Astakhov V. Tribology of Metal Cutting
Подождите немного. Документ загружается.


Improvements of Tribological Conditions 347
Table 6.5. Thermal conductivity (k
cf
), thermal diffusivity (α
cf
), kinematic viscosity (ν
cf
),
volumetric expansion coefficient (β
cf
) and Prandtl number (Pr) for dry vapor and water as
functions of temperature.
θ
(
◦
C
)
k
cf
×10
2
(
W/
(
m
◦
C
))
α
cf
×10
6
m
2
/s
ν
cf
×10
6
m
2
/s
β
cf
×10
4
(
1/
◦
C
)
Pr
Air
20 2.59 21.4 15.06 24.10.703
50 2.83 25.7 17.95 30.90.698
100 3.21 33.6 23.13 26.80.688
150 3.56 42.1 28.94 23.60.683
200 3.93 51.4 34.85 21.10.680
250 4.27 61.0 40.61 19.10.677
300 4.60 71.6 48.33 17.40.674
350 4.91 81.9 55.46 16.00.676
Water (along saturation line)
20 59.8 14.3 1.006 1.81 7.02
30 61.8 12.9 0.805 3.21 5.43
40 63.5 15.3 0.659 3.87 4.31
50 64.8 15.7 0.556 4.49 3.54
60 65.9 16.0 0.478 5.11 2.98
70 66.8 16.3 0.415 5.70 2.55
60 67.4 16.6 0.365 6.32 2.21
90 68.0 16.8 0.326 6.95 1.95
100 68.3 16.9 0.295 7.52 1.75
that in this case Re > 0.01
h
bv
= 170
(
θ
hs
−100
)
1.86
(6.13)
Equations (6.12) and (6.13) are valid only for the temperature difference (∆θ
vp
= θ
hs
−
θ
st
) corresponding to convective boiling. For water and water-based cutting fluids, this
condition is valid when 105
◦
C ≤ θ
hs
≤ 120
◦
C. For other conditions, the heat transfer
coefficient is calculated as
If 120
◦
C ≤ θ
hs
≤ 235
◦
C, then h
bv
= 3.36 ×10
6
(
θ
hs
−100
)
−1.43
(6.14)
If θ
hs
> 235
◦
C, then h
bv
= 3 ×10
3
W/m
2 ◦
C
(6.15)
Equations (6.13)–(6.15) are valid only when water is boiling under the atmospheric
pressure and under natural convection.
As discussed in the previous section, forced convection often takes place in real cutting
systems. As such, the cutting fluid is forced to move over hot surfaces of tool, chip and
workpiece. Such a movement brings changes in the boiling process. First, the natural

348 Tribology of Metal Cutting
value of the contact angle (Fig. 6.4) is changed. Second, the moving fluid detaches the
partially formed bubbles from the surface being cooled before they reach the diameter
defined by Eq. (6.11). Therefore, the flow of the cutting fluid makes the boiling process
weaker so that heat transfer due to evaporation reduces. When the velocity of the cutting
fluid is high, the heat transfer due to evaporation becomes negligibly small and thus
“normal” heat transfer due to forces of convection takes place as discussed in the previous
section.
The opposite is true, however, when film boiling takes place. Here, the flow of the cutting
fluid increases heat transfer because this flow blows a blanket or film of vapor which
prevents the liquid from wetting the surface. Under the condition of film boiling, Zones
2–4 in Fig. 6.5 shift to the right, i.e. towards higher temperature differences. The higher
the cutting fluid velocity, the greater the shift. To the first approximation, the heat transfer
coefficient h
cb
in this case, can be calculated accounting for the combined contribution
of the forced convection and boiling as
If h
bv
< 0.5h
cf
, then h
cb
= h
cf
(6.16)
If 0.5 ≤ h
bv
≤ 2h
cf
, then h
cb
=
4h
cf
+h
bv
5h
cf
−h
bv
h
cf
(6.17)
If h
bv
> 2h
cf
, then h
cb
= h
bv
(6.18)
Cooling action of air–cutting fluid mixture (mist). As discussed at the beginning of
this chapter, the cost of cutting fluid is approximately 16–17% of the life-cycle oper-
ational cost of machining. This cost continues to rise. It includes the costs associated
with procurement, filtration, separation, disposal and record keeping. Already the costs
of disposal of a cutting fluid are higher than the initial cost of this fluid, and they are still
rising. Even stricter regulations are under consideration for cutting fluid usage, disposal
and worker protection. As a result of all of this, the cutting fluid in wet machining oper-
ations is a crucial economic issue. An alternative, machining with “minimum quantity
lubricant,” or MQL, is gaining acceptance as a cost-saving and environmental-friendly
option in place of some wet machining processes. For example, spray cooling provides
the benefits of the cutting fluid used in flood applications with the added performance
of a high-velocity air–cutting fluid mixture. Therefore, it is of interest to consider heat
transfer in the interactions of hot tool and workpiece surface and air–cutting fluid mixture.
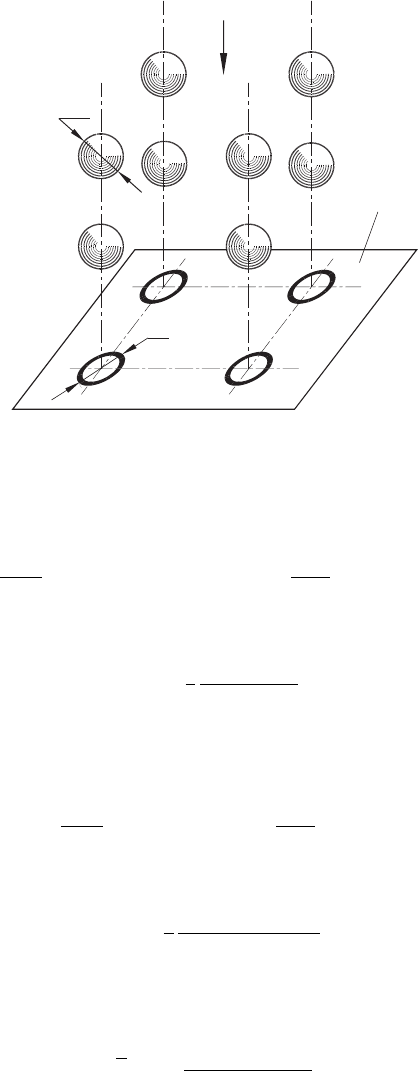
In cooling using an air–cutting fluid mixture, it becomes a case of two-phase cooling
medium. Figure 6.6 shows spherical droplets having diameter d
dr
that moves with veloc-
ity ν
air
towards a hot surface [47]. It is assumed that the concentration of droplets in the
unit volume of the mixture is uniform. The temperature of the hot surface is θ
hs
and that
of the mixture is θ
mx
. When a droplet meets the surface, it deforms so that the diameter
of contact is D
ct
= m
ct
d
dr
. As such, m
ct
1.
The thermal energy entering a droplet first heats it to the saturation temperature, θ
st
and then causes its evaporation. For the first part, heating, of this two-stage process the
Improvements of Tribological Conditions 349
v
air
A
hs
D
ct
d
dr
Fig. 6.6. Graphical representation of droplets moving towards a hot surface.
balance equation is
πD
ct
4
(
θ
st
−θ
mx
)
τ
1h
h
cf
= C
p−cf
ρ
cf
πd
3
dr
6
(
θ
st
−θ
mx
)
, (6.19)
where τ
1h
is the time of heating. Thus this time can be expressed as
τ
1h
=
2
3
C
p−cf
ρ
cf
d
dr
h
cf
m
cf
(6.20)
For the boiling and evaporation
πD
ct
4
(
θ
hs
−θ
st
)
τ
2h
h
vb
=
πd
3
dr
6
ρ
cf
r
vo
, (6.21)
where τ
2h
is the time of boiling and evaporation, which can be expressed as
τ
2h
=
2
3
ρ
cf
r
vo
d
dr
h
vb
m
2
ct
(
θ
hs
−θ
st
)
(6.22)
As the mean heat transfer coefficient for the whole process can be represented as
h
1−2
=
h
cf
τ
1h
+h
vb
τ
2h
τ
1h
+τ
2h
(6.23)
350 Tribology of Metal Cutting
then substituting Eqs. (6.20) and (6.22) into Eq. (6.23), one can obtain
h
1−2
=
h
cf
h
vb
C
p−cf
(
θ
hs
−θ
st
)
+r
vo
r
vo
h
cf
+C
p−cf
h
vb
(
θ
hs
−θ
st
)
(6.24)
The number of droplets falling simultaneously on the hot surface having an area A
hs
depends on the concentration K
dr
of liquid in the two-phase mixture. As it was assumed,
the concentration of droplets in the unit volume (m
3
) of the mixture is uniform, this
number is calculated as
n
dr−V
=
6K
dr
πd
3
dr
(6.25)
and on the surface of unit area (m
2
)itis
n
dr−A
=
3
√
n
dr−V
2
=
1
d
2
dr
6K
dr
π
2
/
3
(6.26)
As the area of the total surface A
hs
taken by one droplet is 0.25πm
2
ct
d
2
dr
, the total area
taken by all the droplets at any time instant is
A
hs−dr
= 0.25πm
2
ct
d
2
dr
n
dr−A
A
hs
(6.27)
The heat transfer process with the heat transfer coefficient
h
1−2
takes place over the
area A
hs−dr
of the total surface area A
hs
. On the rest of this surface (over the area
A
hs
− A
hs−dr
), the heat transfer takes place with the air contained in the two-phase
mixture. The heat transfer coefficient in this process is designated as h
air
. The total
combined heat transfer coefficient for the two-phase mixture is calculated as
h
c−mix
=
h
1−2
A
hs−dr
−h
air
A
hs
−A
hs−dr
A
hs
(6.28)
or
h
c−mix
= 1.2K
2
/
3
dr
m
2
ct
h
1−2
−h
air
+h
air
(6.29)
Heat transfer coefficients in this equation are calculated using the above-described
methodologies.
When boiling takes place in rather thin surface films of the cutting fluid, whose thick-
ness is close to the diameters of bubbles formed on boiling (d
bo
, Eq. (6.11)), then the
heat transfer coefficient h
bv
would depend on the thickness of the surface film, δ
sf
.
Improvements of Tribological Conditions 351
Reznikov and Resnikov [47], for the surface films having a thickness δ
sf
≤ 5 ×10
−3
m,
proposed the following correction factor
χ
cor
= 1.25 exp
−40δ
sf
(6.30)
which is to be used as a multiplier in Eqs. (6.13)–(6.15). Because in the practice of
machining the thickness δ
sf
= (0.5–10) ×10
−3
m, then for many practical cases χ
cor
=
1.20–1.23.
Cooling action due to heat pipe embedded in the tool. Heat pipes are sometimes
used to cool down the cutting tools [48,49]. Although the theory of heat pipes is well
developed [50,51], there is no simple methodology to calculate the cooling action of heat
pipes that can be used in the practice of tool design.
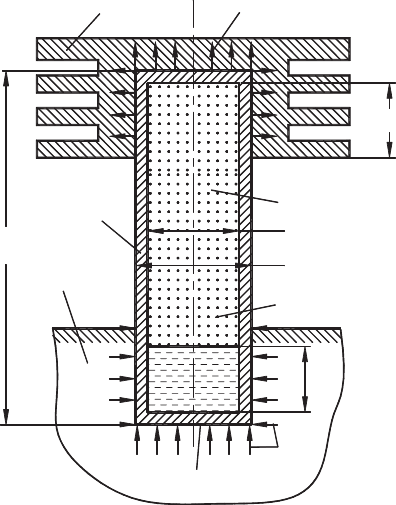
The heat pipe (Fig. 6.7) basically consists of a thick-walled hermetic container (1) made
up of a high thermal conductivity material. The container encloses some amount of the
easy-to-evaporate working fluid. One end (often called as the evaporator) of the container
is embedded in the unit to be cooled (2) while the other end (often called as the condenser)
contains a heat exchanger (radiator) (3) to release the thermal energy to the surrounding.
Heat applied by a heat source at the evaporator region vaporizes the working fluid in that
section. This also creates a pressure difference that makes the vapor to flow from the
h
v1
q
h1
q
in
h
v2
q
out
L
hp
d
h−ex
d
h−in
q
hs2
q
hs1
1
2
3
Fig. 6.7. Model of a vertical heat pipe.

352 Tribology of Metal Cutting
evaporator to the condenser where it condenses releasing the latent heat of vaporization.
The condensed working liquid then returns to the evaporator. This process is capable of
transporting the heat from a hot region to a cold region. The heat pipe transports large
amount of heat with a small temperature difference.
The balance equation for internal surface of the container is constructed assuming that
this container is thermo insulated so that there is no heat exchange with the surrounding
regions between the “hot” and “cold” zones. Thus,
h
bl
A
bl
(
θ
hs1
−θ
st
)
= h
cd
A
cd
(
θ
st
−θ
hs2
)
, (6.31)
where h
bl
and h
cd
are the heat transfer coefficients in the boiling and condensation
zones, respectively, θ
hs1
and θ
hs2
are the average temperatures in the boiling and con-
densation zones, respectively, A
bl
and A
cd
are the areas of boiling and condensation
zones, respectively.
The left side of this equation is the amount of thermal energy that enters from the walls
of the container into the boiling liquid in the “hot” zone while the right side is that released
through the walls in the “cold” zone. For the vertical container shown in Fig. 6.7
A
bl
= πd
h−in
h
ν1
+0.25πd
2
h−in
(6.32)
A
cd
= πd
h−in
h
ν2
+0.25πd
2
h−in
(6.33)
To determine the heat transfer coefficient in the boiling zone, one can use Eq. (6.12)
representing it in the following form
h
bl
= Θ
1
(
θ
hs1
−θ
st
)
m
vo
(6.34)
where
Θ
1
= c
bv
k
p
vo
wf
b
eq−wf
Pr
n
vo
r
wf
ρ
wf
ν
wf
m
vo
(6.35)
Values of the physical characteristic of working liquid and its vapor in Eq. (6.35) are
taken at temperature θ
st
.
The heat transfer coefficient on condensation can be determined using a simplified model.
The condensed liquid usually appears as liquid droplets (poor wetting) or film. In the
cooling of cutting tools, good wetting surfaces for heat tube are usually used so as
to consider the surface condensation. All the thermal energy formed on condensation
of vapor is transferred into the surface (of temperature θ
hs
which is lower than the
temperature of vapor, θ
st
) through the film of condensed liquid. Because the flow in this
film is normally laminar, it can be accepted that heat transfer takes place due to thermal
conductivity. Consider an infinitively small element ∆x of the surface film condensed on
a vertical surface of a body (Fig. 6.8). As thickness of the film, δ
x
is small, the temperature
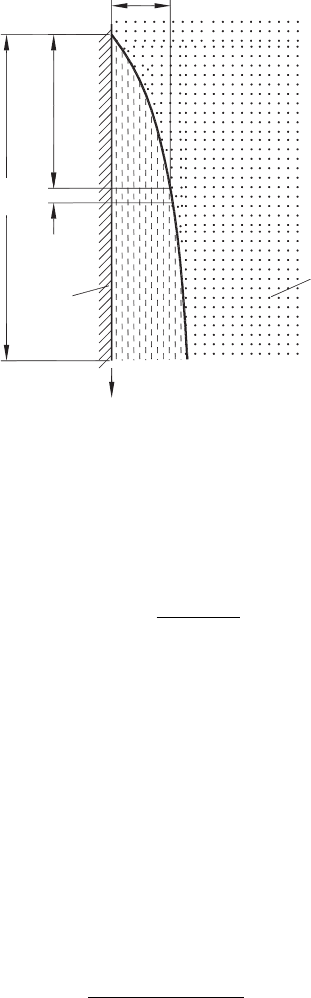
Improvements of Tribological Conditions 353
L
fl
x
∆x
X
q
s
t
q
hs
d
x
Fig. 6.8. Model of film condensation.
gradient across this film can be thought of as
(
θ
st
−θ
hs
)
δ
x
and thus, according to the
Fourier law of heat conduction, the specific heat flux through this film is
q
x
= k
wf
(
θ
st
−θ
hs
)
δ
x
, (6.36)
where k
wf
is the thermal conductivity of the working fluid corresponding to the average
temperature 0.5
(
θ
st
−θ
hs
)
.
Because the specific heat flux is q
x
= h
x
(
θ
st
−θ
hs
)
, the heat transfer coefficient is
calculated as
h
x
= k
wf
/δ
x
(6.37)
As it follows from Eq. (6.37), the determination of the heat transfer coefficient reduces
to that of the thickness of the film condensed on the surface, δ
x
. Nusselt, compiling a
great body of experimental data, proposed the following equation [47]
δ
x
=
4k
wf
µ
wf
(
θ
st
−θ
hs
)
ρ
2
wf
r
wf
g
x
1
/
4
, (6.38)
where µ
wf
is dynamic viscosity of the working fluid, µ
wf
= ν
wf
ρ
wf
.
354 Tribology of Metal Cutting
Substituting Eq. (6.38) into Eq. (6.37), one can obtain
h
x
= 1.25
ρ
2
wf
r
wf
k
3
wf
µ
wf
(
θ
st
−θ
hs
)
x
1
/
4
(6.39)
For practical calculations, it is necessary to determine the average heat transfer coeffi-
cient,
h
cd
over the total length L
fl
, of the hot surfaces in contact with the condensed film
of the cutting fluid as
h
cd
=
1
L
fl
L
fl
0
h
x
dx = 1.67
ρ
2
wf
r
wf
k
3
wf
µ
wf
L
fl
(
θ
st
−θ
hs
)
1
/
4
(6.40)
As before, ρ
wf
,k
wf
, and µ
wf
in Eq. (6.40) should be considered at the temperature
0.5(θ
st
−θ
hs
).
Equation (6.40) is obtained for a vertical wall. If vapor is condensed on a surface inclined
at an angle ϕ
cd
to a horizontal plane, then the heat transfer coefficient is calculated as
h
cd
= 1.67
ρ
2
wf
r
wf
k
3
wf
sin ϕ
cd
µ
wf
L
fl
(
θ
st
−θ
hs
)
1
/
4
(6.41)
and for a horizontal tube having diameter d
fl
it is calculated as
h
cd
= 1.28
ρ
2
wf
r
wf
k
3
wf
µ
wf
d
fl
(
θ
st
−θ
hs
)
1
/
4
(6.42)
By analogy with Eqs. (6.34) and (6.35), the heat transfer coefficient for condensation
can be represented as
h
bl
= Θ
2
(
θ
st
−θ
hs2
)
−0.25
(6.43)
where
Θ
2
= 1.67
ρ
2
wf
r
wf
k
3
wf
sin ϕ
hp
µ
wf
h
ν2
1
/
4
, (6.44)
where ϕ
hp
is the inclination angle of the heat pipe, ρ
wf
,k
wf
and µ
wf
are considered at
the temperature 0.5(θ
st
−θ
hs2
).

Improvements of Tribological Conditions 355
Substituting Eqs. (6.34) and (6.43) into Eq. (6.31), one can obtain
θ
hs1
=
Θ
2
Θ
1
A
cd
A
bl
1
m
vo
+1
(
θ
st
−θ
hs2
)
0.75
m
vo
+1
+θ
st
, (6.45)
where the ratio
Θ
2
Θ
1
depends on the properties of the evaporated fluid, saturation temper-
ature and the temperature on the inner wall of the hot tube in the condensation zone.
Because for a given type of the working fluid, the saturation temperature θ
st
is completely
determined by the pressure in the container, p
in−c
(MPa) then
Θ
2
Θ
1
= f(p
in−c
θ
hs2
), i.e. the
efficiency of the hot pipe is determined by the level of vacuum inside the container, type
of the working fluid and by cooling intensity of its condensation zone. Approximation
of the available experimental data allowed [47] to obtain the following relation
θ
hs2
−θ
st
≤ 14.2p
−0.15
in−c
(6.46)
It is understood that Eq. (6.45) is an approximation because the real thermal process
in the heat pipe is more complicated compared to that considered in the derivation of
this equation. However, it can be used in many engineering calculations involved in the
cutting tool design. An example of a tool design with the heat tube is shown in Fig. 6.9.
The cutting insert (1) is cooled by the heat pipe (2) imbedded in the holder (3). The heat
from the cutting insert is transferred to the “hot” end of the heat pipe and then transferred
by the vapor of the working fluid to the “cold” end of the heat pipe, where it is dissipated
by the radiator (4). As seen, the tool design is very simple. The use of such tools can be
very efficient in dry cutting. Moreover, the efficiency of such tool can be significantly
improved if radiator (4) is a part of the machine tool circulation cooling system.
Embrittlement action. The embrittlement action of the cutting fluid reduces the strain at
fracture of the work material. This action is based on the Rebinder effect [52]. This effect
was invoked as early as in 1936 as the basis of “oiliness” of boundary lubricants. Con-
ducting tension studies of tin specimens, Rebinder observed that a boundary-lubricant
film of oleic acid lowered the stress and stress at fracture. Microscopic examination of
1
3
2
4
Fig. 6.9. Tool with a built-in heat tube.

356 Tribology of Metal Cutting
the test specimens showed a great increase in the number of active slip planes. He con-
cluded that this resulted from the penetration (absorption) of the surface active acid into
microcracks which lowered the strength of the specimen.
Rebinder’s studies were directly concerned with the metal cutting process. Conducting a
great number of cutting tests under different cutting conditions and with different cutting
fluids, he observed microcracks formation and “healing.” The latter was particularly
pronounced in machining ductile materials, where great plastic deformation of the layer
being removed is observed. The results of Rebinder’s study showed that the absorbed
films prevent the closing of microcracks (healing due to plastic deformation of the work
material). Because each microcrack in the machining zone serves as a stress concentrator,
smaller energy was required for cutting. Pursuing this direction, Epifanov [53] found
that the penetration of the foreign atoms (from cutting fluid decomposition) produced an
embrittlement effect in a manner similar to hydrogen embrittlement. He concluded that
it thus facilitated by a resulting decrease in plasticity.
Our current understanding of the Rebinder effect is that the alternation of the mechanical
and physical properties of materials is due to the influence of various physiochemical
processes on the surface energy [52]. The energies of the process which lead to the
formation of a new fracture surface was considered by Griffith [54], who developed the
equation which defines the thermodynamic requirements for fracture
σ
f
=
"
2γ
sf
E
πc
cr
, (6.47)
where σ
f
is the fracture stress, γ
sf
is the surface energy, E is the Young’s modulus and
c
cr
is the length of some pre-existing crack.
Griffiths’ equation assumes that the only process that absorbs energy during fracture is
the energy required to form a new surface. As follows from this equation, the surface
energy reduces from a certain level γ
sf −1
to a new level γ
sf −2
due to the penetration of
the foreign atoms (from cutting fluid decomposition) then the energy required for fracture
reduces by
γ
sf −1
/γ
sf −2
1/2
. At this point one may wonder if pre-existing cracks exist
at the surface of ductile work materials. Multiple observations on the surface conditions
show that there are many cracks existing on the surface of even very ductile materials. For
example, Fig. 6.10 shows a micrograph of partially formed chip (ductile work material –
AISI steel 4330), where a number of very deep cracks exists on the surface of the layer
being removed.
The Rebinder effect takes place for practically all solids and structures. Its occurrence
depends on many physiochemical factors like the chemical composition of solid and
fluid, deformation and fracture conditions (strain, velocity, state of stress, material struc-
ture), etc. Depending upon a particular combination of the conditions, the Rebinder
effect manifests itself in different ways: from lowering the shear flow stress to a signif-
icant reduction of strength (both the stress and strain at fracture). The thermodynamic
condition for the Rebinder effect to lower the strength of a material is that the inten-
sity of surface interactions should be comparable with the energy of atomic, ion and/or
