Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

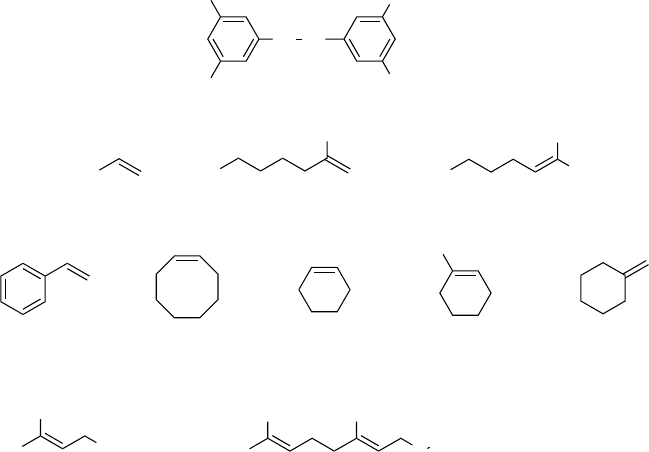
very high selectivity, but relatively slowly, and a higher catalyst loading (about 5 mol%)
is required for full conversion.
4.3.2.4 Rhenium Compounds as Catalysts
Rhenium compounds, and in particular methylrhenium trioxide (MTO), have
attracted considerable attention in oxidation catalysis [36, 37]. Unlike many other
transition metal compounds, their activity in promoting nonproductive decompo-
sition of hydrogen peroxide is low. The epoxidizing activity of MTO was originally
discovered by Herrmann et al. [41, 42]. In the initial studies, selectivity for epoxide
formation was often relatively low because of (Lewis-) acid-catalyzed ring-opening.
The addition of bases proved beneficial with regard to epoxide yield, but reduced the
activity of the catalyst. Typically, hydrogen peroxide in high concentration had to
be used. As an alternative, the urea-hydrogen peroxide clathrate could be used as the
n-C
6
H
13
25 (99), 4 h
a)
H
3
CCH
3
CH
3
H
3
C
CH
3
74 (95), 1 h
H
3
C
H
3
CO
CH
3
CH
3
R
Epoxidations with 60 % H
2
O
2
in 2,2,2-trifluoroethanol (TFE),
using bis[3,5-bis(trifluoromethyl)phenyl] diselenide as pre-catalyst
- epoxide yield [%], selectivity [%], reaction time -
> 99 (> 99), 0.5 h
12 (23), 4 h
99 (> 99), 1 h
a)
98 (99), 1 h
> 99 (> 99), 0.5 h
74 (84), 2 h
H
3
COH
CH
3
R = CH
3
, TMS, Ac:
95 (95) 0.5 h
99 (> 99), 1 h
Reaction conditions: 2 mmol alkene, 4 mmol 60 % H
2
O
2
, 0.25 mol-% diselenide,
0.2 mol-% NaOAc in 2 mL trifluoroethanol, T = 20
o
C.
a)
No NaOAc added, since epoxides are stable.
Se Se
CF
3
CF
3
F
3
C
F
3
C
pre-catalyst:
Scheme 4.8
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
133
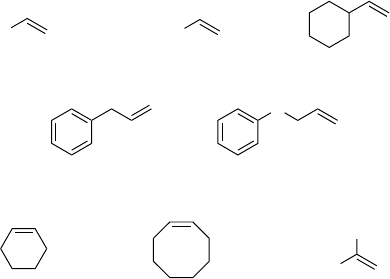
terminal oxidant. Sharpless et al. later on found that the addition of an excess of
pyridine, and in particular 3-cyanopyridine (relative to the catalyst MTO) afforded
both higher reaction rates and selectivities, and provided the possibility of using
aqueous hydrogen peroxide [43, 44]. Shortly after this discovery, pyrazole was shown
to be even more effective [45, 46].
The synthetic e ffort necessary for the preparation of MTO makes commercially
available inorganic rhenium compounds such as perrhenic acid (HReO
4
)an
attractive alternative. Unfortunately, the catalytically active perrhenic acid is to an
even large r extent plagued by subse quent ring opening of the epoxide products – an
effect of its even higher a cidity (relative to MTO). Simple addition of bases is not a
viable solution, as the result ing perrhenat e salts are catalytically inactive. Sheldon
et al. discovered that a combination of perrhenic acid with tert iary arsines forms a
catalytic system which is able to efficiently epoxidize a v ariety of alkenes with
aqueous hydrogen peroxide. With regard to the solvent, TFE once again proved the
best and gave high epoxide yields at high reaction rates (Scheme 4.9) [47]. As little as
0.1 mol% catalyst loading is sufficient to epoxidize, for example, cyclooctene in
excellent yield. At somewhat higher catalyst loading (0.5 mol%), terminal a lkenes
such as 1-octene or 1-decene can be epoxidized in good yield as well. Less
satisfactory results were ob tained with very electron-rich alkenes such as phenyl
allyl ether (26%).
n
-C
6
H
13
n
-C
9
H
19
CH
3
72, 0.5 (2), 45 min
n
-C
8
H
17
O
Epoxidations with 60 % H
2
O
2
in 2,2,2-trifluoroethanol (TFE),
catalyzed by HReO
4
and Ph
2
AsMe
- yield [%], catalyst loading [mol-%] (As:Re), reaction time -
78, 0.5 (2), 60 min
76, 0.5 (2), 45 min
72, 0.5 (2), 120 min 26, 2 (2), 60 min
64, 0.1 (20), 20 min
99, 0.1 (2), 30 min 61, 0.2 (2.5), 20 min
Reaction conditions: 5 mmol alkene, 10 mmol 60 % H
2
O
2
, HReO
4
and Ph
2
AsMe
as indicated, in 5 mL trifluoroethanol, reflux under N
2
, ca. 75
o
C.
Scheme 4.9
134
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
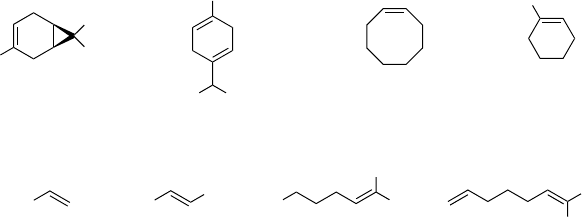
4.3.2.5 Fluoroketones as Catalysts
Sheldon et al. observed that the addition of catalytic amounts of hexafluoroacetone to
HFIP as solvent quite significantly improved the uncatalyzed epoxidation of alkenes
observed in this solvent [38] (the epoxidation of alkenes with H
2
O
2
in HFIP and in the
absence of further catalysts is presented in Section 4.3.1, Scheme 4.4). As already
mentioned, perfluoroketones have gained in importance as epoxidation catalysts, as
they react reversibly with hydrogen peroxide to give a perhydrate, which is the active
oxygen transfer agent (Scheme 4.5) [40]. The results obtained in HFIP, using 5 mol%
of hexa fluoroacetone as catalyst, are summarized in Scheme 4.10 [38].
At ambient temperature and pressure, hexafluoroacetone is a (toxic) gas, and
catalyst recovery is impractical. As a nonvolatile alternative, Sheldon et al. employed
perfluoroheptadecan-9-one. After completion of the epoxidation, this catalyst can be
recovered from halogenated solvents such as dichloroethane or TFE by simple
cooling of the reaction mixture [39]. Furthermore, this long-chain perfluorinated
ketone has the potential for immobilization in fluorous phases. When applied to
epoxidation with perfluoroheptadecan-9-one as catalyst, TFE gave yields of epoxide
comparable to those obtained in dichloroethane. However, to achieve good yields of
acid-sensitive epoxides, buffering by Na
2
HPO
4
was necessary.
Fluorinated alcohols, and in particular HFIP, have also proven beneficial for
epoxidations of alkenes using persulfate (Oxone) as the terminal oxidant and
fluoroketones as catalysts [9, 10]. Under these conditions, the fluorinated ketones
are converted to dioxiranes, which are the active epoxidizing species. Typical catalysts
n
-C
6
H
13
Epoxidations with 60 % H
2
O
2
in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP),
catalyzed by hexafluoroacetone
- yield [%], reaction time -
> 99, 6 h
Reaction conditions: alkene, 1 M in HFIP; 2 eq. 60 % H
2
O
2
; 0.05 eq. Na
2
HPO
4
;
5 mol-% hexafluoroacetone; room temperature.
a)
Bisepoxide, 3 % cymene was also formed.
b)
2,3-Epoxide was formed exclusively.
H
3
C
CH
3
CH
3
CH
3
H
3
CCH
3
n
-C
5
H
11
CH
3
H
3
C
89, 1 h
CH
3
CH
3
> 99, 2.5 h
b)
H
3
CCH
3
CH
3
94, 2 h
> 99, 3 h
97, 6 h
a)
> 99, 16 h92, 16 h
Scheme 4.10
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
135
are fluorinated derivatives of 2-nonanone or 2-decanone. However, as a terminal
oxidant different from hydrogen peroxide is required, a detailed discussion of these
catalytic epoxidations is not within the scope of this review.
4.4
Sulfoxidation of Thioethers in Fluorinated Alcohol Solvents
In 1998, B
egu
e et al. reported that the oxidation of thioethers with 1–2 equiv. of 30%
aqueous hydrogen peroxide in HFIP at room temperature affords the corresponding
sulfoxides in high yields and in high selectivity (Scheme 4.11) [9, 10, 48, 49]. Most
remarkably, no sulfones were formed under these conditions – even the re-submis-
sion of sulfoxides to the reaction conditions did not result in further oxidation to
the sulfone. Once again, hydrogen bond donation from the fluorinated alcohol to
hydrogen peroxide most likely facilitates electrophilic oxygen transfer to the
thioether. On the other hand, hydrogen bonding to the product sulfoxide renders
the latter inert toward further oxidation (Scheme 4.11). Double bonds (C¼C) remain
unaffected under the reaction conditions, and no N-oxidation of, for example,
pyridines occurred. The latter can once again be ascribed to involvement of the
pyridines lone pair in hydrogen bonding to the solvent. Overall, the sulfoxidation
proceeds under neutral conditions, and acid-sensitive functional groups (such as
glycosides, Scheme 4.11) are typically not affected. The sulfoxidation procedure can
be combined with a fluoroalcohol-promoted ring-opening of epoxides by thiophe-
nols, providing b-hydroxy sulfoxides in a one-pot procedure [48].
Under the same reaction conditions (i.e., fluorinated alcohol solvent, room
temperature, 2 equiv. aqueous hydrogen peroxide), thiols are cleanly oxidized to
disulfides (Scheme 4.12) [50].
4.5
Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol Solvents
In 2000, Neumann and Neimann reported that the treatment of certain ketones with
60% aqueous hydrogen peroxide in HFIP as solvent, and in the absence of further
catalysts, results in lactone formation (Scheme 4.13) [21]. After 20 h reaction time at
60
C, good lactone yields were obtained from cycloalkanones. 2-Octanone, as a
prototypical acyclic alkanone, reacted sluggishly, whereas no lactone formation was
observed for acetophenone (Scheme 4.13).
Our independent study of e-caprolactone formation from cyclohexanone revealed
that this process is Brønsted-acid catalyzed [30]. As summarized in Table 4.2, basically
no conversion of the starting cyclohexanone occurred within 4 h in acid-free HFIP.
Unlike alkene epoxidation (see Section 4.3.2.2), the catalytic activity was not specific
for the arsonic acid, but all Brønsted acids tested were catalytically active in HFIP as
solvent. Para-toluenesulfonic acid was identified as the most practical catalyst and
136
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
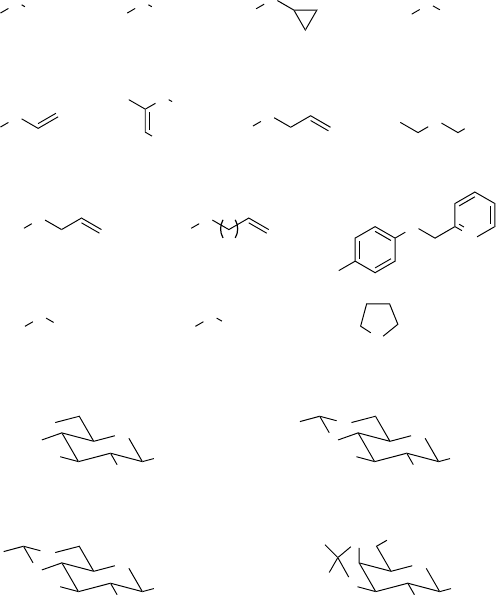
Sulfoxidations in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)
or 2,2,2-trifluoroethanol (TFE):
- yield of sulfoxide [%], reaction time -
a)
Bégué et al., ref. 48. Reaction conditions: sulfide 2 mmol, 30 % H
2
O
2
4 mmol,
2. 5 mL HFIP, room temperature.
b)
Bégué et al., ref. 49. Reaction conditions: sulfide 2 mmol, 30 % H
2
O
2
4 mmol,
2. 5 mL HFIP, room temperature.
c)
Bégué et al., ref. 51. Reaction conditions: sulfide 100 mmol, 30 % H
2
O
2
110 mmol,
50 mL TFE, 0
o
C - room temperature.
d)
Bégué et al., ref. 49. Reaction conditions: sulfide 0.5 mmol, 30 % H
2
O
2
1 mmol,
2. 5 mL HFIP, 25
o
C; product obtained as 1:1-mixture of diastereomers.
Ph CH
3
S
Ph Et
S
Ph
S
Ph Ph
S
Ph
S
Ph
S
SPhPh
n-Bu n-Bu
S
t-Bu t-Bu
S
Et
S
Ph
F
3
C
S
n-C
12
H
25
S
Ph
S
9
H
3
C
S
N
91, 8 h
c)
97, 5 min
a)
93, 5 min
a,b)
99, 5 min
a)
94, 15 min
a,b)
98, 5 min
a)
99, 5 min
a,b)
98, 5 min
a)
95, 5 min
b)
98, 5 min
b)
93, 10 min
b)
92, 5 min
a)
97, 20 min
a)
82, 5 min
a)
O
AcO
AcO
S-Ph
OAc
AcO
O
O
O
Ph
S-Ph
OBz
BzO
O
O
O
Ph
S-Ph
OBn
BnO
O
S-Ph
OBz
O
OBz
O
H
3
C
H
3
C
86, 16 h
d)
96, 4.5 h
d)
97, 3 h
d)
92, 4.5 h
d)
Scheme 4.11
4.5 Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol Solvents
j
137
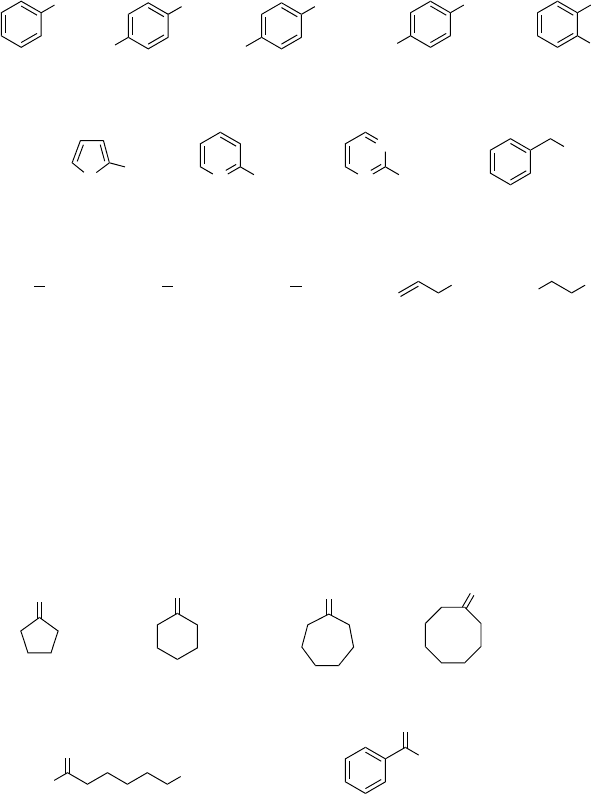
Oxidation of thiols and thiophenols to disulfides
in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) or 2,2,2-trifluoroethanol (TFE):
- yield of disulfide [%], reaction time -
a: Reaction conditions: thiol/thiophenol 1 mmol, 30 % H
2
O
2
1.1 mmol,
1 mL HFIP, room temperature.
b: Reaction conditions: thiol/thiophenol 1 mmol, 30 % H
2
O
2
1.1 mmol,
1 mL TFE, room temperature.
SH
a: 99, 10 min
b: quant., 4 h
SH
a: 97, 10 min
b: 98, 5 h
SH
a: 99, 10 min
b: 96, 4.5 h
SH
a: 98, 10 min
b: 97, 3 h
SH
a: 96, 10 min
b: 94, 2 h
H
3
CCl
MeO NO
2
O
SH
NSH N
N
SH
SH
a: 98, 10 min
b: 96, 3 h
a: 92, 10 min
b: 97, 1 h
a: 96, 10 min
b: 94,04.5 h
a: 98, 10 min
b: 99, 1 h
n
-C
4
H
9
SH
HO
SH
n
-C
5
H
11
SH
SH
t
-C
4
H
9
SH
a: 98, 10 min
b: 95, 1 h
a: 98, 10 min
b: 92, 1.5 h
a: 99, 10 min
b: 93, 4 h
a: 94, 10 min
b: 94, 3 h
a: 95, 10 min
b: 96, 6 h
Scheme 4.12
Oxidation of ketones to lactones and esters in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP):
- yield of lactone/ester [%], reaction time -
a: Neumann and Neimann, ref. 21. Reaction conditions: ketone 1.2 mmol, 60 % H
2
O
2
2 mmol,
1 mL HFIP, 60
o
C.
b: Berkessel and Andreae, ref. 30. Reaction conditions: ketone 19.3 mmol, 50 % H
2
O
2
24.7 mmol,
1 mol-%
p
-toluenesulfonic acid, 24 mL HFIP, 60
o
C.
O
O
O
O
H
3
C
CH
3
O
CH
3
O
a: 88, 20 h
a: 82, 20 h
b: 92, 40 min
a: 68, 20 h
a: 60, 20 h
a: 7, 20 h a: 0, 20 h
Scheme 4.13
138
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
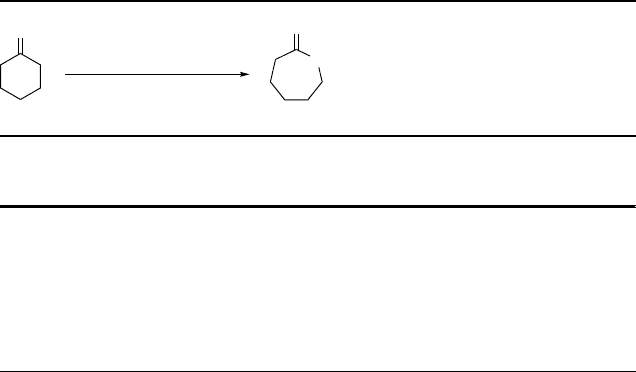
afforded 92% of the lactone product after only 40 min reaction time at 60
C. Note
that only 1 mol% of the catalyst was used, at a very moderate excess of hydrogen
peroxide (1.3 equiv. relative to ketone, 50% aqueous H
2
O
2
). The mechanism of acid
catalysis under these conditions is discussed below in Section 4.5.1.
4.5.1
Acid-Catalyzed Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol
Solvents – Mechanism
Our detailed study of the mechanism of cyclohexanone conversion to e-caprolactone
revealed that it differs from that of the classical Baeyer-Villiger reaction [53].
Unstrained ketones such as cyclohexanone are well known to form various peroxidic
adducts with hydrogen peroxide [54]. In HFIP, only the formation of the spiro-
bisperoxide 7,8,15,16-tetraoxadispiro[5.2.5.2]hexadecane could be observed. This
spiro-bisperoxide is a compound that has been known since the 1940s [55]. In fact,
it is an isomer of two equivalents of caprolactone, but numerous earlier attempts
to isomerize it to e-caprolactone in standard organic solvents under Brønsted- or
Lewis-acid catalysis failed to produce reasonable yields of lactone. In HFIP, however,
Brønsted-acids induce the spontaneous and quantitative rearrangement to two
equivalents of e-caprolactone. In summary, the oxidation of cyclohexanone with
hydrogen peroxide is a Baeyer-Villiger-like transformation, as it produces the same
lactone product, but proceeds via a different mechanism involving the spiro-bisper-
oxide as an intermediate (Scheme 4.14) [53].
Typically, v-hydroxycaproic acid is formed as a by-product (which may be over-
looked, as its GC-detection requires prior silylation). The time-course of the reaction
clearly indicated that this hydroxyacid does not result from subsequent hydrolysis of
Table 4.2
1.28 mmol cyclohexanone,
1.3 eq. 50 % aq. H
2
O
2
,
1 mol-% catalyst
2 mL solvent,
60
o
C, 4.5 h
O
O
O
Solvent Catalyst Reaction
time [min]
Conversion of
ketone [%]
Yield of
lactone [%]
HFIP None 240 <1 <1
HFIP Ph-AsO
3
H
2
240 Quant. 85
HFIP Ph-PO
3
H
2
75 86 84
HFIP HCl 65 90 65
HFIP CF
3
CO
2
H6565 90
HFIP p-TsOH 40 Quant. 92
1,4-Dioxane Ph-AsO
3
H
2
240 <1 <1
4.5 Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol Solvents
j
139
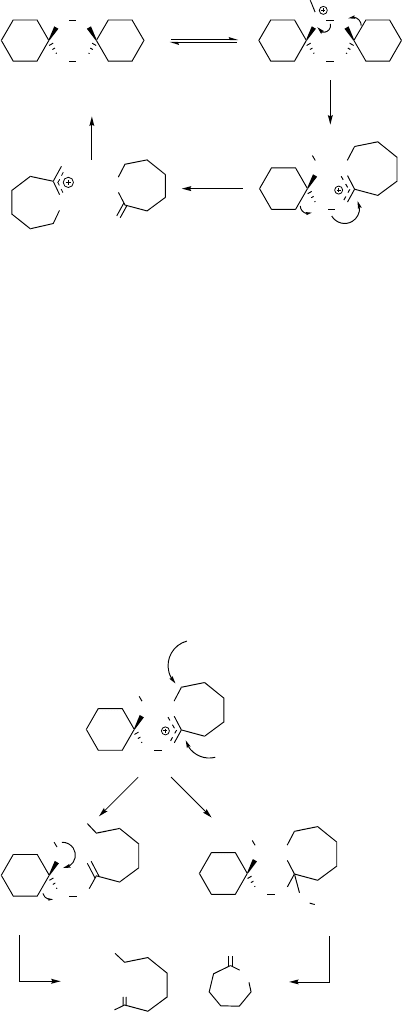
the lactone product, but that it is formed by a separate pathway. Most likely, the
lactonium cation shown as an intermediate in lactone formation (Scheme 4.14) is the
branching point: attack of water results in ring opening and hydroxy acid formation
(Scheme 4.15). We could show by
18
O-isotopic labeling (H
2
18
O) and mass-spectro-
metric analysis that the attack of the water molecule occurs at the acyl carbon atom
(path B, Scheme 4.15) and not in the sense of a nucleophilic attack at the ring carbon
atoms (path A, Scheme 4.15) [53]. In the former case, the oxygen label should end up
in the hydroxy function of the by-product, whereas incorporation into the carboxyl
group should result from pathway B. In fact, exclusive incorporation into the carboxyl
group was observed experimentally.
OO
OO
H
OH
2
, path A
OH
2
, path B
OO
OO
H
O
H
path Bpath A
OO
OO
H
HO
HO
HO
O
path A path B
O
O
+
Scheme 4.15
OO
OO
H
OO
OO
OO
OO
H
+ H
+
O
O
+
2 x e-caprolactone
a
b
O
OH
- H
+
Scheme 4.14
140
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
4.5.2
Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol Solvents – Catalysis
by Arsonic and Seleninic Acids
It was noted in Sections 4.3.2.2 and 4.3.2.3 that arsonic acids and seleninic acids are
efficient catalysts for the epoxidation of alkenes. For both types of catalyst, significant
enhancement of catalyst activity and selectivity was observed in fluorinated alcohol
solvents compared to, for example, 1,4-dioxane.
Arsonic acids: Arene arsonic acids, together with polymer-bound variants, have also
been applied to the catalysis of the Baeyer-Villiger oxidation of ketones by Jacobson
et al. as early as 1979 [56]. Typically, 1,4-dioxane was used as the solvent, together with
high-concentration hydrogen peroxide (90%). Using an up to fivefold excess of
hydrogen peroxide, the Baeyer-Villiger oxidation of a number of substrates can be
performed efficiently. For example, with a catalyst loading of about 10 mol%,
2-methylcyclohexanone yields 80% of methyl e-caprolactone after 7 h at 80
C.
Apparently, no fluorinated alcohol solvents were tested in this study [56]. The relative
behavior of the various substrates used led to the conclusion that under these
conditions (1,4-dioxane, arsonic acid catalysts, high-concentration H
2
O
2
), a normal
Baeyer-Villiger oxidation, initiated by the attack of the persarsonic acid on the ketone,
is operating. As mentioned above (Sections 4.5 and 4.5.1), changing to HFIP as
solvent, in combination with simple Brønsted-acid catalysts such as p-TsOH, leads to
a highly efficient catalytic system that operates by a different mechanism, that is, via
intermediate formation of a spiro-bisperoxide and its subsequent rearrangement.
Seleninic acids: Seleninic acids of the type used for alkene epoxidation (cf. Sec-
tion 4.3.2.3) have also been employed as catalysts for the Baeyer-Villiger oxidation of
ketones with hydrogen peroxide, mainly by Syper [57] and by Sheldon et al. [58, 59]. In
most cases, halogenated solvents such as dichloromethane or 1,2-dichloroethane
were used. In a study of solvent effects, Sheldon et al. observed that, once again, TFE
and in particular 1,1,1,3,3,3-hexafluoro-2-propanol are superior to dichloromethane
with regard to selectivity and rate [58]. However, the effects are not as pronounced as in
thecaseof alkeneepoxidation (e.g.,a factorof about 2 in rate betweendichloromethane
and HFIP, and 1.3 for TFE). The Baeyer-Villiger oxidation of a series of ketones and
aldehydes was studied in TFE, and the results are summarized in Table 4.3 [58].
Once again, the relative rates and product distributions follow the pattern typical
for classical Baeyer-Villiger oxidations. Together with the relatively low accelerating
effect of the fluorinated alcohol solvent, it may be concluded that, also under these
conditions of selenium catalysis, a classical mechanism based on perseleninic acid is
operating. Finally, it should be mentioned that diselenides (as pre-catalysts, see
Section 4.3.2.3) with long-chain perfluoroalkyl substituents have been synthesized
and successfully applied to Baeyer-Villiger oxidations in fluorous bi- and triphasic
systems[59].Severalchiral diselenidesfor the in-situ formation of chiral (per)seleninic
acids have been synthesized by Uemura et al. and tested in the asymmetric Baeyer-
Villiger oxidation of a number of ketones [60]. High chemical yields and enantiomeric
excessesup to 19% ee were observed.However, 1,4-dioxane, DME,and THFwere used
assolventsin this study, and no fluorinatedalcoholsolventsappearto have beentested.
4.5 Baeyer-Villiger Oxidation of Ketones in Fluorinated Alcohol Solvents
j
141
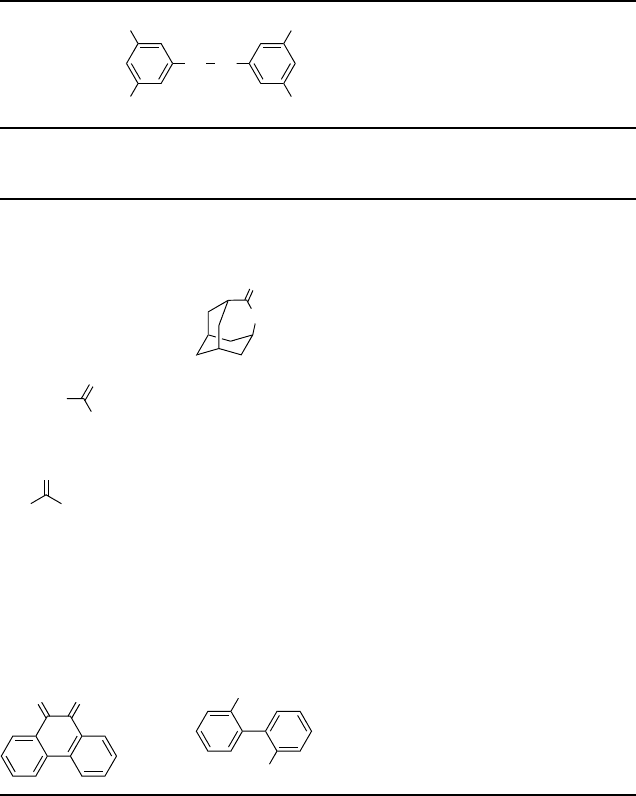
4.6
Epilog
Over recent years, it has become obvious that fluorinated alcohol solvents are
excellent media for effecting various types of oxidations with hydrogen peroxide as
the terminal oxidant. The stability and easy recovery of these solvents, their excellent
Table 4.3
Se Se
CF
3
CF
3
F
3
C
F
3
C
pre-catalyst:
Substrate Product Reaction
time [h]
Conversion
[%]
Selectivity
[%]
Cyclobutanone c-Butyrolactone 1 99 90
Cyclopentanone d-Valerolactone 8 95 94
Cyclohexanone e-Caprolactone 4 95 99
Adamantanone
O
O
1 100 99
p
-Cl-C
6
H
4
CH
3
O
p-Cl-C
6
H
4
O-Ac 24 25 50
p-Cl-C
6
H
4
OH 50
n
-Pr
n
-P
r
O
n-PrCO
2
H242550
n-PrCO
2
-n-Pr 50
3,4,5-(MeO)
3
-C
4
H
2
-CHO 3,4,5-(MeO)
3
-C
4
H
2
-OH 0.25 99 99
a)
p-Me-C
4
H
4
-CHO p-Me-C
4
H
4
-OH 2 99 55
a)
p-Me-C
4
H
4
-CO
2
H45
p-NO
2
-C
4
H
4
-CHO p-NO
2
-C
4
H
4
-CO
2
H 2 98 98
b)
n-C
7
H
15
-CHO n-C
7
H
15
-CO
2
H 3 88 96
b)
Ph-C
2
H
4
-CHO Ph-C
2
H
4
-CO
2
H3>90 99
b)
OO
CO
2
H
HO
2
C
16 >98 95
Reaction conditions: 2 mmol substrate, 1.0 mol% diselenide, 4 mmol 60% H
2
O
2
,in2mL
2,2,2-trifluoroethanol (TFE), T ¼ 20
C.
a) Selectivity to substituted phenol after base hydrolysis.
b) 60
C.
142
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
