All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.


thetarget product which, by nuclear reaction, yields the cosmogenic isotope. Sometimes it is
single. This is so in iron meteorites composed of a metallic alloy of iron and nickel.
Sometimes there are more than one, as in ordinary meteorites where krypton isotopes are
producedbyspallationonrubidiu m, strontium, yttrium, and zirconiu m.
Theprimary£uxofcosmicraysiscomposedofprotons(andsomeHe
þ
ions)( a ndofa llthe
isotopesinthe Universeintheionizedstatebutinverysmall abundances). Ithasbothintensity
(numberofparticlesperunitofsurfaceareaandperunittime)andanenergyspectrum,
because in factthere are N
1
, N
2
, ..., N
3
particles, corresponding to energylevels1, 2, ..., n.As
said,thisprimary£uxofprotonsproducesbarelyanythingother than reactionsatthe meteo-
rite’s surface, since as soonas itpenetratesbyafewcentimeters itgeneratesasecondary £uxof
neutrons, which penetrate more deeply.These neutrons also have an energy spectrum which
changes as they penetrate, dimin ishing, of course. The £ux and energy spectrum of cosmic
radiation may vary with time (weknow neither how, nor the magnitude ofsuch £uctuations).
Whatwemeasure istheresultof£uxaggregatedoverseveralmillion orevenbillionyears.
A further, although generally mi nor, complication is that in addition to cosmic
radi ation there is also a £ux of particles from the Sun. Generally, this £ux is weak and
of low energy, but from time to time it may become intense and of high energy. These
bursts in £ux areknown as solar £ares.They too mayengender spallation reactions.
The e¡ective cross-sections for production of new isotopes by nu clear reactions are also
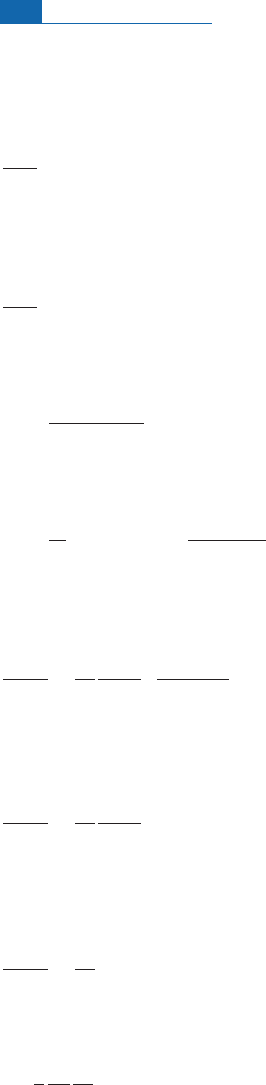
dependent on energy and therefore on penetration inside the meteorite. Figure 4. 15 shows
the production ofvarious isotopesversus depth.
As,inaddition, so meisotopesresultfromspallationonseveraltargetnucleiwhose e¡ects
are cumulative, we can imagine thesheercomplexityofthe phenomenon ifwewishtodeter-
mine all the contributions.That would involve estim ating a mean £ux and its energy spec-
trum and local izing the sample to be measured inside the meteorite. For these reasons, a
simpler wayofmaking the calculation hasbeensought.
59
Ni,
60
Co
(dpm kg
–1
)
36
Cl
(dpm kg
–1
)
Distance (cm)
300
50
100 150
200
100
–10
–20
–30
0
60
Co
36
Cl
59
Ni
Figure 4.15 Production of
36
Cl,
59
Ni, and
60
Co by (n,
) reactions in a supposedly spherical chondritic
meteorite. Distance is measured from the meteorite’s surface towards its center. Cl ¼100 ppm,
Ni ¼1.34%, Co ¼700 ppm.
35
Cl ¼45 barns,
58
Ni ¼4.4 barns,
59
Co ¼37 barns. Initial flux
S
0
¼0.5
neutron cm
3
s
1
.
127 Exposure ages
Radioactive isotopes
In this case, the equation in the preceding subsection must be supplemented by the decay
term l
r
N
r
,whereN
r
isthe numberofradioactiveatoms:
dN
r
dt
¼
r
N
c!r
l
r
N
r
:
Let us posit
r
N
c!r
¼T. It is c onsidered to be constant to simplify things, or else it is
assumedweknow itsmeanvalue.The equationbec omes:
dN
r
dt
¼ T l
r
N
r
:
Integrating gives:
N
r
¼
T C e
l
r
t
l
r
:
where C is the integration constant.Ifatt ¼0, N
r
¼0, C ¼T. Fromthis:
N
r
¼
T
l
r
1 e
l
r
t
¼
r
N
c!r
l
r
1 e
l
r
t
:
Now supp ose we have two isotopes, one stable and one radioactive, produced byspallation
u ndersimilarconditionsofenergyand £ux.We canestablishthe ratio:
N
s
ðtÞ
N
r
ðtÞ
¼
s
r
N
c!s
N
c!r
t
1 e
l
r
t
l
r
:
Ifwe waitlong enough for production and destruction ofth e radioactiveproductto achieve
asteadystate, then e
l
t
!0.Thisgives:
N
s
ðtÞ
N
r
ðtÞ
¼
s
r
N
c!s
N
c!r
l
r
t:
Notice that we have eliminated the £ux factor. If, in addition, the two isotopes are products
ofthe same element, thenN
c!s
=N
c!r
¼ 1 and the equationbecomes:
N
s
ðtÞ
N
r
ðtÞ
¼
s
r
t l
r
;
henc e :
t ¼
1
l
N
s
N
r
r
s
:
In this way, we determine the time elapsed since the meteorite was subjected to cosmic
radiation without knowing anything more about the cosmic radiation but having just the
ratioofthe e¡ective cross-sections.Theexposureageisthetime sincethe meteoritewasbro-
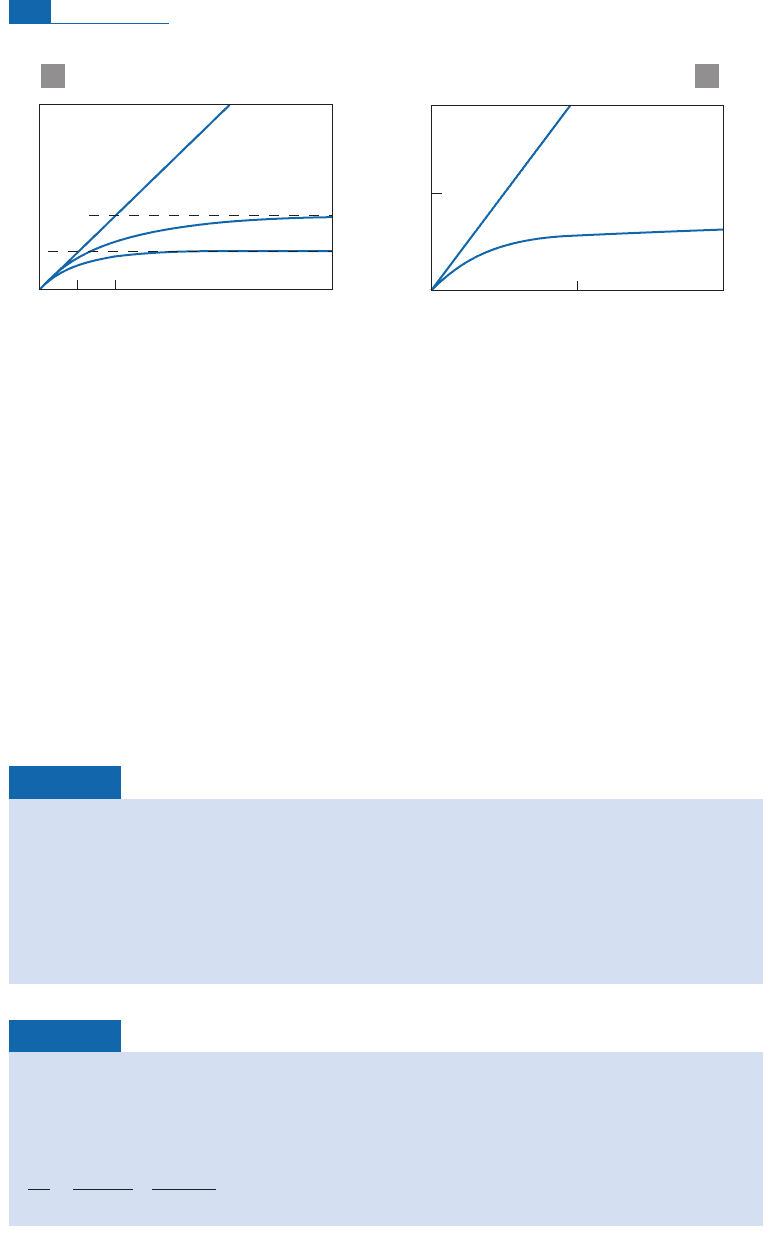
ken intopieces, leading toits exposureto cosmic rays(Figure 4. 16).
128 Cosmogenic isotopes
One of the conditions for successfully applying this method is that the isotopes used
should have e¡ective cross-sections that are s ensitive to the same ranges of energy and £ux
to justify the simpli¢cation of mathematically eliminating £ux. Accordingly, we are inter-
ested in isotopes either of the same element or of adjacent elements in the periodic table.
The pairs mostusedare
3
He^
3
He*,
22
Ne ^
22
Na*,
38
Ar^
39
Ar*,
83
Kr^
81
Kr*,and
41
K^
40
K*
(* indicatesthatthe isotope is radioactive).
The only point that remains undetermined if we are to be able to calculate the age
is the ratio of e¡ective cross-sections and their level of constancy depending on
particl e £ux and energy. The determination of e¡ective cross-sections has, of course,
b ene¢ted from the extraordinary research activity in nuclear energy and we have a
good catalogofe¡ective cross-sectionsfromwhich ithasbeenpossibleto deriveprecise laws.
Exercise
The
83
Kr–
81
Kr method is used to calculate exposure ages (Marti, 1982). The decay constant of
81
Kr is
l
¼0.32 10
5
yr
1
. What is the minimum age we can calculate using the steady state
formula?
Answer
For this, e
lt
must be negligible compared with 1. If we accept that e
lt
must be less than
0.01, then
t
¼1.4 million years.
Exercise
Can we not use this method for ages of less than 1.4 Ma?
Answer
The evolution equation is
N
r
N
s
¼
s
N
c!r
r
N
c!s
1 e
l
t
l
t
:
Number of nuclei
Radiogenic argon
concentration cm
–3
Exposure age (years) Exposure age (years)
Stable
isotopes
Radioactive
isotopes
39
Ar*(radioactive)
38
Ar (stable)
10
–13
2x10
–13
0
1000
2000
λ
–1
2
λ
2
λ
–1
1
λ
1
=
2λ
2
a
b
Figure 4.16 Pairwise chronometry (stable isotope, radioactive isotope) for cosmogenic isotopes. (a) The
general theoretical case; (b) the example of
38
Ar/
39
Ar, which is used extensively in glaciology (see
Oeschger, 1982).
129 Exposure ages
We need to just calculate the curve ð1 e
l
t
Þ=
t
and use the value of the ratio
N
r
/
N
s
.We
know that
N
c!r
is identical to
N
c!s
for krypton. Calculate the curve for the
81
Kr/
83
Kr ratio,
given that
r
/
s
1.6612.
Exercise
The
83
Kr–
81
Kr method can be used for calculating exposure ages in stony meteorites by the
formula:
T
¼
1
l
81
83
83
Kr
83
Kr
cosmogenic
where 1/l ¼0.307 Ma. To be rid of any complications and given that many Kr isotopes are
produced by spallation, the ratio of effective cross-sections is calculated from the formula:
81
83
¼
0:95
2
80
Kr
83
Kr
þ
82
Kr
83
Kr
:
Calculate the exposure age of the Juvinas meteorite if the isotopic measurement of Kr in it
is by convention:
Answer
T
¼10.710
6
years.
Exercise
We use the
41
K–
40
K method to date iron meteorites (Voshage and Hintenberger, 1960). The
only target is therefore iron and we take it that
l
40
¼0.5543 10
9
yr
1
. As the effective cross-
sections are
40
¼9.4 millibarns and
41
¼14.7 millibarns, what is the cosmogenic isotopic
composition of
41
K/
40
K for two meteorites whose exposure ages are 100 Ma and 1 Ga?
Answer
For 100 Ma (
41
K/
40
K)
cosmogenic
¼1.60; for 1 Ga (
41
K/
40
K)
cosmogenic
¼2.038.
Whatarethe main conclusions tobe drawn fromthese measurementsofexposure ages?
Exposureages aregenerallymuchyoungerthantheagesofmeteoriteformation(ormeta-
morphism).Theyrangefromafew millionyearsto afewhundred millionyears.(Ages offor-
mation are closer to 4.5 billion years, as we have seen.) This shows that for most of th eir
lifetime meteorites are inside their parent b ody, where they are shielded from cosmic rays,
and thatthe fracturingof meteoriteshas occur red relativelylate (but longbefore they fall to
Earth !).
84
Kr
78
Kr
80
Kr
81
Kr
82
Kr
83
Kr
86
Kr
1 0.157 0.460 0.0166 0.767 0.968 0.182
130 Cosmogenic isotopes
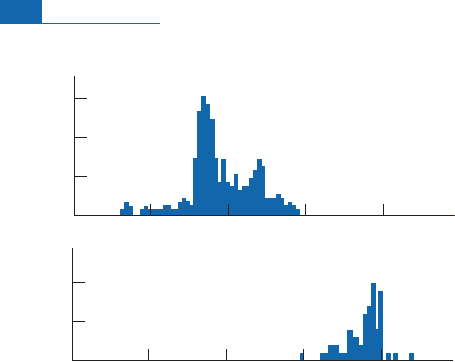
The distribution of meteorite exposure ages seems to display peaks at 5.7 and 20 million
yearsforordinarystonymeteoritesandat700 millionyearsfor iron meteorites(Figu re 4. 17).
The di¡erenc es in exposure ages for iron and chondritic meteorites is problematic.Why
should iron meteorites have so much older exposure ages? It was ¢rst thought that th ey
came from a di¡erent part of the Solar System. It is now thought that the exposure ages of
iron meteorites are older because iron withstands i mpacts better than the silicate assem-
blages that make up chondritic meteorites, which have been subjected to impacts resulting
in more recent fragmentation. In any event, the results show that meteorites have long life-
times in interplanetaryspace.
4.3.2 Terrestrial rocks
The principle for terrestrial rocks (see reviewby Oeschger,1982) is the same as for meteor-
ites.Galact ic co smic radiatio n causes nuclear reactions in rocks.Th e nuclei produced can
bemeasured, andifweknowthe £u xwe can calculatethe duration ofi rradiation.The di¡er-
ence with meteorites is that the intensity of cosmic radiation at the Earth’s surface is far
lowerbecauseithasbeenattenuatedby theabsorptionofprotonsand neutrons inthe atmo-
sphere. It was not until measurem ent sensitivity had been improved that these methods
could be applied to terrestrial rocks. A second di⁄culty is that until now methods using
isotope ratios employed for meteorites, such as
40
K/
41
Kand
83
Kr/
81
Kr, have n ot been
applicable for terrestrial rocks. For the ¢rst m ethod, no equivalent has be en found for iron
meteorites, which do not contain large quantities of initial K, which dilutes the e¡ects
produced by irradiation. For the second metho d, sensitivity is still insu⁄cient, but that
may change. Unlike the case of meteor ites, where the particle £ux is eliminated, here it
mustbe calibrated carefully. Now, the £u x diminishes, ofcourse, as itpenetrates into a rock.
Hence there is a further di⁄culty, which has been solved empirically, that of calibrating the
£ux, or calibratingabsorption.
Iron meteorites
Exposure age (years)
Chondrites
N
N
5
0
10
6
10
7
10
8
10
9
10
10
0
20
30
Figure 4.17 Exposure ages of various types of meteorites.
131 Exposure ages

Exploitable chronometers
In practice, we use the four radioactiveisotopes
14
C*,
10
Be*,
26
Al, and
36
Cl and the two rare
gas es
3
He and
21
Ne, which are stable isotopes. For radioactive elements, we use the formula
establishedfor meteorite exposureages:
N
r
¼
N
c!r
l
1 e
l
r
t
:
Buthere, equilibrium isnotachieved. BynotingT the production rate N
c!r
, we obtain:
N
r
¼
T
l
1 e
l
r
t
:
Forexample, forberyllium,
10
Be ¼
T
l
Be
1 e
l
Be
t
:
The di⁄cultylies in determining the £ux and assuming ittobe constantover time and then
estimatingthe abundanceoftargetnuclei.
For
3
He and
21
Ne, exposure ages are also calculated by the method described for stable
isotopes in meteorites.Thisgives:
N
s
¼ N
c!s
t:
The production rate is written as P ¼ N
c!s
.The gases
3
Hean d
21
Ne accu mulatelinearly
withtim e,therefore
3
He ¼Ptor
21
Ne ¼Pt. Heretoowe needto know the £ux(and to assume
thatitis constant) andtheabsorption laws.
For thetarget,
3
Heproduction does notdepend much on its compositionwhereas
21
Ne is
p roduced byspallation of Mg and depends greatlyon the chemical composition of the tar-
get.To make it uniform, olivine, whose Mg content is more or less constant, is separated
and measurements are madeonthis mineral.
Calibration of prod uction rates, erosion rates, etc.
The particle £ux varies with both altitud e and latitude,
6
as we said when discussing
14
C.
The £ux has therefore to be calibrated in ea ch place where the measurement is made.
There are twoways to do this. First, gene ral laws h avebeen es tablished giving the value of
£ ux by altitude and latitude. To g et some idea of this, let us say that at an altitude of
5000 m, the £ ux is 20 times greater than at se a level. At the poles, at a constant altitude, it
is 60 times greater than atthe equator.T his iswhy £ux is calibrated locally, where possible,
bymea suring
10
Be,
26
Al,
3
He,or
21
Ne contentsonsamples whose agehasbeen determined
byother methods.
Let us look at two important examples that will help in understanding the thinking
behind the method but whichwill show its‘‘fruitful complications’’and so sugg est its future
developments.
6
The first measurements were made in the Antarctic where the flux is greatest and, in addition, the erosion
rate is low.
132 Cosmogenic isotopes

Erosionratemeasurements
Our job is to measure the expo sure age of a basalt £ow from the Piton de la Fou rnaise
volcano on the island of Re
¤
union (Staudacher and Alle
'
gre,1993). This lava £ow is at an
altitude of 2300 m and there is no indicatio n that its altitu de has varied over the course
of time.
We de cide to use the cos mogeni c age method based on
3
He applied to olivine. We
therefore separate the olivine from a rock sampled from the surface and measure the
3
He content of the sample. To obtain the
3
He of cosmogenic origin, we must, of course,
corre ct for
3
He of internal origin. To do this, we measure the
3
He/
4
He ratio of internal
origin on olivine sampl ed at a great distance from the surface. We can calculate the
cosmogenic
3
He content by measuring the (
3
He/
4
He)
total
ratio and the total concentra-
tion i n
3
He.
Exercise
Given (
3
He)
total
,(
3
He/
4
He)
internal
, and (
3
He/
4
He)
total
, establish the formula for calculating
cosmogenic
3
He.
Answer
3
He
cosmo
¼
3
He
total
3
He
4
He
total
3
He
4
He
internal
3
He
4
He
internal
2
4
3
5
:
When all the calculations havebeen made, we ¢nd1.3 10
12
cm
3
g
1
at standard tempera-
ture and pressure of
3
He of cosmogenic origin.The production rate P
0
of
3
He in the olivine
hasbeen calculated for thelatitude and altitude ofthe sampleas 2.2 10
17
cm
3
g
1
per year
atstandardtemperatureand pressure.
Table 4.1 Usable cosmogenic nuclides
Isotope Half-life (yr)
Measurement
method Di⁄culties
Production rate
(atoms/ yr) Sea
level
latitude >558N Time-span
3
He Stable Massspectrometer Di¡uses easily 160(olivine) 1ka ^ 3 Ma
10
Be 1.5 10
6
AMS Atmospheric
contamination
6 (quartz) 3 ka ^ 8 Ma
26
Al 7.16 10
5
AMS Atmospheric
contamination
37 (quartz) 5 ka ^ 2 Ma
36
Cl 3.08 10
5
AMS Atmospheric
contamination
8 (basalt) 5 ka ^ 1Ma
21
Ne Stable Massspectrometer Common neon 45(olivine) 7 ka ^ 10 Ma
14
C 5730 years AMS Contamination 20 (basalt) 1ka ^ 40 ka
41
Ca 103 10
3
AMS Verydi⁄ cultto
measure
^300ka
133 Exposure ages

It is easy thento calculatethe exposure age:
T
cosmo
¼
3
He/P
0
¼59 090 years.
In addition, the ‘‘geological’’ age of the basalt £ow has been calculated by the K^Ar
method as 65 200 2000 years.The surface rock has been irradiated for 65 200 years and
yetits exposure ageisjust 6000 yearsattheyoungest. Howcome?
After examining the various source s of error, we accept that the age di ¡erence is due
to erosion.The rock sampled atthe surface today was in fact located at depth for much of
itshistory. Itonlycametothesurface through ablation ofthe m ate rial aboveit, bye rosion.
Now, we k now that the particle £ux decreases exponentially with the thickness of rock it
haspenetrated.Weneed, then, tomodelthe phenomenon.
We canwritethe rateofproduction of
3
Heintheform:
P ¼ P
0
exp
XðtÞ
L
where X(t)isthethickness counting fromthesurfaceand L isthe attenuation factorofradia-
tionwith depth.
Tosimplify matters, we can assum e thatthe rate oferos ion is c onstant.We canwrite:
X ¼ X
0
2t
where 2is the erosion rateand X
0
the initialdepthofthesa mple.
We canthereforewrite:
d
3
He
dt
¼ P
0
exp
X
0
2tð
L
:
We integrate this equation between 0 and t and then replace X
0
by its value X
0
¼X þ2t
(rememberingthat X is counted downwards).This gives:
3
He ¼
P
0
L
2
exp
X
L
1 exp
2t
L
hi
:
ThenX is the currentdepth coordinate.
Ourobjectiveis todeterminethe erosion rate, 2.
We k n ow t (65 200 years) and P
0
(2.2 10
17
cm
3
g
1
yr
1
at standard temperature and
p ressure), butthe re arestill twounknowns: 2and L.
To measure L, we bore a small core and so take samples at various depths.We isolate the
olivine andmeasurethe cosmogenic
3
Heon eachsample ofit.We can nowdraw theplot:
ln ð
3
HeÞ
cosmo
hi
¼ fðXÞ:
Ifour assumptions aboutthe £ux an d erosion rate are valid, the relation is a straight line of
the form:
ln ð 3He Þ
cosmo
¼
X
L
þ A
where A is aconstant.The slopeis (^1/L).
134 Cosmogenic isotopes

Exercise
A core was taken from the lava of the island of Re
´
union discussed before. Olivine was
extracted at each level and the
3
He content measured. After correcting for
3
He of internal
origin, the following results were found as a function of depth
X
(Table 4.2).
Calculate the attenuation factor
L
.
Notice that depth
X
in this table is expressed in grams per square centimeter, which is an
unusual unit of length! The reason for this is that attenuation is, of course, dependent on the
quantity of matter penetrated and so depends on the density of that matter (attenuation per
unit length penetrated is not the same in rock, soil, or a layer of atmosphere). If attenuation
were expressed per mil length, we would have to multiply it by density, and so the effective
value would be:
length ðcmÞ mass ðgÞ
volume cm
3
ðÞ
which is equivalent to mass per square centimeter. Naturally enough,
L
too will be expressed
in g cm
2
.
Answer
After calculating ln(
3
He) as a function of
X
(do it) we find for the slope:
L ¼ 165 g cm
2
5:
How can we now calculate the erosion rate 2?
Let us go back to the expression as a function of
X
but now start from where
X
¼0, that is, at
the present-day surface. The expression becomes:
3
He ¼
P
0
L
2
1 exp
2
t
L
:
We can develop the exponential in series limiting ourselves to the first three terms.
Remembering that
3
He ¼
T
cosmo
P
0
, we can finally write:
2¼2
L
t
t
T
cosm
t
:
If we note the relative difference between the geological age and cosmogenic age as
T
/
T
:
2¼2
D
T
T
L
t
:
Table 4.2 Relation of
3
He content to depth in olivine
from lava on Re
´
union
X (g cm
2
)
3
He (10
13
cm
3
g
1
)
10.45 1 3
37 .86 9.12
131.95 6.9
217.42 3.58
3 1 8.4 1 1 . 74
135 Exposure ages
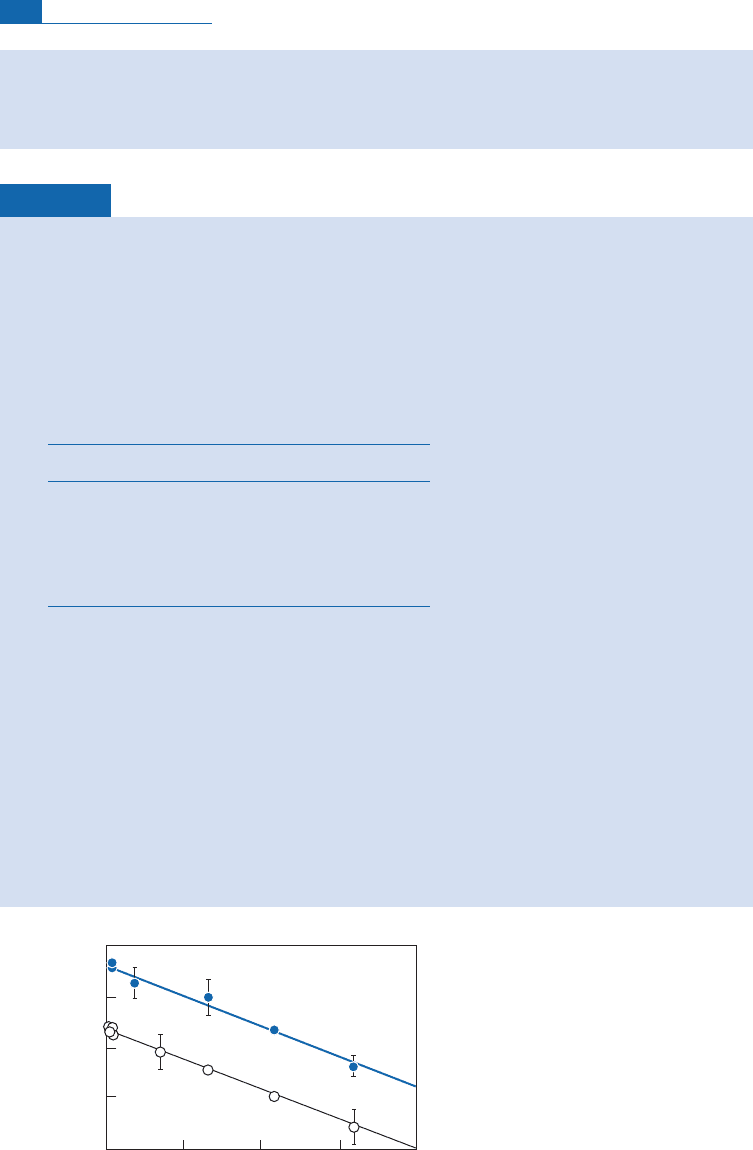
In the present case we find 2¼4.7 g cm
2
yr
1
.
If we accept a density of 2 g cm
3
because the lava sampled is ‘‘bubbly’’ and contains many
cavities, then 2¼2.1 mmyr
1
¼2.1 mm per 1000 yr.
Exercise
Cosmogenic
21
Ne contents were measured on the same samples of olivine from the island
of Re
´
union (Table 4.3). Given that the production rate of
21
Ne is 6.28 10
18
cm
3
g
1
at
the latitude and altitude in question, calculate the attenuation factor
L
and the erosion rate 2.
How do you account for the result for
L
compared with that found for
3
He? Which seems to
you to be the more accurate rate of erosion, that determined with
3
He or that with
21
Ne? To
what do you attribute the difference?
Answer
L
¼ 160 gcm
2
:
It is identical to that for
3
He because the particle flux at the origin of
3
He and
21
Ne is the
same for both. The cosmogenic age of the surface sample is
T
cosmo
¼52 388 years.
2¼ 9:6g cm
2
yr
1
¼ 4:8 mm per 1000 yr:
The
21
Ne age is greater than the
3
He age probably because helium diffuses more readily
than neon and the sample has probably lost a lot of helium. This invites caution when
interpreting helium ages (Figure 4.18).
Table 4.3 Relation of
21
Ne content to depth in olivine
from lava on Re
´
union
X (g cm
2
)
21
Ne (10
13
cm
3
g
1
)
10.45 3.29
37 .86 1.73
1 31.95 1 .59
21 7.42 0.96
3 18.4 1 0.5 2
–28
–27
–29
–30
–31
100 200 300 400
Depth (g cm
–2
)
3
He
21
Ne
In(
21
Ne
c
,
3
He
c
)
Figure 4.18 Attenuation curves for the creation of
3
He and
21
Ne in rocks of Re
´
union given by way of
example.
136 Cosmogenic isotopes
