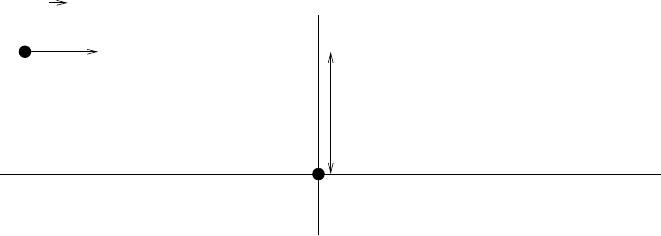
e that passes near a free electron, as illustrated in Fig. 5.6. To simplify
the calculation, we first suppose that the ion is non-relativistic, v c,and
that m
ion
m
e
.Sincem
ion
m
e
, the ion’s movement is nearly unaffected
by the close encounter with the electron so that its trajectory is, to first
approximation a straight line with impact parameter b.
yr = (vt,b)
b
electron
ion
x
Fig. 5.6. Passage of a charged particle in the vicinity of an atom.
The electron feels a Coulomb force due to the the presence of the ion
and therefore recoils after the ion’s passage. The electron’s momentum can
be calculated by integrating the force. The integral is non-zero only in the
direction perpendicular to the trajectory:
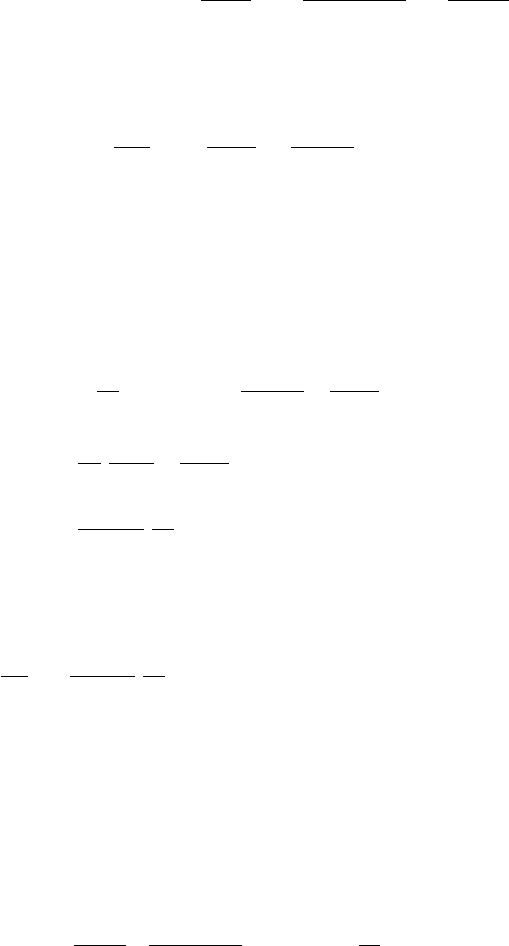
258 5. Radioactivity and all that
p
e
(b, v)=
F
y
dt =
z
ion
e
2
4π
0
∞
−∞
b dx/v
(x
2
+ b
2
)
3/2
=
z
ion
e
2
2π
0
vb
. (5.21)
This formula is valid for values of b that are sufficiently large that during the
passage, the electron recoils through a distance that is small compared to b.
The energy loss of the ion, ∆E, is the kinetic energy of the recoiling electron:
∆E(b, v)=
p
2
e
2m
e
=
z
ion
e
2
4π
0
2
2
v
2
b
2
m
e
. (5.22)
The energy loss is proportional to v
−2
because the slower the ion, the longer
the time that the electron feels the electric field of the ion.
Theenergylossisproportionaltob
−2
so we need to average over impact
parameters. The procedure follows precisely what we did in Chap. 3 when
we calculated reaction probabilities in terms of cross-sections. We consider a
box of volume L
3
containing one electron. The mean energy loss for random
impact parameters is
∆E(v)=
1
L
2
b
max
b
min
2πbdb
2
v
2
b
2
m
e
z
ion
e
2
4π
0
2
=
1
L
2
4π
v
2
m
e
z
ion
e
2
4π
0
2
ln(b
max
/b
min
)
=
(¯hc)
2
L
2
m
e
c
2
4π
β
2
(z
ion
α)
2
ln(b
max
/b
min
) , (5.23)
where β = v
ion
/c and α is the fine structure constant. For N
e
electrons in the
box, the total energy loss is found simply by multiplying by N
e
.Therateof
energy loss, dE/dx, is then found by dividing by the length L of the box
dE
dx
=
(¯hc)
2
n
e
m
e
c
2
4π
β
2
(z
ion
α)
2
ln(b
max
/b
min
) , (5.24)
where n
e
= N
e
/L
3
is the density of electrons in the box.
The energy-loss rate (5.24) has a logarithmic dependence on b
max
/b
min
that must be estimated. The naive expectation b
min
= 0 obviously won’t do.
The source of the problem is that our method for calculating the energy loss
∆E(v, b) gives an infinite energy loss (5.22) for b = 0. In fact, a head-on
collision gives an energy loss of only ∆E
max
=4E
ion
(m
e
/m
ion
)whereE
ion
is
the kinetic energy of the ion. We can then take for an effective value of b
min
that value of b for which (5.22) gives ∆E(v,b)=∆E
max
, i.e.
b
min
∼
z
ion
e
2
4π
0
m
ion
8E
ion
v
2
m
2
e
1/2
= z
ion
α
2
β
2
a
0
(5.25)
where a
0
is the Bohr radius. Since the dependence dE/dx on b
min
is only
logarithmic, we can expect that this estimate will give reasonable results.
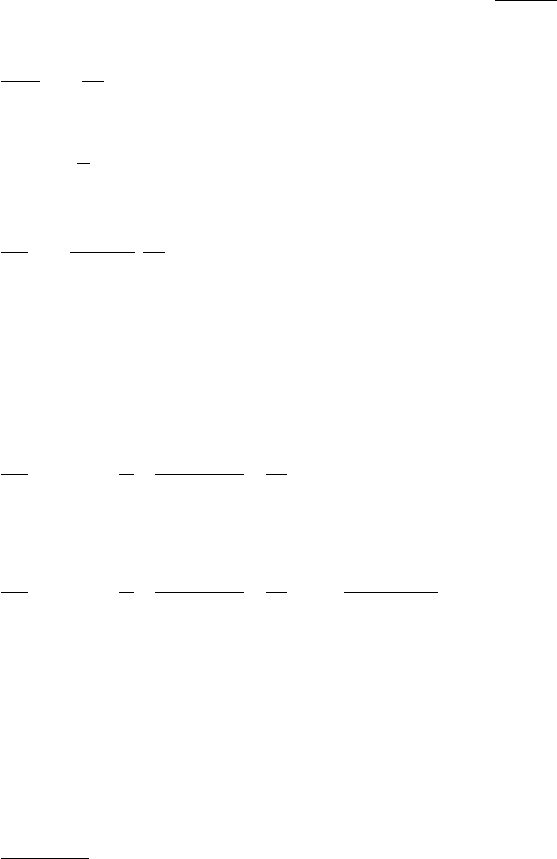
5.3 Passage of particles through matter 259
The naive result b
max
= L also is incorrect but for more subtle reasons
concerning the fact that the electron is bound to an atom rather than free as
we have assumed. In order for the ion to lose energy, the perturbation on the
electron due to the passage of the ion must excite the electron from its ground
state to a higher energy state. This is only possible in quantum mechanics if
the perturbation varies over a time τ that is short compared to the inverse of
the Bohr frequency of the transition, ω
f
−ω
i
where f and i refer to the initial
and final states. For atomic systems, ω
f
−ω
i
∼ αc/a
0
. The characteristic time
for variations of the perturbation is V/
˙
V where V = e
2
/4π
0
√
x
2
+ b
2
is the
perturbing potential. The condition is most stringent by taking x =0:
b
max
v
<
a
0
cα
, (5.26)
i.e.
b
max
∼
β
α
a
0
. (5.27)
This gives our estimate of the energy-loss rate
dE
dx
=
(¯hc)
2
n
e
m
e
c
2
4π
β
2
(z
ion
α)
2
ln[β
3
/(z
ion
α
3
)] β>z
1/3
ion
α. (5.28)
The condition β>z
1/3
ion
α is just b
max
>b
min
. For slow ions, β<z
1/3
ion
α,we
expect little energy loss because the perturbation is not fast enough to excite
atoms.
For β>z
1/3
ion
α, the energy loss is proportional to the inverse square of the
velocity and to the electron density. Eliminating the electron density in favor
of the mass density ρ ∼ m
p
(A/Z)n
e
we have
dE
dx
= ρz
2
ion
Z
A
4π(¯hc)
2
α
2
m
p
m
e
c
2
1
β
2
ln[β
3
/(z
ion
α
3
)] , (5.29)
An improved treatment due to H. Bethe and F. Bloch differs only in the
logarithmic term
dE
dx
= ρz
2
ion
Z
A
4π(¯hc)
2
α
2
m
p
m
e
c
2
1
β
2
ln
2m
e
c
2
β
2
γ
2
I
− β
2
, (5.30)
where I ∼ Zα
2
m
e
c
2
, is the mean ionization energy of the electrons in the
atom. Compared with (5.29), the argument of the logarithm is now ∼ β
2
/α
2
.
The Bethe–Bloch formula (5.30) applies as long as β>z
ion
α.Atslower
speeds, the perturbation does not excite the atoms of the medium and energy-
loss is suppressed. In fact, the ion attaches electrons from the medium so that
the effective value of z
ion
is less than the charge of the naked ion [55].
The order of magnitude of the energy-loss rate is given by the bracketed
combination of fundamental constants in (5.30):
4π(¯hc)
2
α
2
m
p
m
e
c
2
=0.313 MeV (g cm
−2
)
−1
. (5.31)
260 5. Radioactivity and all that
Multiplied by the density and dividing by β
2
, this gives the order of magni-
tude of dE/dx. The logarithmic factor increases this by a factor ∼ 10 so that
for most materials we have
ρ
−1
dE
dx
∼
1 MeV (g cm
−2
)
−1
β
2
z
2
ion
(Z/A)
0.5
. (5.32)
This quantity, ρ
−1
dE/dx, evaluated for z
ion
= 1 is called the stopping power
of a material. For ρ ∼ 1gcm
−3
and Z/A ∼ 1/2, we see that the energy loss
rate is of order 1 MeV cm
−1
/β
2
.
For β ∼ 1, the Bethe–Bloch formula predicts a roughly constant stop-
ping power, rising only logarithmically with energy. Particles that have ener-
gies giving an energy-loss near the minimum value, ∼ 2MeV (g cm
−2
)
−1
,are
so-called minimum ionizing particles. Most cosmic ray muons reaching the
Earth’s surface are roughly minimum ionizing and these particles are often
used to quickly calibrate energy-loss detectors (see below).
1
6
10
1
10
100
βγ
0.01
10 10
24
Stopping Power (MeV/(g/cm2)
Bethe−Bloch Bremsstrahlung
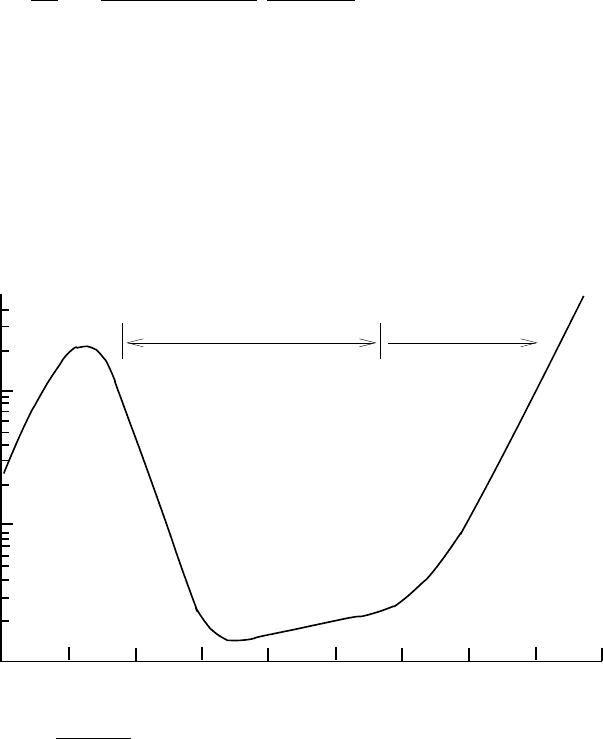
Fig. 5.7. The stopping power for positive muons in copper as a function of
βγ = v/c/
1 − v
2
/c
2
[1]. For 0.04 <βγ<400 the stopping power follows the
Bethe–Bloch formula (5.30). At higher energies the energy loss is dominated by
bremsstrahlung. At low energy, the stopping power is less than predicted by Bethe–
Bloch because positive ions attach electrons, so their effective charge is less than
the naked charge. Nuclei follow the same Bethe–Bloch formula as muons though the
limits of its validity are different. At low energy, the formula works for β>z
ion
α
while for βγ 1 the energy loss is dominated by nuclear inelastic collisions rather
than by bremsstrahlung.

5.3 Passage of particles through matter 261
Figure 5.7 shows the calculated stopping power [1]. The Bethe–Bloch
formula (5.30) is applicable for 0.05 <βγ<500. The stopping power falls like
β
−2
until β ∼ 1 and then rises logarithmically. For β<αz
ion
the formula fails
because the slowly moving ions capture atomic electrons from the medium,
lowering the effective value of z
ion
.Forγ 1, radiation (bremsstrahlung)
eventually becomes important. In Fig. 5.7, this effect is calculated for positive
muons where the effect is important for γ>1000, i.e. E>100 GeV. The
muon is the only particle other than the electron for which bremsstrahlung is
important. Energy loss for hadrons and nuclei with E>GeV is dominated
by discrete inelastic scatters on nuclei (Sect. 3.1.5), liberating nucleons and
creating hadrons.
The energy-loss can be integrated to give the range of a particle, i.e. the
distance traveled before stopping.
R(E)=
E
0
dE
dE/dx
. (5.33)
For dE/dx ∝ β
−2
the integral takes a simple form:
R(E)=E
dE
dx
(E)
−1
∝ E
2
. (5.34)
An α-particle (z
2
ion
=4)ofE ∼ 5MeV has β
2
=2E/m
α
c
2
∼ 2.5 ×
10
−3
giving a stopping power of ∼ 3000 MeV (g cm
−2
)
−1
.Theα-particle will
therefore penetrate only ∼ 0.03 mm of a light material like plastic.
Charged particle detectors. Detection of the ionization caused by the
passage of charged particles is the basis of most types of particle detectors
used in nuclear and high-energy physics. The simplest class consists of gas-
filled ionization chambers as illustrated in Fig. 5.8. The liberated electrons
and ions drift toward charged electrodes where they create an electric pulse.
Other types of energy-loss detectors are listed in Table 5.4. Semiconductor
silicon and germanium ionization detectors are very useful because, being
denser than gasses, they can stop charged particles, yielding a measurement
of their total kinetic energies. Scintillators (Fig. 5.9) where a small portion
of the energy loss is transformed to visible photons are often used because of
their simplicity and low cost.
Cherenkov radiation is created by charged particles moving at a velocity
greater than the light velocity c/n in a medium of refraction index n. While
of great use in high-energy particle physics, they are rarely used in nuclear
physics because of most particles are non-relativistic. One of their most im-
portant uses is in massive detectors constructed to detect solar neutrinos
(Sect. 8.4.1).
Under special conditions, the energy loss of charged particles can create
visible tracks in a medium, as in nuclear emulsions, cloud chambers, and
bubble chambers.
