Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

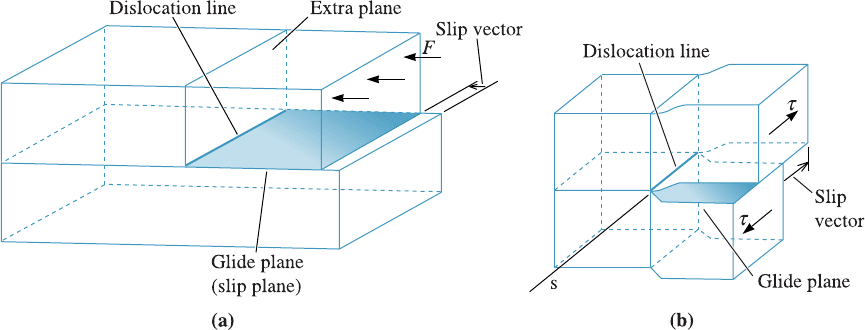
A schematic for slip line, slip plane, and slip vector (Burgers vector) for an edge
and a screw dislocation are shown in Figure 4-7. The Burgers vector and the plane are
helpful in explaining how materials deform.
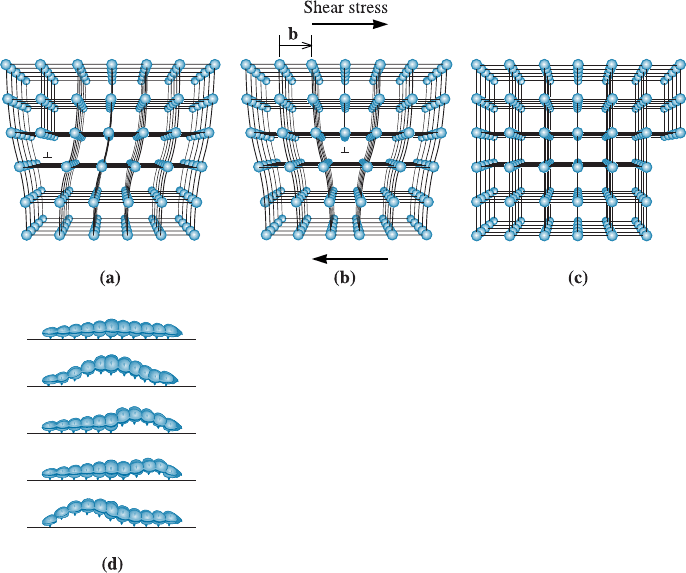
When a shear force acting in the direction of the Burgers vector is applied to a
crystal containing a dislocation, the dislocation can move by breaking the bonds be-
tween the atoms in one plane. The cut plane is shifted slightly to establish bonds with
the original partial plane of atoms. This shift causes the disloc ation to move one atom
spacing to the side, as shown in Figure 4-8(a). If this process continues, the dislocation
moves through the crystal until a step is produced on the exterior of the crystal; the
crystal has then been deformed. Another analogy is the motion by which a caterpillar
moves [Figure 4-8(d)]. A caterpillar will lift some of its legs at any given time and use
that motion to move from one place to another rather than lifting all the legs at one
time. The speed with which dislocations move in materials is close to or greater than the
speed of sound! Another way to visualize dislocation motion is to think about how a
fold or crease in a carpet would move if we were trying to remove it by push ing it
across rather than by lifting the carpet. If dislocations could be introduced continually
into one side of the crystal and moved along the same path through the crystal, the
crystal would eventually be cut in half.
Slip The process by which a dislocation moves and causes a metallic material to de-
form is called slip . The direction in which the dislocation moves, the slip direction, is the
direction of the Burgers vector for edge dislocations as shown in Figure 4-8(b). During
slip, the edge dislocation sweeps out the plane formed by the Burgers vector and the
dislocation. This plane is called the slip plane. The combination of slip direction and
slip plane is the slip system. A screw dislocation produce s the same result; the dis-
location moves in a direction perpendicular to the Burgers vector, although the crystal
deforms in a direction parallel to the Burgers vector. Since the Burgers vector of a screw
dislocation is parallel to the dislocation line, specification of Burgers vector and dis-
location line does not define a slip plane for a screw dislocation. As mentioned in
Chapter 3, there are new software packages that have been developed and use of these
can be very e¤ective in visualizing some of these concepts.
Figure 4-7 Schematic of slip line, slip plane, and slip (Burgers) vector for (a) an edge
dislocation and (b) for a screw dislocation. (Adapted from J.D. Verhoeven, Fundamentals of
Physical Metallurgy, Wiley, 1975.)
C H A P TE R 4 Imperfections in the Atomic and Ionic Arrangements100
During slip, a dislocation moves from one set of surroundings to an identical set of
surroundings. The Peierls-Nabarro stress (Equation 4-2) is required to move the dis-
location from one equilibrium location to another,
t ¼ c expðkd=bÞð4-2Þ
where t is the shear stress required to move the dislocation, d is the interplanar spacing
between adjacent slip planes, b is the magnitud e of the Burgers vector, and both c and k
are constants for the material. The dislocation moves in a slip system that requires the
least expenditure of energy. Several important factors determine the most likely slip
systems that will be active:
1. The stress required to cause the dislocation to move increases exponentially with
the length of the Burgers vector. Thus, the slip direction should have a small
repeat distance or high linear density. The close-packed directions in metals and
alloys satisfy this criterion and are the usual slip directions.
2. The stress required to cause the dislocation to move decreases exponentially with
the interplanar spacing of the slip planes. Slip occurs most easily between planes
of atoms that are smooth (so there are smaller ‘‘hills and valleys’’ on the surface)
and between planes that are far apart (or have a relatively large interplanar
spacing). Planes with a high planar density fulfill this requirement. Therefore,
Figure 4-8 (a) When a shear stress is applied to the dislocation in (a), the atoms are
displaced, causing the dislocation to move one Burgers vector in the slip direction (b).
Continued movement of the dislocation eventually creates a step (c), and the crystal is
deformed. (Adapted from A.G. Guy, Essentials of Materials Science, McGraw-Hill, 1976.)
(d) Motion of caterpillar is analogous to the motion of a dislocation.
4-3 Dislocations 101

the slip planes are typically close-packed planes or those as closely packed as
possible. Common slip systems in several materials are summarized in Table 4-1.
3. Dislocations do not mov e easily in materials such as silicon or polymers, which
have covalent bonds. Because of the strength and directionality of the bonds, the
materials typically fail in a brittle manner before the force becomes high enough
to cause appreciable slip. In many engineering polymers dislocations play a rel-
atively minor role in their deformation. The deformation in polymers occurs
mainly as polymer chains become untangled and then are stretched.
4. Materials with ionic bonding, including many ceramics such as MgO, also are
resistant to slip. Moveme nt of a dislocation disrupts the charge balance around
the anions and cations, requiring that bonds between anions and cations be
broken. During slip, ions with a like charge must also pass close together, caus-
ing repulsion. Finally, the repeat distance along the slip direction, or the Burgers
vector, is larger than that in metals and alloys. Again, brittle failure of ceramic
materials typically occurs owing to the presence of flaws such as small holes
(pores) before the applied level of stress is su‰cient to cause dislocations to
move. It is possible to obtain some ductility in certain ceramics using special
processing techniques.
The following examples illustrate the calculation of the magnitude of the Burgers
vector and identification of slip planes.
TABLE 4-1 9 Slip planes and directions in metallic structures
Crystal Structure Slip Plane Slip Direction
BCC metals f110g h111i
f112g
f123g
FCC metals f111g h110i
HCP metals f0001g h100i
f11
20g
f10
10g
9
=
;
See
Note
f10
11g
h110i
or h1120i
MgO, NaCl (ionic) f110g h110i
Silicon (covalent) f111g h110i
Note: These planes are active in some metals and alloys or at elevated
temperatures.
EXAMPLE 4-4
Dislocations in Ceramic Materials
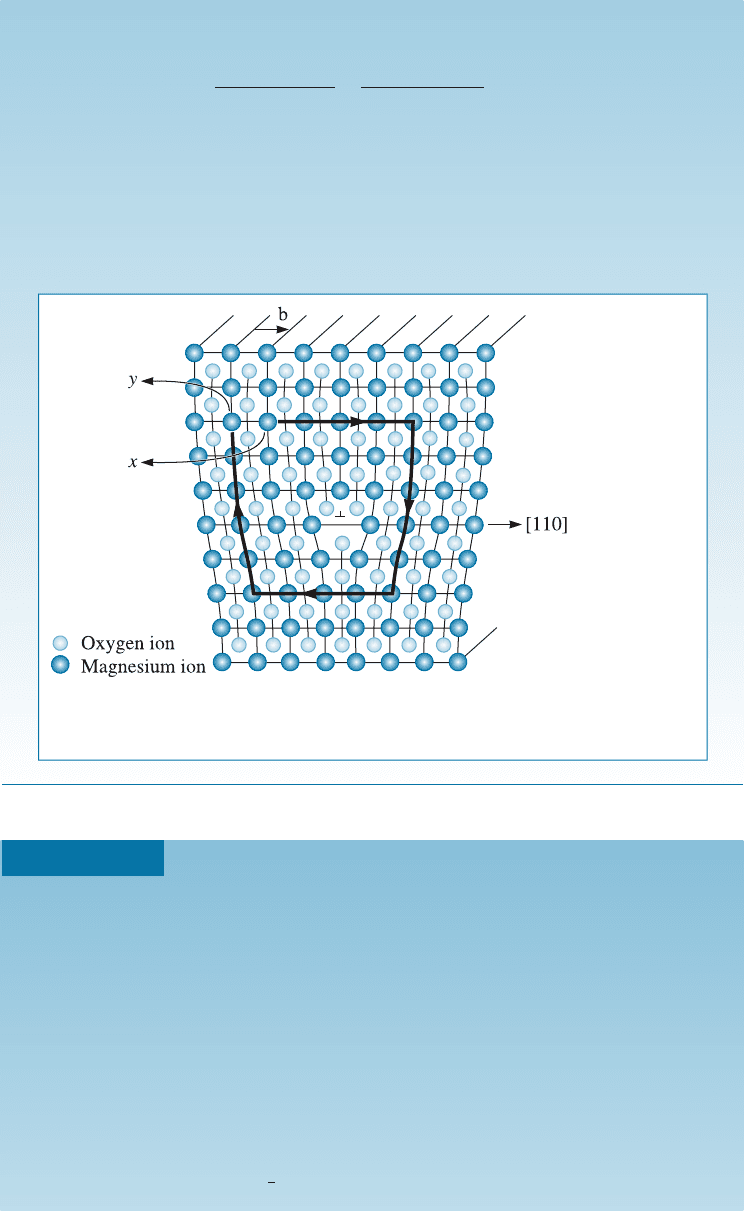
A sketch of a dislocation in magnesium oxide (MgO), which has the sodium
chloride crystal structure and a lattice parameter of 0.396 nm, is shown in
Figure 4-9. Determine the length of the Burgers vector.
SOLUTION
In Figure 4-9, we begin a clockwise loop around the dislocation at point x,
then move equal atom spacings to finish at point y. The vector b is the Burgers
vector. Because b is a [110] direction, it must be perpendicular to f110g planes.
C H A P TE R 4 Imperfections in the Atomic and Ionic Arrangements102
The length of b is the distance between two adjacent (110) planes. From
Equation 3-7,
d
110
¼
a
0
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
h
2
þ k
2
þ l
2
p
¼
0:396
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1
2
þ 1
2
þ 0
2
p
¼ 0:280 nm
The Burgers vector is a h110i direction that is 0.280 nm in length. Note, how-
ever, that two extra half-planes of atoms make up the dislocation-one com-
posed of oxygen ions and one of magnesium ions (Figure 4-9). Note that this
formula for calculating the magnitude of the Burgers vector will not work for
non-cubic systems. It is better to consider the magnitude of the Burgers vector
as equal to the repeat distance in the slip direction.
Figure 4-9 An edge dislocation in MgO showing the slip direction and Burgers
vector (for Example 4-4). (Adapted from W.D. Kingery, H.K. Bowen, and
D.R. Uhlmann, Introduction to Ceramics, John Wiley, 1976.)
EXAMPLE 4-5
Burgers Vector Calculation
Calculate the length of the Burgers vector in copper.
SOLUTION
Copper has an FCC crystal structure. The lattice parameter of copper (Cu) is
0.36151 nm. The close-packed directions, or the directions of the Burgers vec-
tor, are of the form h110i. The repeat distance along the h 110i directions is
one-half the face diagonal, since lattice points are located at corners and centers
of faces [Figure 4-10(a)].
Face diagonal ¼
ffiffiffi
2
p
a
0
¼ð
ffiffiffi
2
p
Þð0:36151Þ¼0:51125 nm
The length of the Burgers vector, or the repeat distance, is:
b ¼
1
2
ð0:51125 nmÞ¼0:25563 nm
4-3 Dislocations 103

Figure 4-10 (a) Burgers vector for FCC copper. (b) The atom locations on a (110)
plane in a BCC unit cell (for Examples 4-5 and 4-6, respectively).
EXAMPLE 4-6
Identification of Preferred Slip Planes
The planar density of the (112) plane in BCC iron is 9:94 10
14
atoms/cm
2
.
Calculate (1) the planar density of the (110) plane and (2) the interplanar
spacings for both the (112) and (110) planes. On which plane would slip nor-
mally occur?
SOLUTION
The lattice parameter of BCC iron is 0.2866 nm or 2:866 10
8
cm. The (110)
plane is shown in Figure 4-10(b), with the portion of the atoms lying within the
unit cell being shaded. Note that one-fourth of the four corner atoms plus the
center atom lie within an area of a
0
times
ffiffiffi
2
p
a
0
.
1. The planar density is:
Planar density ð110Þ¼
atoms
area
¼
2
ð
ffiffiffi
2
p
Þð2:866 10
8
cmÞ
2
¼ 1:72 10
15
atoms=cm
2
Planar density ð112Þ¼0:994 10
15
atoms=cm
2
ðfrom problem statementÞ
2. The interplanar spa cings are:
d
110
¼
2:866 10
8
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1
2
þ 1
2
þ 0
p
¼ 2:0266 10
8
cm
d
112
¼
2:866 10
8
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1
2
þ 1
2
þ 2
2
p
¼ 1:17 10
8
cm
The planar density and interplanar spacing of the (110) plane are larger than
those for the (112) plane; therefore, the (110) plane would be the preferred slip
plane.
C H APT E R 4 Imperfections in the Atomic and Ionic Arrangements104

4-4 Significance of Dislocations
First, dislocations are most significant in metals and alloys since they provide a mecha-
nism for plastic deformation, which is the cumulative e¤ect of slip of numerous dis-
locations. Plastic deformation refers to irreversible deformation or change in shape that
occurs when the force or stress that caused it is removed. This is because the applied
stress causes dislocation motion that in turn causes permanent deformation. When we
use the term ‘‘plastic deformation’’ the implication is that it is caused by dislocaiton
motion. There are, however, other mechanisms that cause permanent deformation. We
will see these in later chapters. The plastic deformation is to be distinguished from elastic
deformation, which is a temporary change in shape that occurs while a force or stress
remains applied to a material. In elastic deformation, the shape change is a result of
stretching of interatomic bonds, however, no dislocation motion occurs. Slip can occur
in some ceramics and polymers. However, other factors (e.g., porosity in ceramics, dis-
entanglement of chains in polymers, etc.) dominate the near room-temperature
mechanical behavior of polymers and ceramics. Amorphous materials such as silicate
glasses do not have a periodic arrangement of ions and hence do not contain disloca-
tions. The slip process, therefore, is particularly important in understanding the mechanical
behavior of metals. First, slip explains why the strength of metals is much lower than the
value predicted from the metallic bond. If slip occurs, only a tiny fraction of all of the
metallic bonds across the interface need to be broken at any one time, and the force re-
quired to deform the metal is small. It can be shown that the actual strength of metals is
10
3
to 10
4
times smaller than that expected from the strength of metallic bonds.
Second, slip provides ductility in metals. If no dislocations were present, an iron
bar would be brittle and the metal could not be shaped by metalworking processes,
such as forging, into useful shapes.
Third, we control the mechanical properties of a metal or alloy by interfering with
the movement of dislocations. An obstacle introduced into the crystal prevents a dis-
location from slipping unless we apply higher forces. Thus, the presence of dislocations
helps strengthen metallic materials.
Enormous numbers of dislocations are found in materials. The dislocation density,
or total length of dislocations per unit volume, is usually used to represent the amoun t
of dislocations present. Dislocation densities of 10
6
cm/cm
3
are typical of the softest
metals, while densities up to 10
12
cm/cm
3
can be achieved by deforming the material.
Dislocations also influence electronic and optical propertie s of materials. For ex-
ample, the resistance of pure copper increases with increasing dislocation density. We
mentioned previously that the resistivity of pure copper also depends strongly on small
levels of impurities.
4-5 Schmid’s Law
We can understand the di¤erences in behavior of metals that have di¤erent crystal
structures by examining the force required to initiate the slip process. Suppose we apply
a unidirectional tensile stress to a cylinder of metal that is a single crystal (Figure 4-11).
We can orient the slip plane and slip direction to the applied force (F ) by defining the
angles l, and f. The angle between the slip direction and the applied force is l , and f is
the angle between the normal to the slip plane and the applied force. Note that the sum
of angles f and l can be but does not have to be 90
.
4-5 Schmid’s Law 105
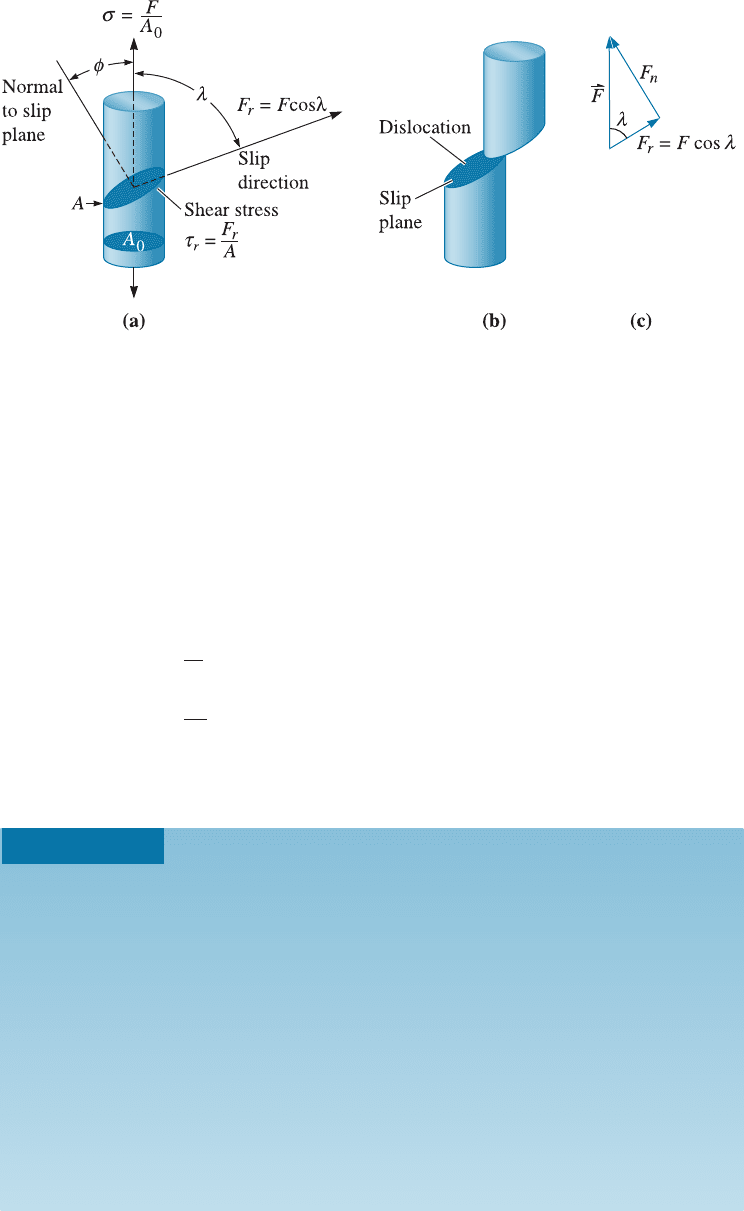
In order for the dislocation to move in its slip system, a shear force acting in the slip
direction must be produced by the applied force. This resolved shear force F
r
is given by:
F
r
¼ F cos l
If we divide the equation by the area of the sl ip plane, A ¼ A
0
=cos f, we obtain the
following equation known as Schmid’s law,
t
r
¼ s cos f cos l; ð4-3Þ
where
t
r
¼
F
r
A
¼ resolved shear stress in the slip direction
s ¼
F
A
0
¼ unidirectional stress applied to the cylinder
The example that follows illustrates an application of Schmid’s law.
Figure 4-11 (a) A resolved shear stress t is produced on a slip system. (Note: (f þ l) does not
have to be 90
.) (b) Movement of dislocations on the slip system deforms the material.
(c) Resolving the force.
EXAMPLE 4-7
Calculation of Resolved Shear Stress
Apply Schmid’s law for a situation in which the single crystal is at an ori-
entation so that the slip plane is perpendicular to the applied tensile stress.
SOLUTION
Suppose the slip plane is perpendicular to the applied stress s, as in Figure
4-12. Then, f ¼ 0
, l ¼ 90
, cos l ¼ 0, and therefore t
r
¼ 0. As noted before,
the angles f and l can but do not always add up to 90
. Even if the applied
stress s is enormous, no resolved shear stress develops along the slip direction
and the dislocation cannot move. (You could perform a simple experiment to
demonstrate this with a deck of cards. If you push on the deck at an angle, the
cards slide over one another, as in the slip process. If you push perpendicular to
the deck, however, the cards do not slide.) Slip cannot occur if the slip system is
oriented so that either l or f is 90
.
C H APT E R 4 Imperfections in the Atomic and Ionic Arrangements106
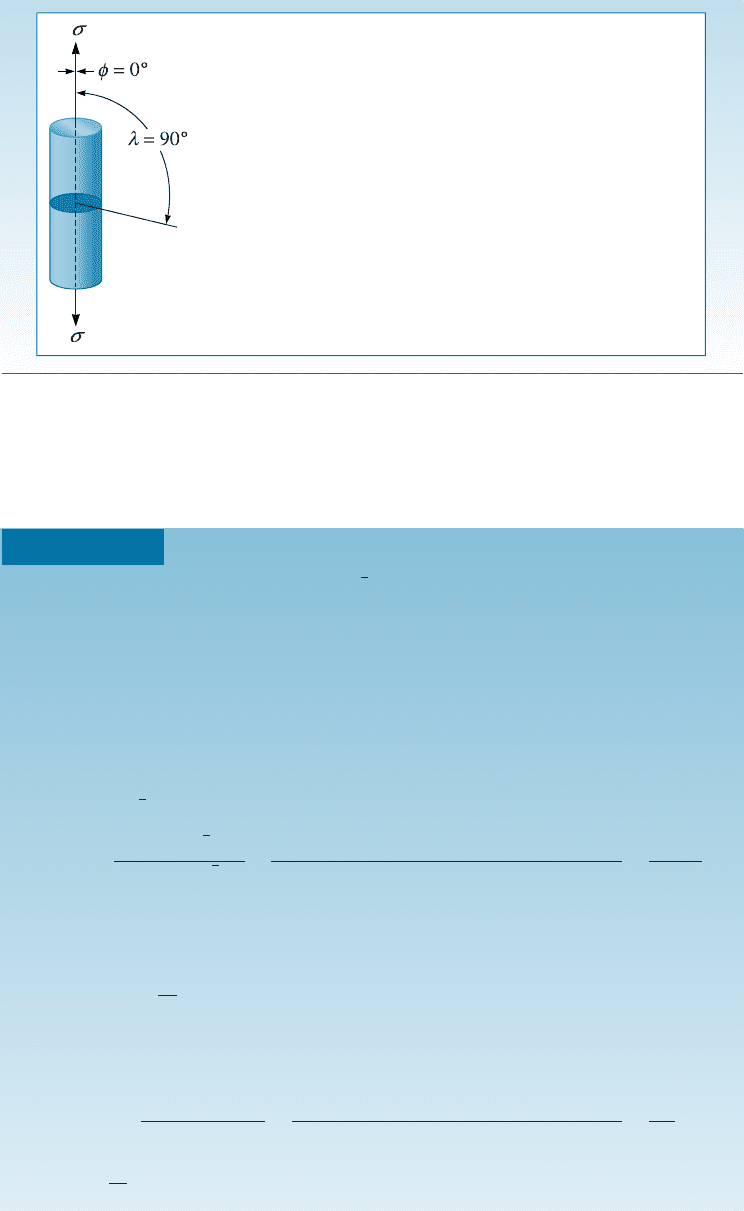
Figure 4-12
When the slip plane is perpendicular to the applied stress s,
the angle l is 90
and no shear stress is resolved. (For
Example 4-7.)
EXAMPLE 4-8 Schmid’s Law Calculation of Resolved Shear Stress
Consider the (111) slip plane and ½0
11 slip direction for a single crystal of
copper. A tensile stress ðsÞ of 21 MPa is applied to this crystal along the [001]
direction. What is the resolved shear stress ðt
r
Þ along the slip direction?
SOLUTION
We will use Schmid’s law:
t
r
¼ s cosðlÞ cosðfÞ
We calculate the angle ðlÞ between the tensile stress direction [001] and the slip
direction ½0
11 from the dot product as follows:
cos l ¼
½001½0 11
k001kk0
11k
¼
½ð0 0Þþð0 ð1ÞÞ þ ð1 1Þ
ð
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
0
2
þ 0
2
þ 1
2
p
Þð
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
0
2
þ1
2
þ 1
2
p
ÞðÞ ðÞ
¼
1
ffiffiffi
1
p ffiffiffi
2
p
The symbol kkstands for the magnitude of the vector which is given by
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
h
2
þ k
2
þ 1
2
p
Since cos l ¼
1
ffiffi
2
p
, the angle l will be 45
.
The normal to the slip plane (111) is [111]. Thus, the angle f betwee n the
tensile stress direction [001] and normal to the slip plane (i.e., [111] is given as
follows).
cos f ¼
½001½111
k001kk111k
¼
½ð0 1Þþð0 1Þþð1 1Þ
ð
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
0
2
þ 0
2
þ 1
2
p
Þð
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1
2
þ 1
2
þ 1
2
p
ÞðÞ ðÞ
¼
1
ffiffiffi
3
p
cos f ¼
1
ffiffi
3
p
, the angle f will be 54:73
.
4-5 Schmid’s Law 107

Thus, the resolved shear stress will be
t
r
¼ð21 MPaÞ
1
ffiffiffi
2
p
1
ffiffiffi
3
p
¼ 8:6 MPa
If we had a combination of slip systems (i.e., di¤erent slip planes and slip di-
rections), we could calculate the values of critical resolved shear stress for each
one. The direction that had the highest resolved shear stress would become ac-
tive first (i.e., dislocation in that particular system will begin to move first).
The critical resolved shear stress t
crss
is the shear stress required to break enough
metallic bonds in order for slip to occur. Thus slip occurs, causing the metal to plasti-
cally deform, when the applied stress ðsÞ produces a resolved shear stress ðt
r
Þ that equals
the critical resolved shear stress.
t
r
¼ t
crss
ð4-4Þ
4-6 Influence of Crystal Structure
We can use Schmid’s law to compare the properties of metals having BCC, FCC, and
HCP crystal structures. Table 4-2 lists three important factors that we can examine. We
must be careful to note, however, that this discussion describes the behavior of nearly
perfect single crystals. Most engineered materials are seldom single crystals and always
contain large numbers of defects. Since di¤erent crystals or grains are oriented in dif-
ferent random directions, we can not apply Schmid’s law to predict the mechanical be-
havior of polycrystalline materials.
Critical Resolved Shear Stres s If the critical resolved shear stress in a metal is very
high, the applied stress s must also be high in order for t
r
to equal t
crss
. A higher t
crss
implies a higher stress is necessary to deform a metal, which in turn indicates the metal
has a high strength! In FCC metals, which have close-packed f111g planes, the critical
resolved shear stress is low—about 0.34 to 0.69 MPa in a perfect crystal; FCC metals
TABLE 4-2 9 Summary of factors affecting slip in metallic structures
Factor FCC BCC HCP
c
a
I1:633
Critical resolved
shear stress (MPa)
0.34–0.69 34–69 0.34–0.69
a
Number of slip
systems
12 48 3
b
Cross-slip Can occur Can occur Cannot occur
b
Summary of
properties
Ductile Strong Relatively brittle
a
For slip on basal planes.
b
By alloying or heating to elevated temperatures, additional slip systems are active in HCP metals,
permitting cross-slip to occur and thereby improving ductility.
Note: For most elements c=a < 1:633, slip occurs on planes other than (0001) and t
crss
is high.
C H APT E R 4 Imperfections in the Atomic and Ionic Arrangements108

tend to have low strengths. On the other hand, BCC crystal structures contain no close-
packed planes and we must exceed a higher critical resolved shear stress—on the order
of 69 MPa in perfect crystals—before slip occurs; therefore, BCC metals tend to have
high strengths and lower ductilities.
We would expect the HCP metals, because they contain close-packed basal planes,
to have low critical resolved shear stresses. In fact, in HCP metals such as zinc that have
a c=a ratio greater than or equal to the theoretical ratio of 1.633, the critical
resolved shear stress is less than 0.69 MPa, just as in FCC metals. As noted in Table 4-2,
for most HCP elements c=a < 1:633. The slip occurs on non-basal planes and t
crss
is high.
For example, in HCP titanium, the c=a ratio is less than 1.633; the close-packed planes are
spaced too closely together. Slip now occurs on planes such as ð10
10Þ, the ‘‘prism’’ planes or
faces of the hexagon, and the critical resolved shear stress is then as great as or greater
than in BCC metals.
Number of Slip Systems If at least one slip system is oriented to give the angles l and
f near 45
, then t
r
equals t
crss
at low applied stresses. Ideal HCP metals possess only
one set of parallel close-packed planes, the (0001) planes, and three close-packed direc-
tions, giving three slip systems. Consequently, the probability of the close-packed planes
and directions being oriented with l and f near 45
is very low. The HCP crystal may
fail in a brittle manner without a significant amount of slip. However, in HCP metals
with a low c=a ratio, or when HCP metals are properly alloyed, or when the temper-
ature is increased, other slip systems become active, making these metals less brittle than
expected.
On the other hand, FCC metals contain four nonparallel close-packed planes of the
form f111g and three close-packed directions of the form h110i within each plane,
giving a total of 12 slip systems. At least one slip system is favorably oriented for slip to
occur at low applied stresses, permitting FCC metals to have high ductilities.
Finally, BCC metals have as many as 48 slip systems that are nearly close-packed.
Several slip systems are always prope rly oriented for slip to occur, allowing BCC metals
to also have ductility.
Cross-Slip Consider a screw dislocation moving on one slip plane that encounters an
obstacle and is blocked from further movement. This dislocation can shift to a second
intersecting slip system, also properly oriented, and continue to move. This is called
cross-slip. In many HCP metals, no cross-slip can occur because the slip planes are
parallel (i.e., not intersecting). Therefore, polycrystalline HCP metals tend to be brittle.
Fortunately, additional slip system s become active when HCP metals are alloyed or
heated, thus improving ductility. Cross-slip is possible in both FCC and BCC metals
because a number of intersecting slip systems are present. Consequently, cross-slip helps
maintain ductility in these metals.
4-7 Surface Defects
Surface defects are the boundaries, or planes, that separate a material into regions, each
region having the same crystal structure but di¤erent orientations.
Material Surface The exterior dimensions of the material represent surfaces at which
the crystal abruptly ends. Each atom at the surface no longer has the proper coordina-
tion number and atomic bonding is disrupted. This is very often an important factor in
making silicon based microelectronic devices. The exterior surface may also be very
4-7 Surface Defects 109
