Baeckvall J.-E. (ed.) Modern Oxidation Methods
Подождите немного. Документ загружается.

4.3
Epoxidation of Alkenes in Fluorinated Alcohol Solvents
The first reports on the use of fluorinated alcohols, and in particular of HFIP in
oxidations with hydrogen peroxide, can be found in the patent literature of the late
1970s and early 1980s [19, 20]. Typically, 60% aqueous hydrogen peroxide was used in
the presence of metal catalysts. A number of reports on alkene epoxidations in
fluorinated alcohols, both in the absence and in the presence of additional catalysts,
have followed.
4.3.1
Alkene Epoxidation with Hydrogen Peroxide – in the Absence of Further Catalysts
In 2000, Neimann and Neumann reporte d on alkene epoxidation by H
2
O
2
in
fluorinated a lcohol solvents without the addition of further catalysts [21]. Shortly
thereafter, in 2001, Sheldonetal.reported about their results, also on alkene
epoxidation in fluorinated alcohol solvents [22]. In the latter study, it became clear
that buffering the reaction mixtures, preferably by addit ion of Na
2
HPO
4
improves
the overall efficiency of the process, presumably by suppre ssing acid-catalyzed
degradation of the product epoxides. Scheme 4.3 summarizes the results obtained
using TFE as solvent, whereas the resul ts for HFIP are summarized in
Scheme 4.4.
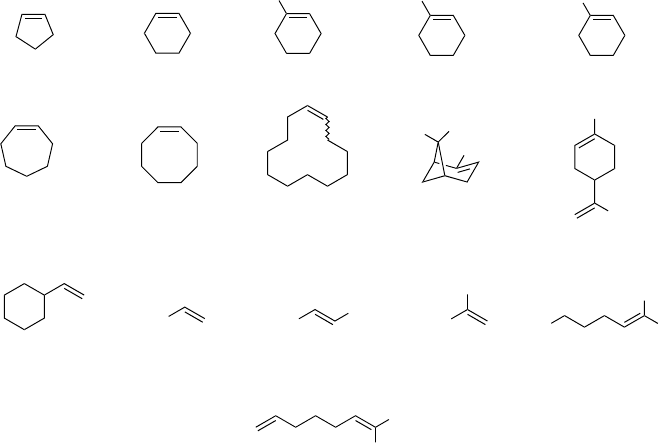
Inspection of Schemes 4.3 and 4.4 reveals that, in the absence of further
catalysts, epoxidation with hydrogen peroxide i n TFE or HFIP is especially
effective for relatively electron-rich alkenes such as cis-cyclooctene, 1-methylcy-
clohexene, or 3-carene. For less electron-rich alkenes such as 1-octene, the
epoxidation is typically slow, and low conversions result even after longer reaction
times and at reflux temperature. Generally speaking, the noncatalyzed epoxidation
has thre e parameters that can be adjusted t o the individual alke ne: (i) HFIP has a
stronger activating ef fect than TFE, (ii) hydrogen peroxide can be used in higher
concentrations, if necessary, and (iii) the reaction temperature can be varied up to
reflux of the solvent. As evidenced by the work of Neim ann and Neumann,styrenes
appear not to provide epoxide in useful yields. In their study, a mixture of products,
resulting f rom ring-opening of the epoxide and from CC-bond cleavage, was
obtained [21].
4.3.1.1 On the Mechanism of Epoxidation Catalysis by Fluorinated Alcohols
Kinetic investigations of the epoxidation of Z-cyclooctene by aqueous H
2
O
2
in HFIP
by Berkessel et al. showed that the reaction follows a first-order dependence with
respect to the substrate alkene as well as to the oxidant, suggesting a monomolecular
participation of these components in the rate-determining step [16, 18, 23]. On the
other hand, a kinetic order of 2–3 with respect to the concentration of HFIP was
observed for several co-solvents. The large negative DS
of 39 cal mol
1
K
1
points
to a highly ordered TS of the rate-determining reaction step: typical DS
-values for
alkene epoxidations by peracids range from 18 to 30 cal mol
1
K
1
[24]. These
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
123
experimental results provide the basis for our calculations in which one to four
molecules of HFIP were added to the transition state of the reaction.
The first quantum-chemical investigation of the mechanism of alkene epoxidation
in fluoroalcohols was carried out by Shaik et al. [25]. In the absence of kinetic data,
a monomolecular mode of activation by the fluorinated alcohols for all reaction
pathways was assumed [25]. In our work [18], we first compared the transition state,
which does not involve HFIP-participation [TS(e,0)], with single-HFIP involvement
[TS(e,1) and TS(e,1)
0
] (Figure 4.6). Particular emphasis was then put on the twofold
HFIP-activated complex (Figure 4.7) for a detailed inspection of the hydrogen-bond-
assisted epoxidation. All relevant characteristics of higher-order activation (as shown
e.g., in Figure 4.8) are already present in the transition states TS(e,2) and TS(e,2)
0
(Figure 4.7).
CH
3
CH
3
H
3
C
CH
3
CH
3
80:20 cis:trans
H
3
C
i
-Pr
t
-Bu
n
-C
6
H
13
n
-C
9
H
19
CH
3
H
3
CCH
3
CH
3
CH
3
CH
3
a: Sheldon et al., ref. 22. Reaction conditions: 5 mmol alkene, 10 mmol 60 % H
2
O
2
, 5 mol-%
Na
2
HPO
4
, 5 mL trifluoroethanol, reflux (ca. 80
o
C).
b: Neumann and Neimann, ref. 21. Reaction conditions: 1.2 mmol alkene, 2 mmol 60 % H
2
O
2
,
1 mL trifluoroethanol, 60
o
C.
b: 99 (100), 20 h
b: 65 (48), 20 h
b: 98 (100), 20 h b: 39 (100), 20 h
b: 14 (100), 20 h
n
-C
5
H
11
CH
3
b: 77(100), 20 h
a: 3 (7), 0.5 h
a: 81 (94), 3 h
(mono- and diepoxide)
a: 85 (94), 3 h
a: 88 (93), 4 h
(69 % isol. yield of 2,3-monoepoxide)
a: 91 (97), 3 h
a: 83 (93), 5 h
a: 67 (96), 20 h
a: 88 (>99), 24 h
a: 28 (97), 22 ha: < 2, 24 ha: 7, 24 h
a: 82 (93), 5 h
Epoxidations in 2,2,2-trifluoroethanol (TFE):
- yield [%], selectivity [%], reaction time -
Scheme 4.3
124
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
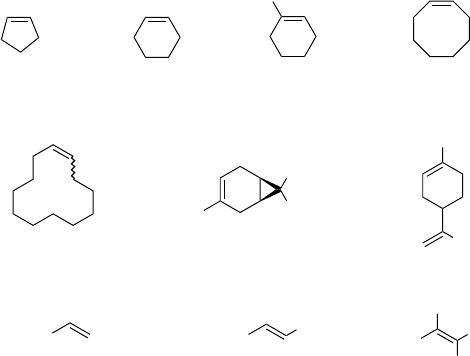
In the 2 : 1 pre-complex C2 composed of HFIP and H
2
O
2
, hydrogen peroxide is
coordinated by the two alcoholic hydroxyl groups in a cyclic fashion, one HFIP acting
as a hydrogen bond donor toward the leaving OH (hydrogen bond length 1.767 Å),
and the other one as a hydrogen bond acceptor (hydrogen bond length 1.906 Å),
deprotonating the hydroxyl group which is transferred to be the epoxide oxygen atom
(C2, Figure 4.7) [18]. The internal hydrogen bond between the two fluorinated
alcohols (hydrogen bond length 1.823 Å) cooperatively increases the hydrogen bond
donor ability of the alcohol molecule which activates the leaving OH group. By this
hydrogen bond pattern, a polarization of the OO bond is achieved (the donated
and accepted hydrogen bonds are not equal in length and angle), and an electron-
deficient oxygen atom is generated, ready for electrophilic attack on the alkene double
bond. In the corresponding transition state (TS(e,2), Figure 4.7) the shorter hydrogen
bond (in which HFIP acts as H-bond donor) is extremely contracted to 1.409 Å
(0.358 Å), whereas the longer hydrogen bond (in which HFIP acts as acceptor) is
slightly decreased in length to 1.864 Å (0.042 Å). Thus, the acidity of the donor
Epoxidations in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP):
- yield [%], selectivity [%], reaction time -
80:20 cis:trans
H
3
C
n-C
6
H
13
a: Sheldon et al., ref. 22. Reaction conditions: alkene 1M in HFIP, 2 eq. 60 % H
2
O
2
, 5 mol-%
Na
2
HPO
4
, room temperature.
b: Neumann and Neimann, ref. 21. Reaction conditions: 1.2 mmol alkene, 2 mmol 60 % H
2
O
2
,
1 mL HFIP, 22
o
C.
c: Berkessel and Adrio, ref. 23. Reaction conditions: cyclooctene 31 µmol, 42 % H
2
O
2
, 23 eq., 1.2 mL
HFIP, 50
o
C.
b: 93 (100), 20 h
b: 91 (61), 20 h
a: 94, 18 h
b: 100 (100), 20 h
c: 95, 0.5 h
b: 80 (100), 20 h
b: 21 (100), 20 h;
59 (100) at 60
o
C, 20 h
n-C
5
H
11
CH
3
b: 73 (100), 20 h;
97 (100) at 60
o
C, 20 h
a: 82, 2 h
a: 6, 21 h;
52 % at reflux, 24 h
CH
3
CH
3
a: 42, 1 h
H
3
C
CH
3
H
3
C
CH
3
b: 100 (57), 20 h
30 % H
2
O
2
H
3
C
CH
3
CH
3
a: 98, 21 h
Scheme 4.4
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
125
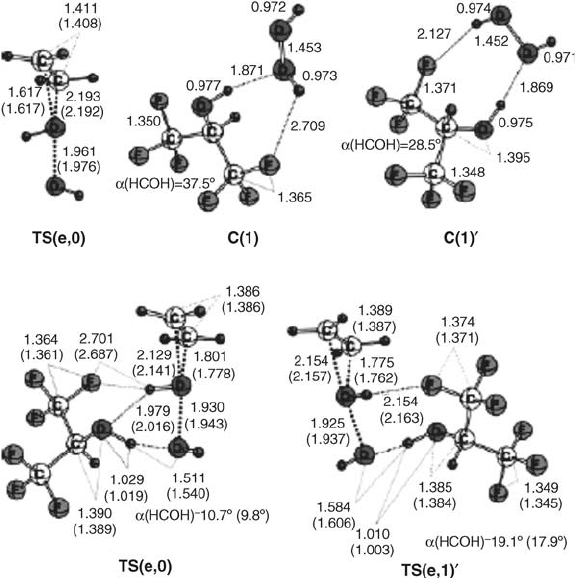
HFIP molecule is cooperatively increased by shortening of the HFIP internal
hydrogen bond from 1.823 Å to 1.692 Å (0.131 Å).
A second potential reaction path (C(2)
0
, TS(e,2)
0
, Figure 4.7) for twofold HFIP
activation was calculated, which differs from TS(e,2) with regard to the hydrogen
bond from H
2
O
2
to HFIP. Here, a fluorine atom of the trifluoromethyl group serves
as hydrogen bond acceptor, and not a second hydroxy function. Both transition states
are similar in energy, but the corresponding pre-complex C(2)
0
, consisting of H
2
O
2
and two HFIP molecules, lies 18.4 kJ mol
1
above C(2).
An analysis of the hydrogen b onding parameters shows that, in all case s wh ere
HFIP donates a hydrogen bond to the oxidant, this hydrogen bond is significantly
contracted in the transition state, u sually by more than 0.3 Å. The resul t of this
significant contraction is the formation of a low-barrier hydrogen bond [26],
characterized by an increase in covalency which effe ctively exerts the pronounced
stabilization of t he highly polar transition states through charge transfer. Hydrogen
bonds between two HFIP molecules show the same trend, being regularly short-
ened by about 0.1 Å. This effect clearly indicates a cooperative enh ancement of
Figure 4.6 Stationary-point structures for the epoxidation of ethene with hydrogen peroxide in
the absence and in the presence of one molecule of HFIP, optimized at RB3LYP/6-31þG(d,p)
(selected bond lengths in Å; RB3LYP/6-311þþG(d,p) results in parentheses).
126
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
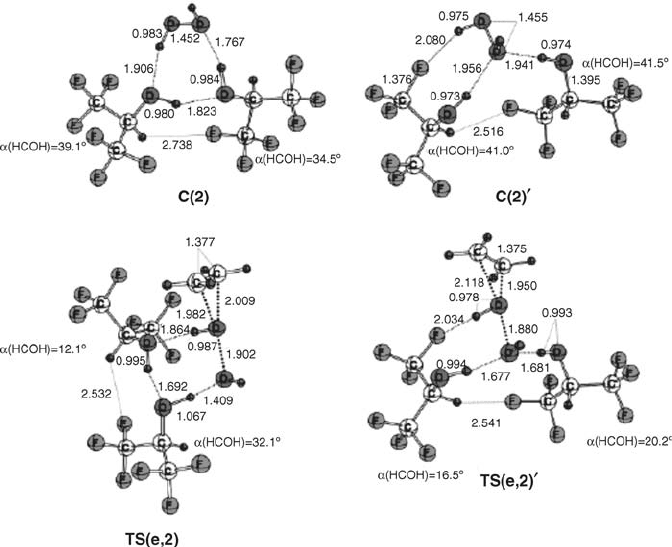
hydrogen bonding. Additionally, we find a reduct ion of the HOCH-dihedral angles
in 14 of t he 16 HFIP molecules within the seven calculat ed reaction pathways [18].
This result is in agreement w ith the ana lysis of the hydrogen-bonding propert ies o f
HFIP, as the hydrogen bond donor ability is maximized toward the sp conformation
of the alcohol.
Proceeding from the transition states to the resulting products, IRC analysis
demonstrates that, along this reaction path, a subsequent and barrier-free, cascade-
like proton transfer toward the formation of the epoxide and water takes place.
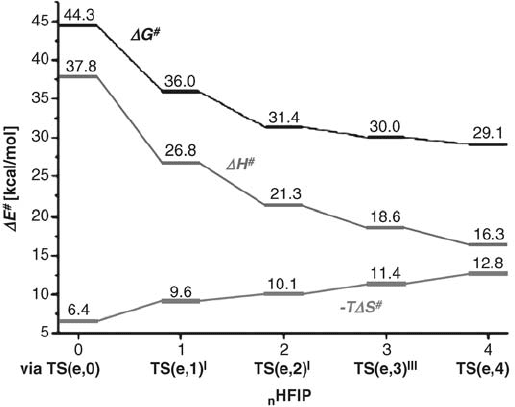
Figure 4.9 shows the overall dependence of the activation parameters on the number
of HFIP molecules involved. Interestingly, the activation enthalpy of the epoxidation
decreases steadily from zero to fourth order in HFIP. As expected, the activation
entropy TDS
shows a continuous increase with increasing numbers of specifically
coordinated HFIP molecules. Because of the increasing entropic contribution, the
value of DG
approaches saturation when three or four HFIP molecules are involved.
For methanol, however, no influence of explicit coordination of the solvent on the
activation parameters of oxygen transfer could be found, so it seems solely to act as
a polar reaction medium. In line with this result, no significant epoxidation catalysis
results from the use of methanol as solvent.
Figure 4.7 Stationary-point structures for the epoxidation of ethene with hydrogen peroxide in the
presence of two molecules of HFIP, optimized at RB3LYP/6- 31 þ G(d,p).
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
127
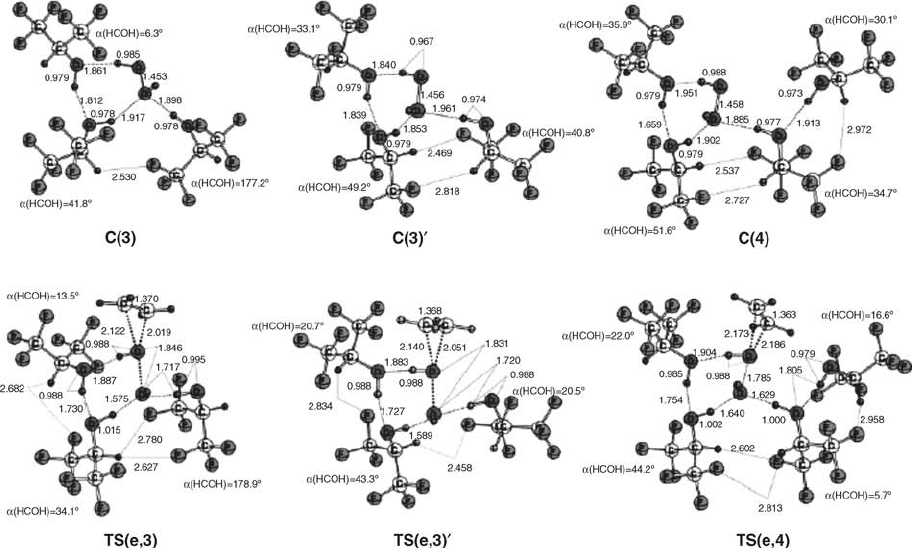
Figure 4.8 Stationary-point structures for the epoxidation of ethene with hydrogen peroxide in the presence of three to four molecules of HFIP, optimized at
RB3LYP/6-31þG(d,p).
128
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
4.3.2
Alkene Epoxidation with Hydrogen Peroxide – in the Presence of Further Catalysts
The catalysts applied to alkene epoxidation in fluorinated alcohol solvents can be
subdivided into those which are metal/chalconide-based and those which are purely
organic in nature (Scheme 4.5). The former comprise arsanes/arsane oxides [27, 28],
arsonic acids [29, 30], seleninic acids/diselenides [31–35], and rhenium compounds
such as Re
2
O
7
and MTO (methylrhenium trioxide) [36, 37]. As shown in Scheme 4.5,
their catalytic activity is ascribed to the intermediate formation of, for example,
perseleninic/perarsonic acids or bisperoxorhenium complexes. In other words, their
catalytic effect is due to the equilibrium transformation of hydrogen peroxide to
kinetically more active peroxidic species.
A second class of catalysts comprises perfluorinated ketones such as he xafl uoro-
acetone [38] and perfluoro-2-octanone [39]. This class of catalyst is assumed to
reversibly form peroxyhemih ydrates whe n exposed to hydrogen peroxide [40]; the
latter are the active oxygen-transfer agents. Oxygen transfer to the su bstrate is
accompanied by formation of the ketones hydrate,which,inequilibrium,regen-
erates the fluoroketone catalyst.
4.3.2.1 Arsines and Arsine Oxides as Catalysts
Inspired by a claim in the patent literature [27], Sheldon et al. identified di-n-butyl-
phenylarsine as a highly active catalyst for the epoxidation of cyclohexene as the
substrate, and with 60% hydrogen peroxide in TFE as the solvent [28]. Among a
series of purely aromatic arsines, electron-rich ones (such as Ph
3
As) were found to be
Figure 4.9 Activation parameters versus number of HFIP molecules for the epoxidation of ethane
within a solution model at 298 K (RB3LYP/6-311þþG(d,p)//RB3LYP/6-31þG(d,p)).
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
129
more active than electron-deficient ones [such as (C
6
F
5
)
3
As]. In fact, the arsines
employed are pre-catalysts, as under the reaction conditions they are promptly
oxidized to the corresponding arsine oxides. The latter are presumed to be the active
catalysts. No mechanistic information regarding the further fate of the arsine oxide
appears to exist. It may be assumed that a species analogous to the peroxyhemihy-
drates generated from perfluoroketones may be formed (Scheme 4.5). However, at
this point in time, this assumption is purely speculative. Scheme 4.6 summarizes the
results obtained in epoxidations with di-n-butylphenylarsine as catalyst. The latter
arsine was identified as the most advantageous catalyst in the preceding screening.
By modifying the n-butyl substituents in di-n-butylphenylarsine to (CH
2
)
2
-C
8
F
17
,
Sheldon et al. obtained a catalyst system that, in principle, can be recycled by extraction
with prefluorinated solvents [28]. Thus far, the partitioning of the corresponding
arsine oxide proved unsatisfactory. Nevertheless, at least partial recovery could be
achieved by crystallization from hexane or acetone.
4.3.2.2 Arsonic Acids as Catalysts
Epoxidation catalysis by arsonic acids (both free and polymer-bound) had been
reported as early as 1979 by Jacobson et al. [29]. However, these early experiments
were typically carried out in 1,4-dioxane as solvent. The latter had been identified as
an optimal solvent, as it is miscible both with the aqueous oxidant and the organic
Active oxidant involved in H
2
O
2
-epoxidations catalyzed by (a) arsonic acids,
(b) seleninic acids/diselenides, (c) methylrhenium trioxide (MTO), and (d) fluoroketones.
AsR OH
O
OH
+ H
2
O
2
, - H
2
O
AsR OOH
O
OH
a.
SeRSeR
SeROH
O
SeROOH
O
O
+ H
2
O
2
, - H
2
O
O
H
2
O
2
b.
CH
3
Re
O
O
O
CH
3
ReO
O
O
O
CH
3
Re
O
O
O
O
O
+ H
2
O
2
, - H
2
O
O
+ H
2
O
2
, - H
2
O
c.
R
F
R
F
O
+ H
2
O
2
R
F
R
F
OOHHO
R
F
R
F
OHHO
H
2
O
O
d.
R
F
: perfluorinated
alkyl residue
Scheme 4.5
130
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
substrate, and because it promotes the swelling of the polystyrene supports used
to immobilize the arsonic acid catalyst [29]. In 2001, we observed that the catalytic
effect of benzenearsonic acid in the homogeneous-phase epoxidation of 1-octene is
potentiated enormously when switching the solvent from 1,4-dioxane to fluorinated
alcohols, preferably HFIP [30]. Our results are summarized in Table 4.1. Inspection
CH
3
CH
3
H
3
C
n
-C
8
H
17
Reaction conditions: 5 mmol alkene, 10 mmol 60 % H
2
O
2
, and amount of di-
n
-butylphenyl arsine
indicated in 5 mL trifluoroethanol, reflux under N
2
(ca. 80
o
C).
a)
5 mol-% Na
2
HPO
4
added as buffer.
b)
preferential epoxidation of trisubstituted double bond, 5 % of
the diepoxide were formed as well.
94 (2), 1 h
97 (1), 1 h
a)
98 (2), 1 h
quant. (2), 1 h
Epoxidations with 60 % H
2
O
2
in 2,2,2-trifluoroethanol (TFE),
catalyzed by di-
n
-butylphenyl arsine
- yield [%], catalyst loading [mol-%], reaction time -
88 (2), 1.5 h
a)
H
3
CCH
3
CH
3
88 (2), 1 h
H
3
C
CH
3
78 (2), 2 h
79 (1), 1.5 h
a,b)
54 (5), 2 h
a)
Scheme 4.6
Table 4.1
n
-C
6
H
13
n
-C
6
H
13
O
1.28 mmol 1-octene,
1.3 eq. 50 % aq. H
2
O
2
,
1 mol-% catalyst
2 mL solvent,
60
o
C, 4.5 h
Solvent Catalyst Conversion of
1-octene[%]
Yield of
epoxide [%]
HFIP None 9 9
HFIP Ph-AsO
3
H
2
Quant. 95
HFIP Ph-PO
3
H
2
77
HFIP HCl 8 7
HFIP CF
3
CO
2
H9 8
HFIP p-TsOH 8 7
1,4-Dioxane Ph-AsO
3
H
2
<1 <1
4.3 Epoxidation of Alkenes in Fluorinated Alcohol Solvents
j
131
of Table 4.1 furthermore reveals that epoxidation catalysis is not just an effect of
acidity, but is specific for the arsonic acid: the closely related benzenephosphonic
acid, hydrochloric acid, and toluenesulfonic acid do not accelerate the epoxidation
beyond the background reaction.
With regard to the mechanism, Jacobson et al. proposed that the arsonic acid
catalyst is transformed to the perarsonic acid by hydrogen peroxide, the perarsonic
acid being the active oxidant (Scheme 4.5) [29]. This may well be the case for arsonic
acid-catalyzed epoxidations performed in 1,4-dioxane as solvent. When using fluo-
rinated alcohols as solvent, we believe that reversible ester formation is involved in
the mechanism (Scheme 4.7). This assumption is based on ESI-MS studies of the
reaction mixtures [23].
4.3.2.3 Diselenides/Seleninic Acids as Catalysts
Under the reaction conditions, diselenides are converted to seleninic acids, which
are the true catalytically active species (Scheme 4.5) [31–34]. The first report on
epoxidation catalysis by a seleninic acid (benzeneseleninic acid, employed in
stoichiometric amount together with hydrogen peroxide) appears to be by Grieco
et al., published in 1977 [33]. The transition to a truly catalytic and highly ef ficient
system was achieved by Sheldon et al. in 2001 [34]. A systematic solvent screening
once again identified TFE as affording the highest turnover frequencies, at high
selectivity. Buffering with sodium acetate proved crucial to prevent ring-opening of
acid-sensitive epoxides. With regard to the substitution pattern on the diselenide pre-
catalyst, 3,5-bis(trifluoromethyl) disubstitution was found to be the best (diselenide
shown in Scheme 4.8). Epoxidation results obtained under optimized conditions are
summarized in Scheme 4.8 [34]. With as little as 0.25 mol% of the diselenide catalyst
precursor, impressive epoxide yields and selectivities were obtained for a number of
di- and trisubstituted alkenes carrying aliphatic substituents. The hydroxyl group
of an allylic alcohol is tolerated, just as a methyl and TMS ether or an acetoxy
substituent. Styrene turned out to undergo epoxidation with low yield and selectivity.
1-Octene, as a prototypical mono-substituted terminal alkene, gave the epoxide with
As OH
O
OH
As O
O
OH
O
H
+ H
2
O
2
, - H
2
O
+ HFIP,
- H
2
O
As OH
O
O
CF
3
CF
3
As O
O
O
CF
3
CF
3
O
H
+ H
2
O
2
, - H
2
O
Scheme 4.7
132
j
4 Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
