All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.


The curve y ¼f(x) has the famous bell shape. It is symmetrical about
x (mean).The stan-
dard deviation
x
is the distance between the point of in£ection and the mean.The maxi-
mum deviation is equaltoabout3
x
or 99.7%.
Remark
The converse of this last observation is important: if a distribution is random and we know its
maximum dispersion
D
, we can then estimate
x
¼
D
/6. This dispersion is known as the maximum
range.
In practice, the distributions and parameters calculated are in creasingly signi¢cant as we
approach the theoretical distribution, that is, as the number of measurements N increases.
Unfortunately, N isn ot alwayshigh.
Whenthe shape ofthehistogram canbe represented byabell-shaped curve, we apply the
results obtained for the ideal distribution with a very large N to such histograms
(Figure 5.3). Let us say that in making this assimilation we are bei ng optimistic about the
uncertainty. In statistics it is shown that this uncertainty is estimated by multiplying the
standard devi ation of the histogram measured ()by1=
ffiffiffiffi
N
p
. The more measurements we
make, the lower the riskof uncertainty, of course, but this decrease obeys the square-root
law. Uncer taintyonx,written
x
, is therefore de¢nedas:
D
x
¼
x
ffiffiffiffi
N
p
:
As, in the absence of other information, the standard deviation is the quadratic mean of
deviations, thetotaluncertainty is therefore:
D
x
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
P
i
ðx
i
xÞ
2
ðN 1Þ
v
u
u
t
2
6
6
4
3
7
7
5
1
ffiffiffiffi
N
p
:
0.4
–2
σ –σ 0
Probability
σ 2σ 3
σ
–3σ
0.3
0.2
0.1
σ
x
x − x
−
Figure 5.2 The normal curve. The probability of a measurement occurring, or its frequency, is plotted against
the value of the measurement on a scale expressed in
x–x
(value corrected by the mean). The distance
between the points of inflection is the dispersion . Note the geometrical signification of 2 and 3.
157 The calculation of uncertainties
Naturally, relativeuncertainty mayalsob e de¢ned here:
D ¼
D
x
x
:
Thisuncertaintyhasnounitand is expressedaspercent, per mil,etc.Thisformulaby which
u ncertainty varies as1=
ffiffiffiffi
N
p
is abs olu te ly f undamenta l. It indic ates that after estimating
dispersionbas ed on thehistogram ofexperimentalvalues, we estimatethe reliabilityofthis
u ncertainty by attributing to it a weight of 1=
ffiffiffiffi
N
p
. If we make 10, 100, 1000, and 10 000
measurements, precision improvesto30%,10%,3%, and1%.
Remark
It can be shown in statistics that if, for a normal distribution, we consider an uncertainty
x
,we
have a 63% chance of the real value lying within this interval. If we take 2
x
, that is a greater
uncertainty, this probability reaches 95%. In the first case we speak of high-risk uncertainty, and in
the second of low-risk uncertainty.
In p ractice, then, we measure uncertainties to the nearest sigm a or two sigmas, by the
exp ressions:
x
ffiffiffiffi
N
p
or
2
x
ffiffiffiffi
N
p
where N is thenumberofmeasurements.
A furthe r pro cedure must be added to these: that of the eliminati on of aberrant values
(o u tli e r s).Somevalues measured are completelyoutoflinewiththehistogram.These‘‘aber-
rant’’ values are thought to arise from some random accident during measurement (typi-
cally a sudden £uctuation in electric current when making a measurement on the mass
spectrometer, or the sampling of an apparently sound rock which proves to be weathered
when analyzed more closely). T he following criterion is used to eliminate these values: all
N
Concentration (ppm)
10
19.0
20.0 21.0
8
6
4
2
12
Figure 5.3 Histogram of 100 measurements of strontium concentration in a series of rocks from a
single site. The histogram and the normal curve approximating it are shown. The mean is 20 ppm, the
dispersion ¼0.5 ppm. We can therefore write concentration
C
¼20 0.5 ppm. Relative uncertainty is
written 0.5/20 ¼2.5%.
158 Uncertainties and results of radiometric dating
the values beyond 3 are eliminated, and then the entire calculation is repeated. The
resulting u ncertainty that has been ‘‘cleaned’’ of extreme outliers is denote d x*or
D
dependingonwhethe r itis an absoluteor relative uncertainty.
We now need to introduce a few useful distinctions (Figure 5.4). The accuracy of a mea-
surement is estimated from th e deviation between the measured value and the true value
sought. Precisionor rep rod uc i bility is the dispersion ofa measurement repeated N times:
itis this dispersion divided by the meanvalue. Reproducibility can be estimated from a his-
togram of measurements. Accuracy can only be estimated if we have independent knowl-
edge (or an estimate) of the true values. The power of resolution in geochronology is the
smallest age di¡erence we can measure with any guarantee of reliability. It is de¢ned as the
quantity signi¢cantly di¡erent from zero that can be estimated between two events:
R ¼t
1
t
2
.To estimate R, we assumethe deviation mustbegreaterthanthesum ofthestan-
dard deviations: R
t
1
þ
t
2
.
Exercise
The
206
Pb/
204
Pb ratio of a rock is measured six times giving: 18.35, 18.38, 18.39, 18.32, 18.33,
and 18.35. What value will we give for the isotopic composition of this rock? What is the
precision achieved? Is it worth making another six measurements given that the reproduci-
bility of each measurement is 3ø?
N
x
measured
values
true
value
N
x
true
value
a
c
x
true
value
x
true
value
b
d
Figure 5.4 The difference between reproducibility and accuracy. (a) Good reproducibility but poor
accuracy; (b) poor reproducibility and poor accuracy; (c) poor reproducibility but good accuracy; (d)
good reproducibility and good accuracy.
159 The calculation of uncertainties
Answer
Supposing the uncertainty values of each measurement are equal, we calculate:
x
¼ 18:353; ¼ 0:025; D
x
¼
x
=
ffiffiffiffi
N
p
¼ 0:010;
D ¼ D
x
=
x
¼ 5:4 10
4
:
We can therefore write
x
¼ 18:353 0:010 with 63% confidence and
x ¼ 18:353 0:02 with
95% confidence.
If we were to make another six measurements, precision would move from 0.01 to 0.0077,
which is a gain of 4 10
4
. But the precision of a single measurement is 3 10
3
, therefore it
is not worthwhile (except in special cases).
Remark
It is worth pondering that the numerical calculation gives more figures after the decimal point for
a much larger number of measurements (to be exact ¼0.024 94). Now, we have written
¼0.025 because the figures 94 are not significant. This approach is in line with the answer to
the famous question: can we measure the length of a table to the nearest tenth of a millimeter
using a measuring rod graduated in centimeters? Common sense is as good a guide to the answer
as mathematics.
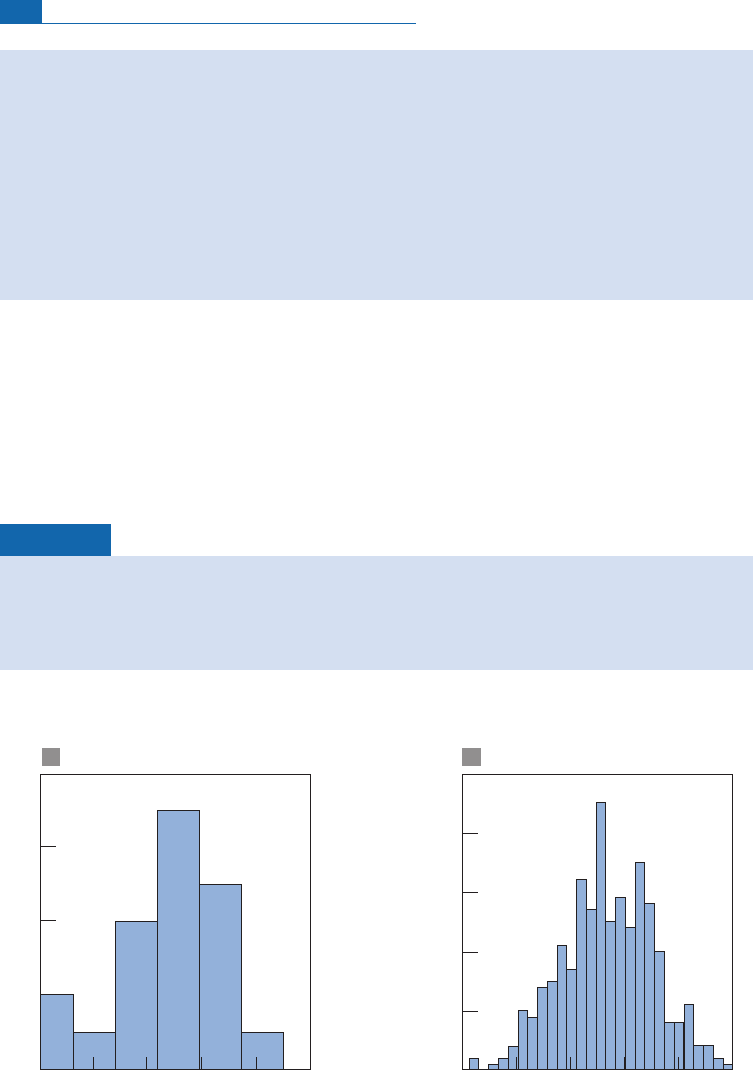
Exercise
Here are two histograms of measurements of
87
Sr/
86
Sr ratios for a single sample
(Figure 5.5). The first consists of 20 measurements with a mean value of 0.709 166, with
x
¼2.31 10
5
. The second consists of 400 measurements with a mean value of 0.709 184
with
x
¼4.34 10
6
.
6
0.709 1
0.709 15
0.709 2
0.709 25
4
2
87
Sr/
86
Sr
Frequency
a
87
Sr/
86
Sr
40
30
20
10
0.709 25
0.709 2
0.709 15
0.709 1
Frequency
b
Figure 5.5 Histogram of
87
Sr/
86
Sr ratios measured on a single sample. (a) 20 measurements, (b) 400
measurements. Notice that the classes become narrower as the number of measurements rises.
160 Uncertainties and results of radiometric dating
(1) What is the uncertainty affecting the measurement?
(2) How many measurements need to be made to get a standard deviation of 1 ppm?
(3) In this last case, do you think the uncertainty should be taken as
x
=
ffiffiffiffiffi
N
p
or 2
x
=
ffiffiffiffiffi
N
p
?
Answer
(1) Intrinsic uncertainty is determined from the (ln ,ln
ffiffiffiffiffi
N
p
) curve as 111 ppm.
(2) It would take 10 000 measurements.
(3) With
x
=
ffiffiffiffiffi
N
p
, the 400 ratio measurement lies outside the uncertainty limits on the 20
measurement expression. However, with 2
x
=
ffiffiffiffiffi
N
p
it is within limits. The latter expres-
sion is therefore required.
Exercise
Two
14
C ages are measured: 3230 70 years and 3260 60 years. Are these ages significantly
distinct? What is the power of resolution of
14
C for 3000 years?
Answer
The two ages are not significantly different as their standard deviations overlap. The power
of resolution of
14
C for 3000 years is 2%, or 60 years, which is a somewhat optimistic
estimate!
5.2.1 Systematic uncertainties
Randomuncertainties are deviationsofmeasuredvaluesfrom thetruevalue causedby vag-
aries obeying the l aws ofchance.‘‘Chance is aword that hides our ignorance,’’as the mathe-
matician and great scholar of probability E
¤
mile Borel used to say. But some uncertainties
aresystematic,thatis,theya¡ecttheoutcomeofmeasurementsbythesamefactor,although
that factor is not ne cessarily a known one. These really are random ‘‘errors.’’ Here’s an
example.
As stated, radioactive constants are a¡ected by measurement uncertainty and so are
periodically ‘‘updated,’’ that is, improved.When we calculate an age with one of the dat-
ing formulae we have developed, with the given value of a constant, we invariably intro-
duce the same uncertainty (but the amplitude of the uncertainty is not always the
same). Such un certainty is not really troublesome when the same method is used sys-
tematically. We then draw up a dating scale which can always be adjusted as required.
But whenever we wish to use ages obtained by methods based on di¡erent decay rates
and compare them, uncertainties about decay constants become very troublesome
indeed.
Another systematic uncertainty mayar isefrom the system ofphysical measurements.As
described, international standards are used for calibrating any systematic uncertainties
there may be among laboratories. Avalue is set for these standards although one cannot be
sureitis accurate.Hereagain, this approach, while n ecessary, is notfullysatisfactory when-
ever several methods are used. A nd what if some of the stan dards were wrong? After all,
even the U.S. National Bureau of Standards, the ¢nal arbiter, makes ‘‘errors’’ too and gives
its resultswith margins ofuncertainty!
161 The calculation of uncertainties
5.2.2 Pseudo-random uncertainties
In the problems we dealwith, uncertainty is often a c ombination ofsystematic and random
phenomena.When we spoke in Chapter 3 of the histogram of
40
K^
40
Ar ages, which is
asymm etrical, we said it was better to take not the mean as the most likely age valu e but
the mode (the value most frequently measured) as the asymmetry was probably caused by
di¡usion of the radiogenic isotope
40
Ar, which tends to‘‘lower’’ the age. Supe rimposed on
a random distribution due, say, to measurement uncertainties, we have a systematic trend
ofargon di¡usion. Even when a large number of measurements are made, pseudo-random
distributions are generally asymmetrical relative to the normal distribution (Figure 5.6).
Anyofthree typesofparam eter may representthetruevaluesought.
The mean is de¢nedas inthenormaldistributionby:
x ¼
X
i
x
i
N
:
The mode is the value occurring most frequently in the distribution. It is, mathematically,
the most l ikely value.The median is the value dividing the sample measured into two equal
halves.It is what we mightcall thehalfwayhouse.
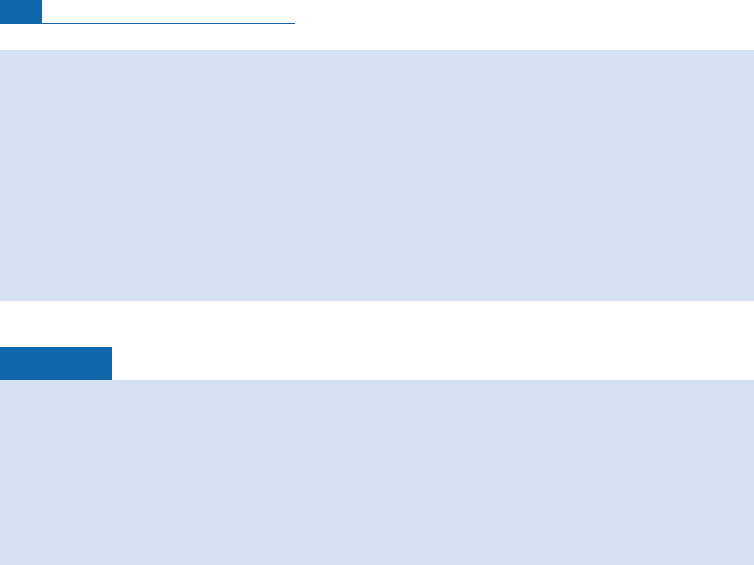
Exercise
The U–He age of a series of magnetites is measured. Table 5.1a shows the distribution of
apparent ages and Table 5.1b the distribution of uranium contents.
(1) Draw the corresponding histograms.
(2) Calculate the mean age and mean U content. Calculate the modes and medians.
(3) What is your geological interpretation of these results?
Answer
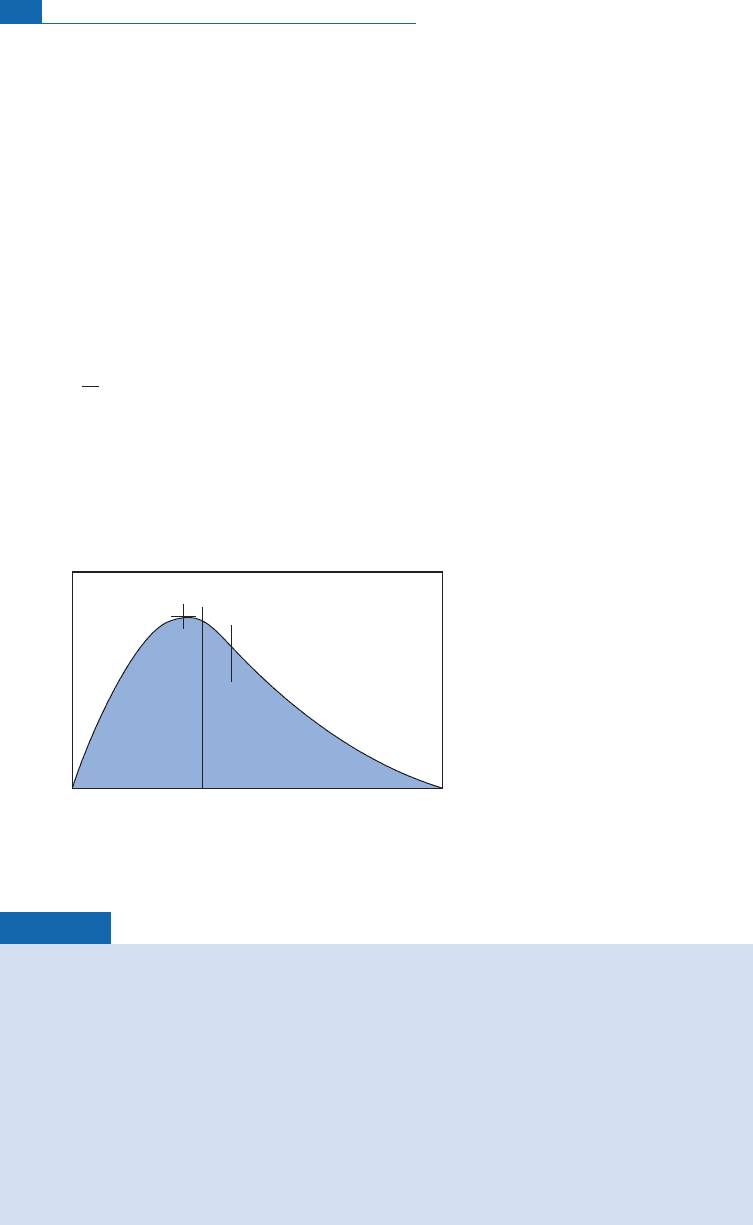
Mean age ¼26.3 Ma; median age ¼27 Ma; modal age ¼30 Ma.
Mean U content 25 ppb; median U content 25 ppb; modal U content 25 ppb.
The most likely geological age is 30 Ma since the distribution of uranium is virtually normal,
which is evidence that it has not been disrupted subsequently. The asymmetry seems to result
Frequency
X
mode median
mean
Figure 5.6 The difference between mode, median, and mean illustrated on an asymmetrical distribution.
162 Uncertainties and results of radiometric dating
solely from the diffusion of helium (Figure 5.7), which tends to lower the ages. Here, then, the
mode is the preferred value, although there is nothing to show that the maximum value of
32 Ma is not closest to the truth.
There is nothi ng automatic, then, about the choice of parameter (mean, mode, or med-
ian) that must be chosen to g et closest to th e truth.This must be decided in each individual
case by a qualitative analysis (here geochemical or geological).This is a characteristic fea-
ture of pse udo - statistics. Letus l aydown a rule ofprocedure: we s hal l u s e th e parameter s
employe d i n statist icsbutt h ei r meani ng w i ll be d is c u s s e d i nterm s ofge olog yand in par t i -
cular the randomor non-randomcharacterofthep henomenaconsidered.
Uncertainty is often expressed by the variance V
x
, the standard deviation
x
,andthe
uncertainty
x
=
ffiffiffiffi
N
p
¼ Dx. But here too uncertainties may be asymmetrical, as
mentioned in the introductor y example.We might also write 300 Ma
þ15
5
. That means the
ageliesbetween 315 and 295 Maanddepends onwhatultimately causes the uncertainty.
Table 5.1a Distribution of apparent ages
T (Ma)
32 3 0 28 26 24 22 2 0 18
Numberofsa mples 3 10 7 65432
2
4
6
8
10
18 20 22 24 26 28 30 32
N
Age (Ma)
2
4
6
8
10
40 30 20 10
N
U (ppb)
Figure 5.7 Histogram of age and uranium content measurements (see Tables 5.1a and 5.1b).
Table 5.1b Distribution of uranium content
U (ppb )
42.5 3 7.5 32.5 27.5 22.5 17.5 1 2.5 7.5
Numberofsa mples 1 2 7 10 10 6 3 1
163 The calculation of uncertainties

5.2.3 Composite uncertainty
We de¢ne the possible estimated deviation between the measured value and the true
value by the absolute uncertainty x. This uncertainty is therefore expressed in the same
units as x. We also de¢ne relative uncertainty x/x. This has no units and is expressed
in per cent, per mil, etc. Both types of uncertainty are very important in radiometric
dating. Absolute uncertainty determin es what time interval we can measure, which is
physically essential, of course, and is re£ected in the expression of the power of resolu-
tion. Relative uncertainty represents the measu rement quality, which is a very useful
p ointer too.
In what follows, we take the standard deviation of the measurement as the esti mate of
u ncertainty.
When a process leading to a measurement consists in a series ofoperations, the calcula-
tion ofthe¢naluncertainty involvessome quitestrictrules.
Addition ofoperations
Iftheprocessisanadditionof operationsx ¼au þbv wherea and b mayb e positiveor nega-
tive, thenwe addvariances:
V
2
x
¼ a
2
2
u
þ b
2
2
v
þ 2ab
2
u;v
whereV
u,v
isthe correlationbetweenvariances u an d v, thatis, the covariance:
V
u;v
¼
X
i
ðx
i
xÞðy
i
yÞ
N 1
¼
2
u;v
:
If b is negative and a positive, th e covariance is subtracted. But be careful, the covariance
itselfmaybe either positiveor negative.The covariance canbewritten:
2
u;v
¼
u;v
u
v
and thelinearcorrelative coe⁄centis:
u;v
¼
2
u;v
u
v
:
Thevalue of
u,v
liesbetween 0 and1 (Figure5.8).
Ifx ¼u þv,wecanwrite:
x
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2
u
þ
2
v
þ 2
u;v
u
v
q
:
Notice thatwhen covarianceishigh andis subtracted
x
maybeverylow. Thislow variance
isthe resultofacompensation e¡ect and is misleading.
Thus, for example, when we wish to c alculate the erroron the radiogenic (
207
Pb/
206
Pb)*
slope afte r making an isotopic measurement of lead, this error is very slight because of the
close correlation between the errors on
206
Pb/
204
Pb and
207
Pb/
204
Pb created by the high
u ncertaintyon
204
Pb.
164 Uncertainties and results of radiometric dating
Multiplication ordiv ision operations
Ifthe process involves multipl icationordivision operations:
x ¼ a u v
orsimilarly
x ¼
au
v
:
Once againvariances are required, butthistimethey mustbe weighted:
V
2
x
x
2
¼
2
u
u
2
þ
2
v
v
2
þ
2
2
u;v
uv
for multiplication
V
2
x
x
2
¼
2
u
u
2
þ
2
v
v
2
2
2
u;v
uv
for division:
(Toobtainthis exp ression, justshiftto logs andwe comebacktoadditions.)
x
x
¼ x
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2
u
u
2
þ
2
v
v
2
2
2
u;v
uv
s
¼
x
;
thatis,reduceddispersion.Inthis case,covarianceis addedfor multiplication andsubtracted
fordivision. In fact, this use of var iance is essential when uncertaint ies are correlated.
Ifthereisno correlation, we can deal directly withstandard deviations, aswith di¡erentia l
deviations (obeyingtherulesofdi¡erentiation).
Foraddition
x ¼ au bv
wewrite:
x
¼ a
u
þ b
v
:
u
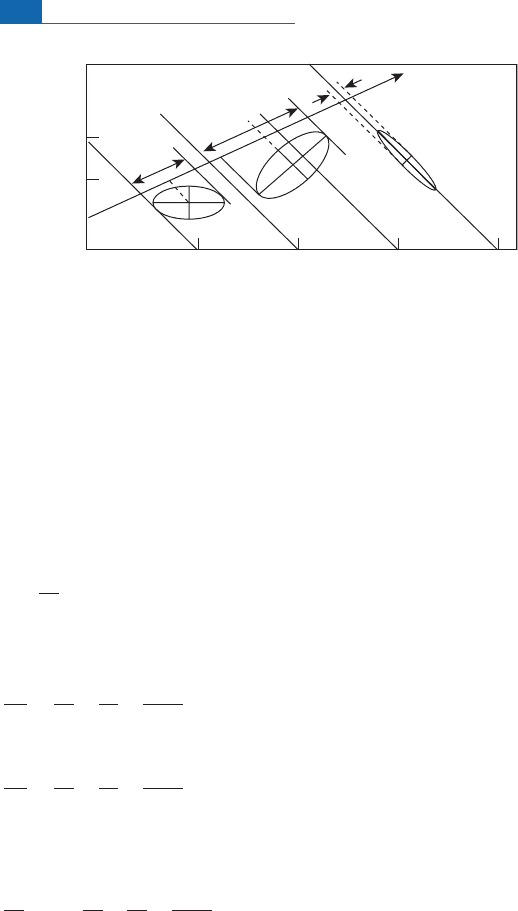
X
v
= 0
_
0.6
=
–0.95
2 4 6 8
2
1
∼
ρ
ρ
ρ
Figure 5.8 Diagram showing how errors on
u
and
v
in a product
uv
may be correlated and how the
factor
u,v
varies (see text for definition of symbols).
165 The calculation of uncertainties

For multiplication
x ¼ a uv
wewrite:
x
x
u
u
þ
v
v
:
Letusexaminethescopeofapproximationonasimple examplewherex ¼u þv.If
u,v
¼0it
can be seen that
x
corresponds to the length of the diagonalof the rectangle of dimensions
u
and
v
:
x
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2
u
þ
2
v
q
:
If we make the approximation:
x
u
þ
v
it holds good if
u
and
v
are quite di¡erent. If
they are of the same order of magnitude, we can make a maximum error of 2=
ffiffiffi
2
p
¼ 1:4,
which is not bad for an uncertainty given that we gain in ease ofestimation compared with
the calculation i nvolvingvariance.
Notice that both expressions consist in di¡erentiating and then replacing the di¡erences
by ¢nite increases wh ich are taken to be equal to . This practice is generalized when the
u ncertaintyofavalue stems from a mathematical formula.
We di¡erentiateandth en replacethe di¡erentialsby the values.
Ifx ¼uv:
x
¼ ðu; vÞ¼u
v
þ v
u
QED:
Likewiseifx ¼u
n
:
x
¼jnju
n1
x
and so on.
Thisistheapproachweshalladoptinwhatfol lows, unlessotherwisestated.Ifwehavesev-
eral measurements in each case, thenwe mustsystematically replace by D ¼ =
ffiffiffiffi
N
p
.
Noticethattobe abletoadduncertaintieswhen theyareofdi¡erenttypes,they must allbe
expressed in the same units. A convenient way to do this in geochronology is to express
them all as ages (as we have alreadydone for
14
C).This makes it easier to compare di¡erent
geochrono meters.
5.3 Sources of uncertainty in radiometric dating
Letus recal l thestages wegothrough in determininga geological age:
we collectthesamples tob e analyzed:rocks, minerals, wood, etc.;
weanalyzethese samples i n thelaboratory for their isotopic and che mical ratios;
weusethese measurements tocalculate an age;
wesituatethis agewithin ageologicalscenariowhichwe construct.
Eachstageis a¡ected by potentialuncertainties (Figure5.9).
166 Uncertainties and results of radiometric dating
