All?gre Claude J. Isotope Geology
Подождите немного. Документ загружается.

Measu ring the rate ofupli ft
As sai d, the particle £ux at 500 meters altitude is 20 times higher than the £ux at sea level.
Suppose, then, wehave a lavathathas £owed atsea level.Subsequently the terrain itoverlies
hasbe en liftedtoan altitude of 500m.
Of course, the l ava would be irradiate d much more over the course ofuplift.This type of
mo del canbe calculatedinthesamewayasbefore:
d
3
He
dt
¼ P
0
exp
XðtÞ
L
where X(t)isthealtitudefor which zerois takenat100mand L istheattenuationfactori n the
atmosphere. Ifwe call the rateofupliftU, weget:
U ¼ 2
T
cosmo
t
t
L
t
:
As canbeseen,T
cosmo
¼the ageofexposureandisgreater thanthe‘‘geological’’age.
Naturally, eros ion has n ot been allowed for in this example.Ifwe add the erosion rate, we
will need other constraints and other chron ometers to solve the problem because we wil l
then havetwounknowns, 2and U.
Here, weareatthefrontierofcurrentresearch.
Intere stand l imits ofthese methods
The methods are of interestforanumberofreasons:
erosion ratescanbe determined(providedtheyaren ottoohighbecausesensitivityislost,
ofcourse);
we can date the time a surface comes tobe expos ed to the air, for example a fault plane or
thewallofa collapsedc aldera;
the rate ofupliftofmountains canbe measured.
There are many limitations, the main one for terrestrial rocks being that £ux is
weak (except at high altitudes near the poles). Very sensitive methods are therefore
required.
Ofcourse, the‘‘boxes’’musthaveremained closedas fordating with natural radioactivity.
For reliable results, measurements must be made by various methods such as
21
Ne or
10
Be
or by combinations of methods (
26
Al/
10
Be) as proposed by La l (Lal et al., 1958;Laland
Peters,1967).The various methods are dependent on the chemical composition of rocks or
minerals, ¢rst because the target must not contain too many non-cosmogenic natural iso-
topes, and second because reaction yields depen d on the chem ical c ompos ition of the
target.
Theproduction of
21
Ne,for instance, canbewritten:
ð
21
NeÞ
cosmo
¼ C
1Mgþ 0:36 Al þ 0:19 Si
þ0:04 Ca þ 0:01 ðFe þ NiÞ
inwhichC is aconstant.
137 Exposure ages

Lastly, of course, the £uxes must be calibrated in terms of altitude, l atitu de, and their
variations overtime andaveragedout (seethe reviewbyLal,1988).
4.4 Cosmic irradiation: from nucleosynthesis to stellar
and galactic radiation
We speakofcosmogenic isotopes as something special.But, in fact, all the isotopes in nat-
u re are cosmogenic.They were born of nuclear reaction s in the cosmos by what i s called
nucleosynthesis.Wehave mentioned this pheno menon several times. Itanswers the ques-
tion of the alchemists of old: how were the chemic al elements created? Forour purposes,
we need to complete this question: how were the various is otopes form ed and in what
proportions? In the manufacture of an atom, what is important, as we have said, is
the nucleus, because it is in the nucleus that all th e mass is concentrated, the electrons
being captured subsequently to populate the surrounding orbitals. Here, then, i s a ¢rst
p art of the answer: the chemical elements are the outcome of nuclear reactions. Nuclear
reactions produce both isotopes and chemical elements. Such reactions are not merely
the invention of nuclear physicists, they occur naturally throughout the Universe, where
the same causes produce the same e¡ects, governed by the laws of nuclear reactions.
Upon exa min ation, the table of chronometers based on cosmogenic isotopes lo oks very
similar to the table of extinc t radi oactivity. Why should the two converge like this? To
an swer this, we need to broaden ou r ¢eld of view and raise the more general issue of
nucleosynthesis.
Nucleosynthesis and the theory explaining it are the foundation of modern astro-
physics. It is also the starting point of what i s called chemistry of the cosmos or co smo -
chemistry. This is hardly the place to develop this theory in full as it would take us to
the heart of astronomy and very far from our present subject matter. None the less, it is
worth exp ounding brie £y a few important concepts,
7
particularly as astronomy and the
earth sciences have moved clo ser on these topics over the last 20 years (reread Chapter 1
onthis).
The chemical elements were made in the stars by nuclear reactions. The levels of
energy involved (MeV or GeV) are so great that only the st ars can be the sites of such
sy nthesis on so great a scale. These are the only environments in the Universe where
the‘‘ambient energies’’are intense enough and extensive enough for nuclear reactions to
be gene rated creating new chemical species in such large masses. The alchemists of old
were out by a factor of a million. They wanted to transform matter with burning coals,
that is, with energies of the o rder of the electronvolt (eV) whereas it takes energies of the
order of MeV at least to change nuclei and so atoms. With their athanors
8
they could
7
It is worth reading the few well-documented, introductory books on this, particularly D. D. Clayton
(1983), Principles of Stellar Evolution and Nucleosynthesis, Chicago University Press or C. Cowley
(1995), Introduction to Cosmochemistry, Cambridge University Press.
8
Athanors are receptacles used by alchemists to do their experiments.
138 Cosmogenic isotopes

alter the atoms’ outer electron shells, wh ereas making an atom involves altering the
nucleus.
The idea that the nucleosynthesis of chemical elements occurred mainly in stars, ¢rst
hituponbyAtkinson and Houte rma ns (1929)andthenbyGa mow (1946), was con¢rmed
on ly in 1952 by the astronomer Merrill, who observed the presence of technetium-98
around a star. Now, all the isotopes of thi s ele ment are radioactive, with a period of less
than 1 million years. If this element is foun d near an isolated star in the Universe, it must
have been created recentlyby the starotherwise it would be‘‘dead,’’destroyed by radi oac-
tive decay.
The theoryof nucleosynthesis was developed synthetically in1957 in apioneering paper
known as B2FH by Margaret Bu rbidge, Je¡ Bu rbi dge, Willy Fowler,andFred Hoyle
(Burbidge et al.,1957).
9
The major stages are as follows. It allbegins withthe proton, thatis,
the hydrogen atom, synthesized at the ti me of the Big Bang. After that, it all happens by
nuclear reactions in the stars, but not just any stars. L et us look at the successive stages in
nuclear terms.
4.4.1 The transition from the proton to helium
This is no s traightforward transitio n. It ¢rst involves the intermediate products D
and
3
He. The entire process is written as a series of nuclear reactions in an
avalanche:
1
H þ
1
H !
2
D þ
þ
þ
2
D þ
1
H !
3
He þ
3
He þ
3
He !
4
He þ2
1
H
where is aneutrinoand isgammaradi ation.
These nuclear reactions occur in ordinary stars like the Sun. It is the most wi despread
activity in stars. Itrequires temperaturesofseveral million degrees.
10
4.4.2 The synthesis of elements
Helium-4 ( radiation) is an exceptionally stable building block in nuclear terms
(two protons and two neutrons). The nuclear reactions involving this nucleus are
written:
4
He þ
4
He !
8
Be*.
9
Al Cameron of Harvard developed a similar theory at the same time but failed to publish any summary
in a major scientific journal but just gave conferences and lectures on the subject.
10
It is these reactions we cannot manage to ‘‘calm’’ to produce ‘‘domestic’’ energy, we can only produce
them artificially in hydrogen bombs.
139 Cosmic irradiation: from nucleosynthesis to stellar and galactic radiation

Beryllium-8* is an unstable isotope which reacts quickly to give, in its turn,
12
Cbythe
equation:
8
Be*þ
4
He !
12
C.
Therefore, all told:
3
4
He !
12
C.
This is what is called helium fusion, that is, the formation of
12
C.This type of nuclear reac-
tion continues.Wehave:
12
C þ
4
He !
16
O þ
16
O þ
4
He !
20
Ne þ
20
Ne þ
4
He !
24
Mg þ.
Alongside the addition of successive ‘‘blocks’’of
4
He, fusion of carbon and oxygen nuclei
o ccurs, these nucleithemselvesbeing formedbythe additionofth reeand four
4
He:
12
C þ
12
C !
24
Mg þ
12
C þ
12
C !
23
Na þp
12
C þ
12
C !
20
Ne þ
4
He
12
C þ
12
C !
23
Mg þn
12
C þ
12
C !
16
O þ2
4
He
or
16
O þ
16
O !
32
S þ
16
O þ
16
O !
31
P þp(
1
H)
16
O þ
16
O !
31
S þn
16
O þ
16
O !
28
Si þ
4
He
16
O þ
16
O !
24
Mg þ2
4
He.
Trigge ring these nuclear reactions requires considerable energies to overcome the natural
repulsion between positively charged nuclei.The ¢rst stages begin at 100 million degrees
and endin stages atcloseto1billion degrees.
140 Cosmogenic isotopes
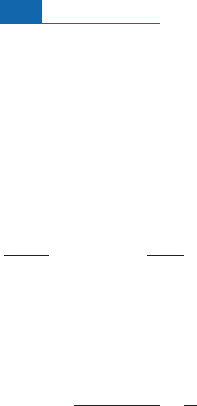
4.4.3 The iron peak
This phase of fusion (addition) of ever-heavier nuclei has its limits becaus e with successive
synthesis we arrive at minimum energy per nucleon. And the element corresponding to
th is minimum is iron. In other words, when there is fusion of nuclei whose mass is greater
thanthatofiron, the resulting nuclei areless stablethanthe initialones.This stabilityof iron
underlies a statistical process of nuclear reactions. Fusion reactions occur in an anarchic
fashion and are o¡set by destruction reactions, which may even go as far as releasing
4
He
nuclei.We are dealing here with temperatures of 6 billion degrees. But all of these reactions
have a natural limit: we cannot ‘‘exce ed’’ the atomic number of nuclei close to iron, nickel,
and cobalt, which areverystable tooin nuclearterms.Accordingly, such nuclei accumulate
and are very abundant. Hence we get what is known as the iron peak, corresponding to an
exceptional abundanceof iron andofthe elementson either side of it (Figure 4. 1 9). But how
can we go‘‘further’’and make elementswith atomic numbers greater thanthat of iron, hea-
vier than iron? Howcanwe cross this seem ingly impassablestabilitybarrier and makevery
heavy elements? After all, these elements do occur, be th ey strontium, r ubidiu m, neody-
mium, or uranium.Thisispossiblethroughtherelationship of neutron addition.
4.4.4 Neutron addition
Neutrons are electrically neutral particles. They react in nuclear terms by adding
to the nucleus without doing too much‘‘damage.’’On the (Z, N) plot the addition of n eutrons
creates a new isotope with more neutrons and shifts the nucleus towards the rightofthe plot.
When neutron addition ac cumulates, ever-heavier isotopes of the element are created. On
the (Z, N) plot, the new nuclei move horizontally ever further to the right. There comes a
A
Iron (Fe)
4
He
12
C
Nuclear
fusion
Neutron
addition
B/A (MeV)
3
0
8
16 24 30 60 90 120 150 180 210 240
9
8
7
6
5
4
Statistical
equilibrium
Figure 4.19 The curve of binding energies of nuclei by nucleon (
B
/
A
) versus mass number (
A
).
141 Cosmic irradiation: from nucleosynthesis to stellar and galactic radiation

point, then, wh en the new nucleus lies outside the valley of nuclear stability. It is therefore
unstable. To return to the valley of stability it disintegrates by
radioactivity. The neutron
changes into aproton. By the sametoken, Z increases. A newchemical elementhas therefore
b een made. New isotopes and newchemical elements canbe madeby neutron addition.This
is a general outline. In actual fact, the neutron addition process has two variants depending
on the relative number of neutrons and the equilibrium established between the addition of
neutrons and radioactivity.
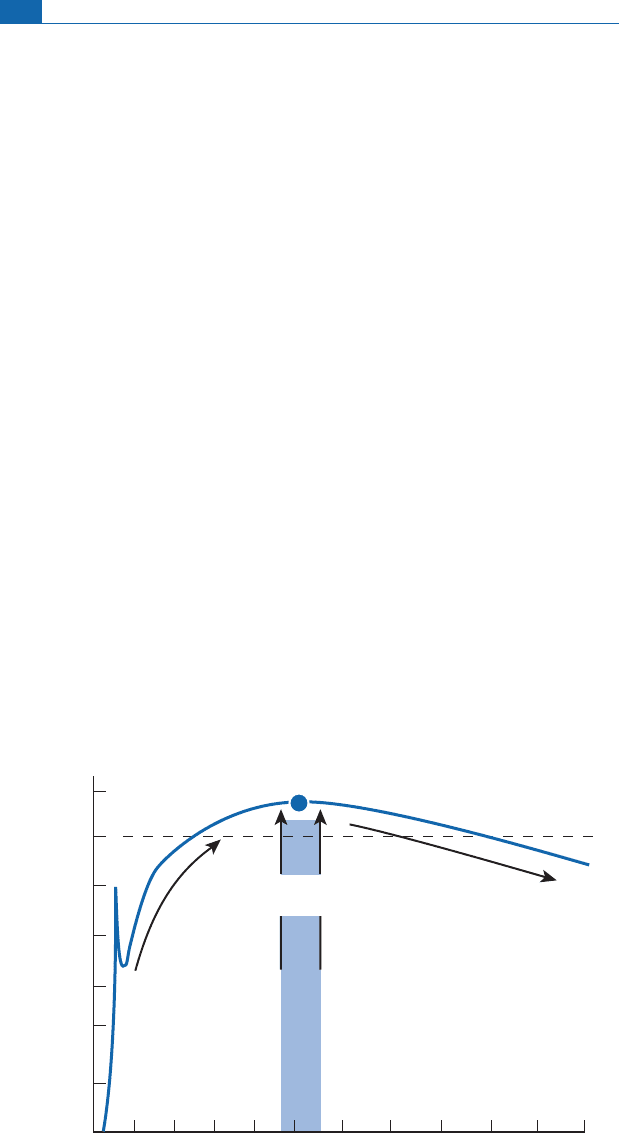
Iftheneutron£uxisweak,theradioactivityofisotopestotherightofthevalleyofstability
is abarrier. Su ch isotopes disintegrate as soon as they form and we zigzag across the (Z, N)
plot(Figure4.20).
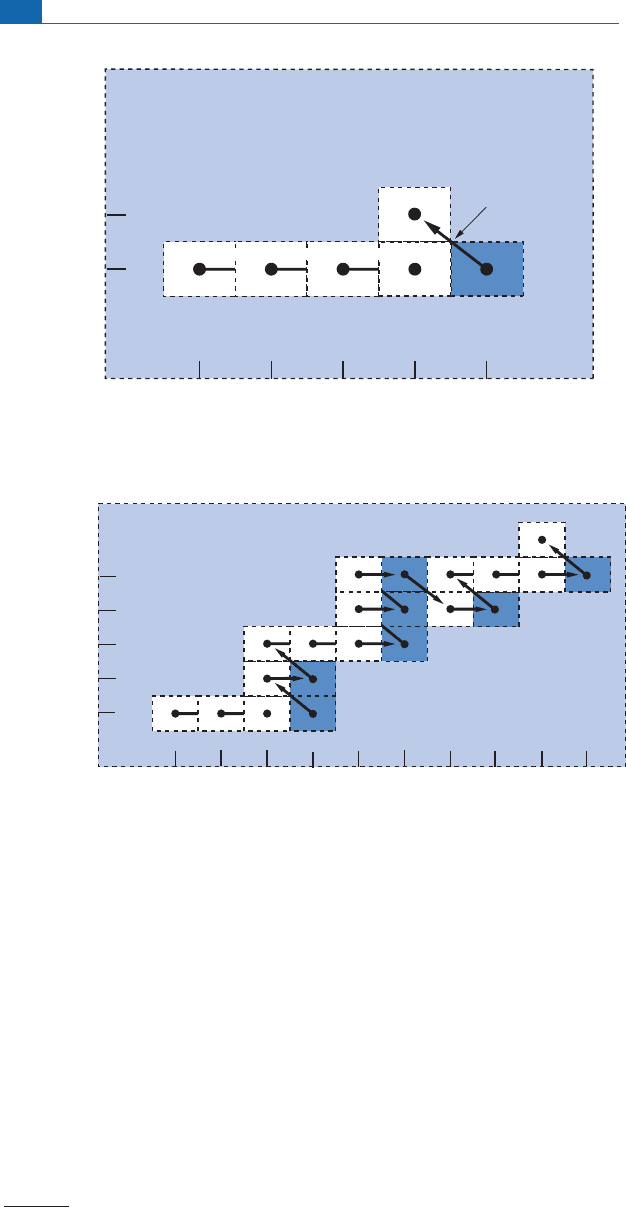
Iftheneutron£ux isveryhigh, radioactivityhasno timeto disintegratefully the radioac-
tive nuclei which are‘‘loaded’’ with extra neutrons and in turn give rise to other radioactive
isotopes which move further to the right of the (Z, N) plot (Figure 4.21 ). Of course, decay
also occurs and a £ux of new nuclei is formed, with large r Z numbers. New chemical ele-
ments havebeen synthesized.
The two ¢gures (Figures 4.20 and 4.2 1 ) clearly show the two processes which are termed
s - proces s (slow) and r- process (rapid), respe ctively.
A few simple rules can be laid down to check whethe r an isotope of a heavy element
ha s been synthesized by an s-process or an r-process o r by both. A stable isotope to the
rightofaradioactiveisotope(shortperiod)cannothavebeenformedbyans-process
since the radioactive isotope is a barrier to any furthe r horizontal rightwards shift.
If itoccursin nature,then itmus thavebeen formedbyanr-process.However, anyadjacent
stable isotope m ay be the outcome of an s-process. Conversely, any stable isotope
locatedon the samenegative diagonal as an isotope ofanotherelementoflower mass can-
n ot have been created by an r-process as it is ‘‘shielded,’’ protected by the other isotope
(Figure 4.21).
Averysimpleand conveni entequationwhichthe s- proc essobeys is:
N
A1
N
A
¼
A
A1
where N is the abundanc e, the e¡ective cross- section of neutron absorption, and A and
A^1aretwoisotopes with decreasing massnumbers.
Theabundance ratiosofthetwoisotopes arein inverseproportiontotheire¡ective cross-
sectionsof neutron absorption. Letu s show this simply.The kinetic equation ofproduction
of isotope A is written:
d N
A
ðÞ
dt
¼
A1
N
A1
A
N
A
ðÞ
where
n
is the neutron £ux, N
A^1
and N
A
are the numbers of atoms of atomic numbers A
and A^1,an d
A^1
and
A
arethe e¡ective cross-sections.
This is a classic d estruction^production equation like the one written for
14
C. If equili-
brium is attained:
d N
A
ðÞ
dt
¼ 0:
142 Cosmogenic isotopes
Therefore:
A1
N
A1
¼
A
N
A
:
Ourassertion hasbeen demonstrate d.
Nowconsiderthe transitionby
decay:
d
A
Z
N
dt
¼ N
A1
A1
Z
N l
A
Z
N
:
Proton
number
P
NN+1 N+2 N+3 N+4
Neutron number
P+1
β radioactivity
1 neutron 2 neutrons 3 neutrons
Unstable
Proton
number
26
Neutron number
27
28
29
30
Iron
Cobalt
Nickel
Copper
Zinc
30 31 32 33 34 35 36 37 38 39
Figure 4.20 The s-process on the proton–neutron plot. Top: theoretical pathway of neutron reactions.
In blue, the first radioactive isotope. There is a change of elements up to the left by
decay. Bottom:
example of s-process pathways on the proton–neutron plot allowing the creation of elements heavier
than iron. After Broecker (1986 ).
143 Cosmic irradiation: from nucleosynthesis to stellar and galactic radiation
Proton
number
P
NN+1 N+2 N+3 N+4 N+5 N+6 N+7 N+8 N+9
Neutron number
Proton number
Zirconium 40
Molybdenum 42
Yttrium 39
Strontium 38
Rubidium 37
Krypton 36
Bromine 35
Selenium 34
Niobium 41
Arsenic 33
Germanium 32
Gallium 31
48 49 50 51 52 53 54 55 56
Neutron number
Stable isotope produced by r-process
Stable isotope not produced by r-process
Radioactive isotope on pathway of r-process
Neutron capture by r-process
β radioactivity during r-process
β radioactivity after r-process
Figure 4.21 The r-process on the proton–neutron plot. The neutron flux is intense enough for
considerable horizontal shift to the right before radioactivity becomes a barrier. But, of course, all
radioactivity plays a part before the barrier effect kicks in. After Broecker (1986).
144 Cosmogenic isotopes
Production ofthebarr ier radioactive isotope is notedwithan asterisk (*), and l is the d ecay
con stant.
d
A
Zþ1
N
dt
¼ l
A
Z
N
N
A
A
Zþ1
N
:
If equilibrium occurs, the radioactive term between the two equations is eliminated,
giving:
A1
A1
Z
N ¼
A
A
Zþ1
N:
This is the same relation asbefore.
The abundance of isotopes of type s (i.e., formed by the s-process) heavy elements is
therefore determined by the e¡ective cross- sections of absorption of neutrons. These
cross-sections canbemeasured inthelaboratory (theyarerequiredfor constructingatomic
reactors).
Exercise
Strontium has three isotopes, of masses 88, 87, and 86. Their effective cross-sections of
neutron absorption are 4.8, 60, and 48 millibarns, respectively. The ‘‘primitive’’ isotope ratios
of strontium are:
88
Sr/
86
Sr ¼8.3754,
87
Sr/
86
Sr ¼0.698, and
88
Sr/
87
Sr ¼11.99.
Can strontium isotopes be synthesized by the s-process?
Answer
Let us first apply the rule
N
1
/
N
2
¼
2
/
1
. We find the ratios
88
Sr/
86
Sr ¼10,
87
Sr/
86
Sr ¼0.8, and
88
Sr/
87
Sr ¼12.5.
Let us compare this with observations. In view of the uncertainties on effective cross-
sections, the agreement is quite good. This is odd because
88
Sr derives from both the s- and
the r-processes, which proves that the r-process is not important here.
Exercise
Table 4.4 gives the abundance
N
, the effective cross-section
, and the product
N
for
samarium isotopes.
Calculate the proportion of the r-process involved in the formation of 147 and 149 isotopes.
Table 4.4 Data for samarium isotopes
Samariu m isotope N
A
%Class
a
c
(millibarn) N
144 2.87 p 119 55 342
147 14.94 rs 1 173 192 1 7 6 0 0 2900
148 11.24 s 258 48 2 930 54 0
149 13.85 rs 1 622 279 22 500 400 0
15 0 7.36 s 370 72 2 770 535
152 2 6.9 0 r or r s 411 71 1 110 0 1900
15 4 22.8 4 r 325 61 7 430 140 0
a
Fordetailsofthe p-process, see nextsubsection.
145 Cosmic irradiation: from nucleosynthesis to stellar and galactic radiation
Answer
If we take the 148 isotope, which is a pure s-process isotope, the (s) isotopes give a
N
value
2930.
We can therefore write:
percentage (r) of 147 ¼
17 600 2930
17 600
100 ¼ 83:35%
percentage (r) of 149 ¼
22 500 2930
22 500
100 ¼ 86:97%:
4.4.5 The p-process
Some isotopes are depleted in neutrons (and so have‘‘surplus’’protons). In the chart of the
nuclides they lie to the left of the valley of stability, o¡ the s-process pathway, and sh ielded
from the r-process.They include for example
84
Sr,
92
Mo,
124
Xe, and
14 4
Sm.Theyare called
p-process nuclides (or p-isotopes) and are said tobe formed by the p-process.The pro duc -
tion process is fairly obscure but we do know that the abu ndanc e of p-isotopes plotted
against (Z) forms a curve which roughly follows that of the s- and r-isotopes except that the
abundance levels are much lower. To get some idea, the abundance ratios for silicon are:
s-process ¼1, r-process ¼0.5 , and p-process ¼0.02.
It can be inferred, then, thatthe p-process is secondaryand tracks the s- and r-process es,
thus supplementing them. Nowadays it is thought that the p-process is mainly caused by
(, ) reactions in supernovae.
4.4.6 The light elements lithium, beryllium, and boron
When the abundance of chemical elements is plotte d against their atomic number
(Figure 4.22), it can be seen that three light elements, lithium, beryllium, and boron, are
u nderabundant compared with their atomic numbers. The He burning nucleosynthetic
p rocess seems to have leapfrog ged the m, with three
4
He nuclei eventually combining to
give
12
C. Andyetthese elements do exist! Howdidtheyco metobe?
Their formation is attribute d totwocauses:
the Big Bangforlithium in part;
spallation reactions in interstellar space caused bygalactic cosmic radiation and acting
on interstellar material.
This explains the abundance cur ve of the chemical elements and isotopes (Figure 4.22).
Butwhatstructuresproducethesereactions? We nowneedto speakaboutthestars.
4.4.7 The stellar adventure: the life and death of stars
Letus nowlookatwhat nucleosynthesis is as theastrophysicistsees it.
The Un iverse is populated by stars clustered into galaxies. These stars are of di¡erent
sizes and brightnesses buttheyare all gigantic nu clear reactors. Nuclear reactions take place
146 Cosmogenic isotopes
