Adlard E.R. (ed.) Chromatography in the Petroleum Industry
Подождите немного. Документ загружается.

Chapter
I
12
a
Sample
2’1
,
ez
j:
Column
--<
1
d
1
Detector
I
b
Sample
!
\
‘I
Gas
Jl
v1
Carrier
._
I
1
-.-
I
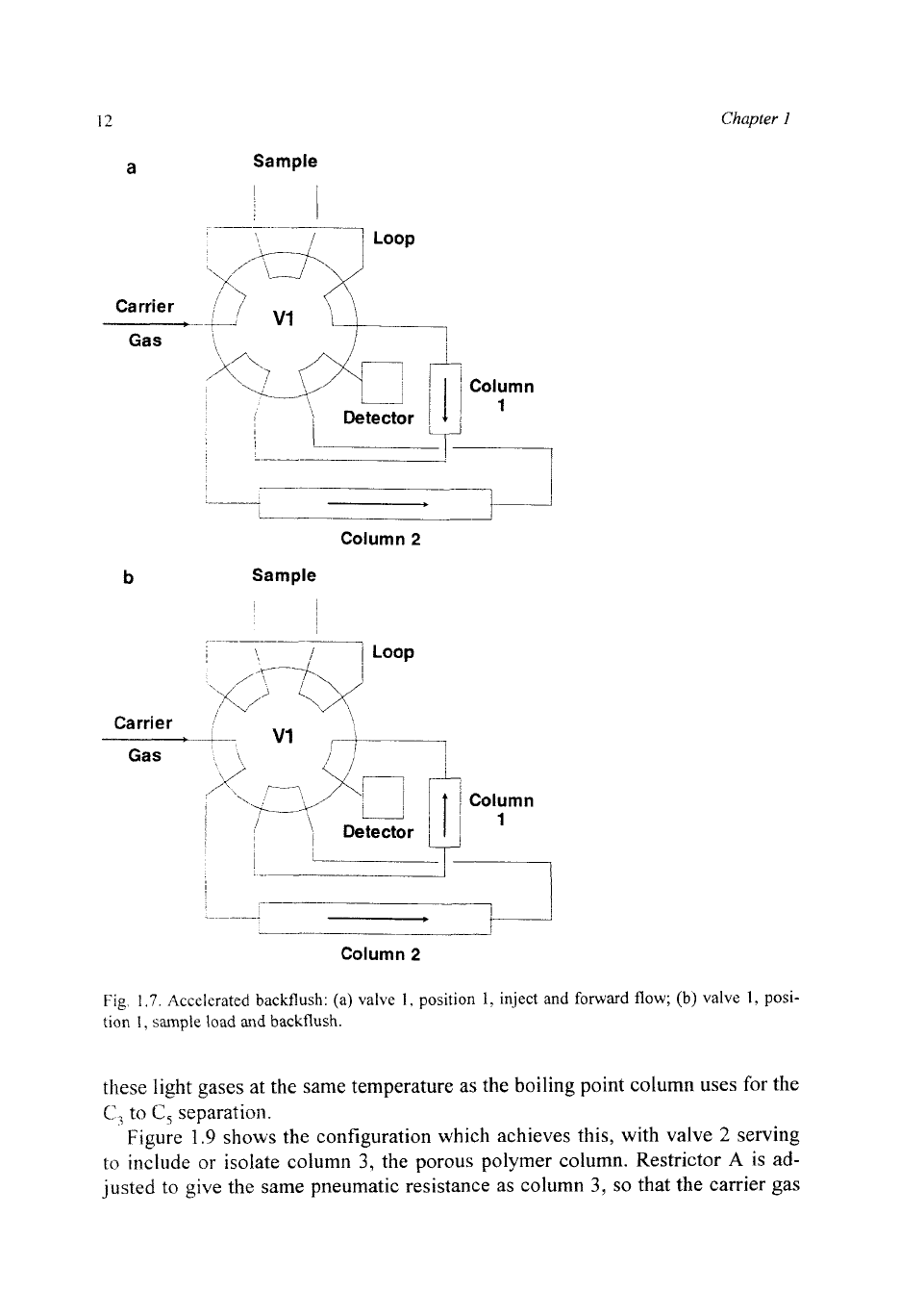
Fig
1.7.
Accelerated backflush:
(a)
valve
1.
position
1,
inject and
forward
flow;
(b) valve
1.
posi-
tion
I.
sample load
and
backflush.
these light gases at the same temperature as the boiling point column uses for the
C3
to
C,
separation.
Figure
1.9
shows
the configuration which achieves this, with valve
2
serving
to
include
or
isolate column
3,
the porous polymer column. Restrictor
A
is
ad-
justed to give the same pneumatic resistance as column
3,
so
that the carrier gas

The
analysis
of
hydrocarbon
gases
I3
0
min
10
min
I
20
min
Fig.
1.8.
Boiling point separation with backflush. Column
1:
0.75 m
X
2
mm
i.d. Column
2:
5.25 m
X
2 mm i.d. Both containing 28% DC 200/500 on 45/60 mesh Chromosorb
P-AW.
Tem-
perature: 100°C. Carrier gas: helium at 28 ml/min.
flow
remains constant. With column
3
in series, the sample is injected via valve
1.
As
before,
C,+
is backflushed to the detector
by
returning valve
1
to the load
position.
As
soon as
all
the
C,
has passed into column
3
(found
by
trial and er-
ror), valve
2
is switched to isolate the light gases,
N,,
CO,,
C,
and
C,
in that col-
Sample
II
WLoop
,
Carrier
Gas
\
I
I
I
I
I
I
Column
2
Column
3
7-
Restrictor
A
Fig.
1.9.
Three
column
analyser. Valve
1,
position
2;
valve 2, position
1
References p.
40

14
Chapter
I
0
min
10
min
20
min
Fig.
1.10.
Boiling point and polymer bead
column.
Column
1:
0.75
m
X
2
mm
i.d.
Column
2:
5.25
m
X
2
mm i.d. Both containing
28%
DC
200/500
on
45/60
mesh Chromosorb
P-AW.
Col-
umn
3:
2.4
m
x
2
mm i.d.
15%
DC
200/500
on
SOB0
mesh Hayesep
N.
Temperature: 100°C.
Car-
rier
gas:
helium
at
28
ml/min.
umn. C,, C, and C, hydrocarbons emerge from columns
2
and
1
to the detector.
After n-C, has eluted, column
3
is
returned on-line by switching valve
2,
and the
light gases elute and are measured. Figure
1.10
shows a typical chromatogram.
The above configuration, with possible minor variations, is widely used for
on-line natural gas analysers, where the sample stream is connected
in
such
a
way that the possibility of contamination of the sample by air is minimal. How-
ever, any sample returned for analysis to a laboratory is prone to air contamina-
tion, which means that
0,
and
N,
must be separated if the presence
of
air is to be
recognized, and accurately measured if the air-free composition is to be recalcu-
lated.
0,
may also be present in a transmitted natural gas if air
or
N,
ballasting is
used as a means of controlling CV
or
WI.
Porous polymer bead columns will not separate air components at the tem-
peratures used for normal analysis; molecular sieves are the only materials able
to
do this, and they in turn retain CO, for
so
long as to make it unmeasurable.
The solution then is to cut the
N,
and
CH,,
with any
0,
which may be present,
onto
a
molecular sieve column, leaving the
CO,
and
C,
on the porous polymer.
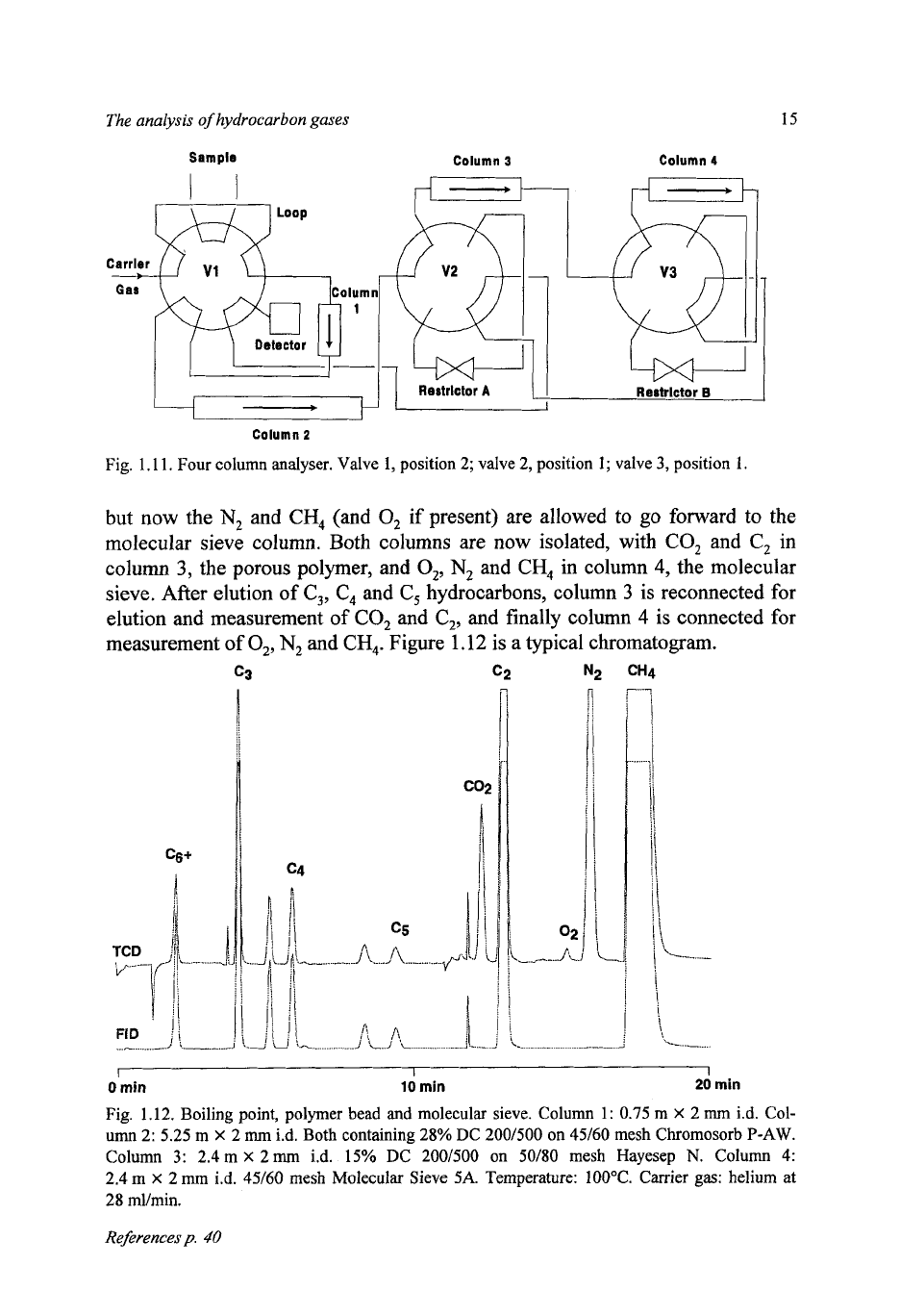
Figure
1.1
1
shows the configuration, where the additional valve
3
includes
or
isolates the molecular sieve.
The procedure is similar to the previous one, starting with all columns in se-
ries.
After backflush
of
C,+, the light gases pass into the polymer bead column,
The
analysis
of
hydrocarbon
gases
15
Sampla Column
3
Carrlrr
Gas
Column
4
Restrlctor
A
(I
Reitrlctor
B
I
Column
2
Fig.
1.1 1.
Four
column analyser. Valve 1, position 2; valve
2,
position
1;
valve
3,
position 1.
but now the
N,
and
CH,
(and
0,
if present) are allowed to
go
forward to the
molecular sieve column. Both columns are now isolated, with
CO,
and
C,
in
column
3,
the porous polymer, and
0,,
N,
and
CH,
in column
4,
the molecular
sieve. After elution of
C,,
C,
and
C,
hydrocarbons, column
3
is reconnected for
elution and measurement
of
CO,
and
C,,
and finally column
4
is connected for
measurement of
0,,
N,
and
CH,.
Figure
1.12
is a typical chromatogram.
c3 c2 N2 CH4
I
I
I
0
min
10
min
20
min
Fig.
1.12. Boiling point, polymer bead and molecular sieve. Column 1: 0.75 m
X
2
mm
i.d. Col-
umn
2:
5.25
m
x
2
mm
i.d. Both containing 28% DC 200/500
on
45/60 mesh Chromosorb
P-AW.
Column
3:
2.4 m
x
2mm i.d.
15% DC 200/500
on
50/80
mesh Hayesep
N.
Column
4:
2.4 m
x
2
mm
i.d. 45/60 mesh Molecular Sieve
5A.
Temperature:
100°C.
Carrier
gas:
helium at
28
ml/min.
Re$rences
p.
40

16
Chapter
1
The above chromatograms were generated, as
is
evident from the conditions,
on a single chromatograph, configured as in Fig. 1.1
I.
The configuration of Fig.
1.9
(chromatogram
in
Fig.
1.10)
was achieved by isolating column
4
throughout,
and that of Fig.
1.7
(chromatogram in Fig.
1.8)
by isolating columns
3
and
4
throughout.
It
would be possible to optimize columns for these configurations
which gave somewhat better
N,/CH,
separation, but it
is
clear that the baseline
separations of all light components seen in Fig. 1.12 could not be matched.
The setting-up procedures described above sound more complicated than they
in
fact are and most instrument suppliers provide ready configured systems.
Once set up, their performance is usually extremely stable, since none
of
the
coiumns are used anywhere near their temperature limit. The molecular sieve
column will lose separation gradually due to slow adsorption of moisture, but
its performance does not influence other timings or separations within the
sys-
tem.
1.2.2.2
Two
detectors
The chromatogram in Fig. 1.12 illustrates the use
of
two
detectors, TCD and
FID in series. The presence of
N,
and
CO,
makes the use of the TCD necessary,
and
it
is sufficiently sensitive for measurement of the lighter hydrocarbons. To
include pentanes, however, means choosing
a
larger sample size than would be
desirable for linear measurement of the major components. Using an
FID
in
series with the TCD avoids this problem, as the FID has much higher sensitiv-
ity
for hydrocarbons.
A
smaller sample size can be used, satisfying linear detec-
tion requirements for major components and ample sensitivity for minor compo-
n
en
ts
.
1.2.2.3
C6~
detail
Separation and measurement of the individual higher hydrocarbons, repre-
sented by the C,+ backflushed peak,
is
needed both to define the composition
and hence the properties of this group, and also to provide detail for other calcu-
lations such as hydrocarbon dewpoint temperature. The complexity of the minor
alkane isomer components increases dramatically with carbon number, and this,
with the presence of cyclo-alkanes and aromatics, means that high resolution
chromatography
is
required.
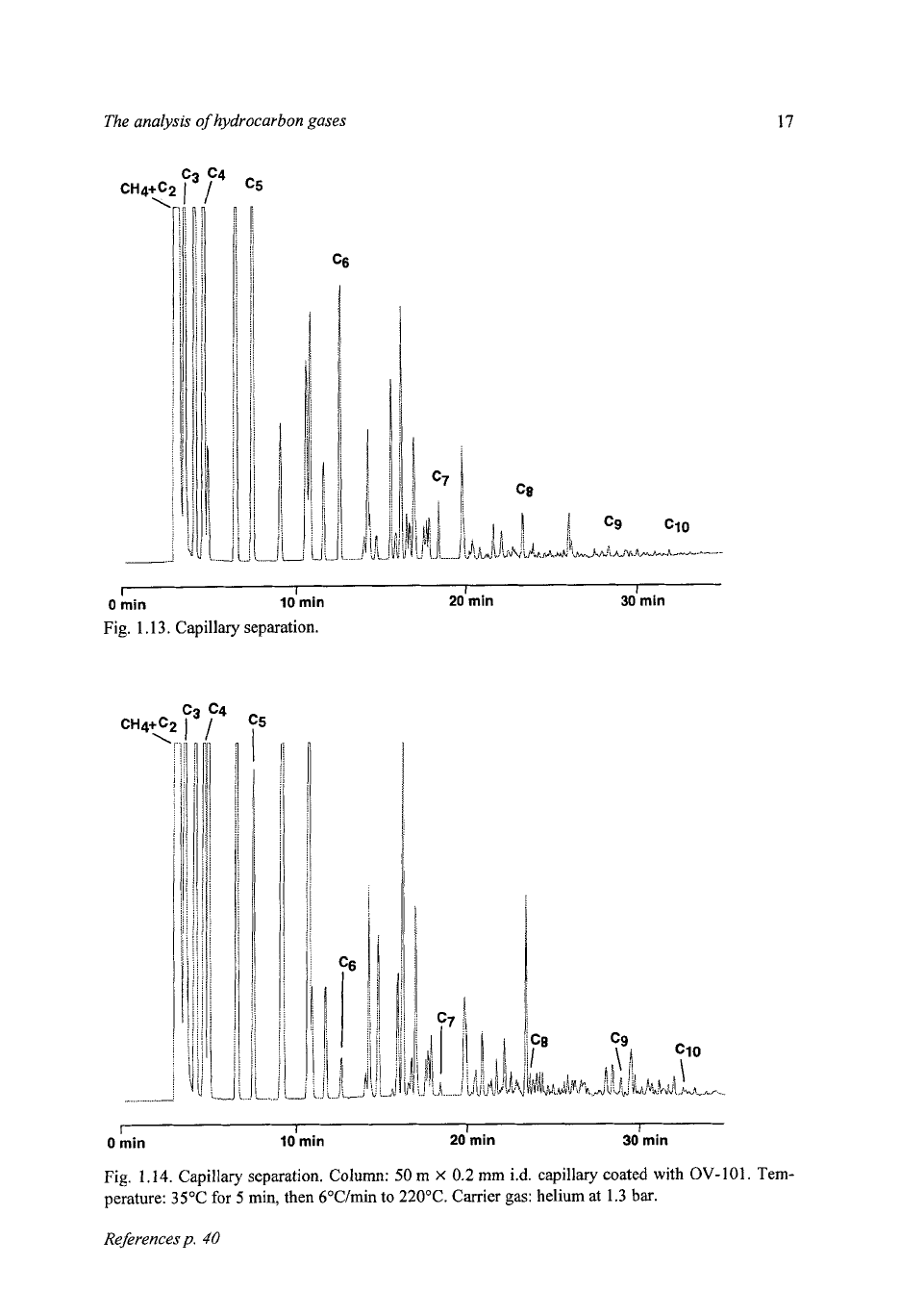
Capillary columns are widely used for liquid hydrocarbon samples, and are
equally adaptable to gas analysis. The sample injected from a conventional gas
sampling valve can be split without fear of sample discrimination, as there is no
phase change on injection. Alternatively, the capillary column can be connected
directly into a micro-volume gas sampling valve, fitted with a sample loop of
some tens
of
microlitres. Chromatograms
of
natural gases with very different
isomer distributions are shown in Figs.
1.13
and
1.14.
The limit of detection for
individual components in this instance
is
around
5
parts per million molar.
The analysis
of
hydrocarbon gases
17
0
min i0'min
Fig.
1.13.
Capillary separation.
I
20
rnin
tomin
I
I
0
min
10
min 20'min 3Omin
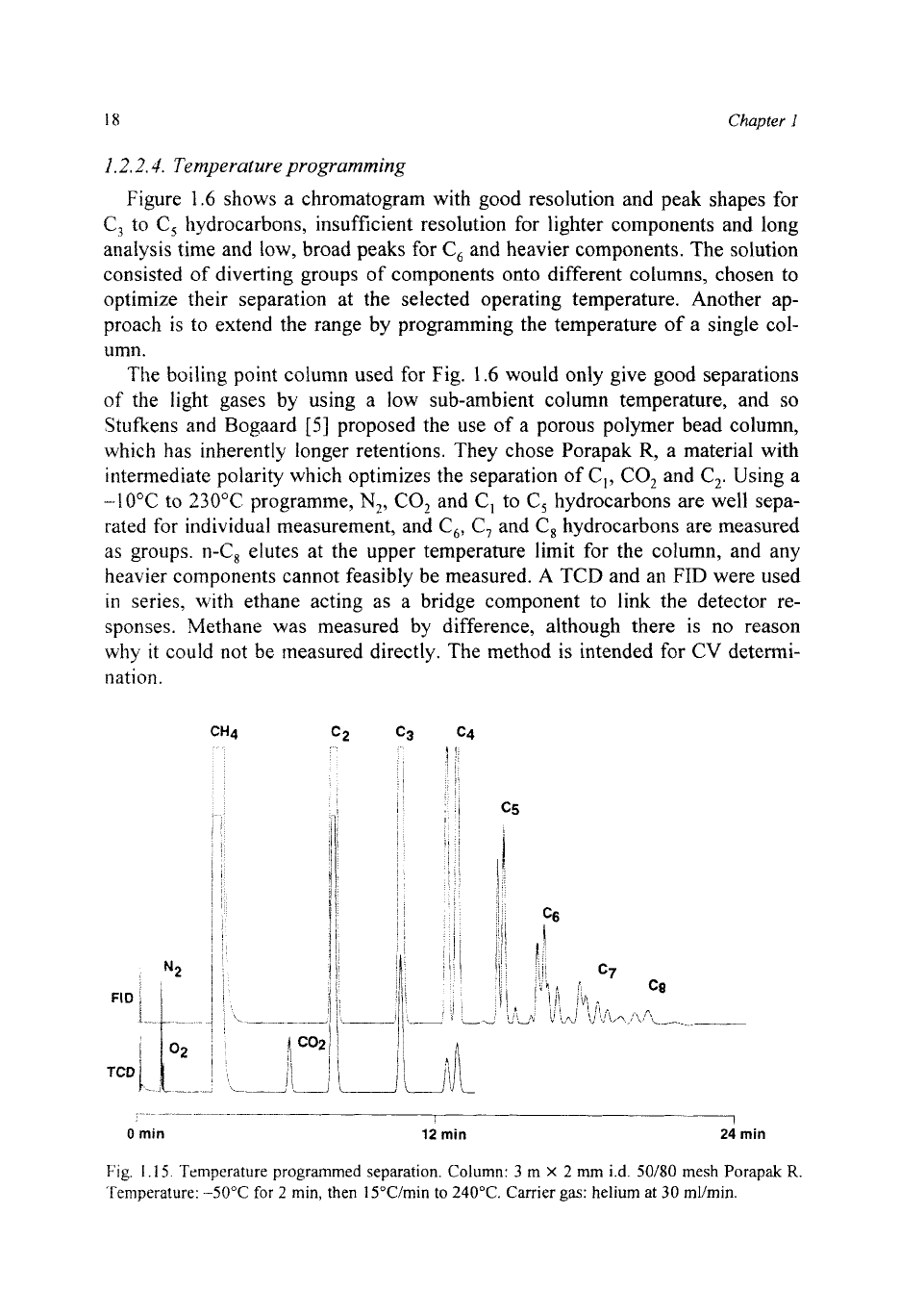
Fig. 1.14. Capillary separation. Column:
50
m
X
0.2 mm
i.d.
capillary coated with OV-101. Tem-
perature: 35°C for
5
min, then 6"C/min to 220°C. Carrier gas: helium at
1.3
bar.
References
p.
40
18
Chapter
I
I.
2.2.4
Temperacure programming
Figure
1.6
shows a chromatogram with good resolution and peak shapes for
C,
to
C,
hydrocarbons, insufficient resolution
for
lighter components and long
analysis time and low, broad peaks for
C,
and heavier components. The solution
consisted of diverting groups of components onto different columns, chosen to
optimize their separation at the selected operating temperature. Another ap-
proach
is
to
extend the range by programming the temperature of a single col-
umn.
The boiling point column used
for
Fig.
1.6
would only give good separations
of the light gases by using a
low
sub-ambient column temperature, and
so
Stufkens and Bogaard
[5]
proposed the
use
of a porous polymer bead column,
which has inherently longer retentions. They chose Porapak
R,
a material with
intermediate polarity which optimizes the separation of
C,,
CO,
and
C,.
Using
a
-.IO"C
to 230°C programme,
N,,
CO,
and
C,
to
C,
hydrocarbons are well sepa-
rated for individual measurement, and
C,, C,
and
C,
hydrocarbons are measured
as groups.
n-C,
elutes at the upper temperature limit
for
the column, and any
heavier components cannot feasibly be measured. A
TCD
and an
FID
were used
in
series, with ethane acting as a bridge component to link the detector re-
sponses. Methane was measured by difference, although there is no reason
why
it
could not be measured directly. The method
is
intended for
CV
determi-
nation.
0
min
12
min
I
24
min
Fig.
1.15
Temperature programmed separation. Column:
3
m
X
2
mm
i.d.
50180
mesh Porapak
R.
Temperature: -50°C
for
2
min, then ISWmin
to
240°C.
Carrier
gas:
helium
at
30
ml/min.

The analysis
of
hydrocarbon
gases
19
Using this method, but with a sub-ambient (-50°C) start to the temperature
programme,
O,/N,
separation is achieved within the same analysis. Figure 1.15
is a typical chromatogram. IS0 6974 [6] is based on this separation, with a
starting temperature of 35"C, which eliminates the need for sub-ambient equip-
ment, but an additional separation on a molecular sieve column is required for
helium, oxygen and nitrogen.
1.2.2.5
Combined systems
The capillary chromatogram in Fig. 1.13 separates the majority of components
in a natural gas, but not those few lighter components which have the largest
concentrations. Figure 1.13 has been optimized for good detection limits over a
wide range, giving quantitative measurement from C, to C,, and beyond. Used in
conjunction with the column switching system illustrated in Fig. 1.1 1, either
C,
or
C,
components can be used as a quantitative bridge between the two analyses.
If the capillary conditions are optimized in order to include quantitative meas-
urement of C,, then the packed column TCD system for light gases can be sim-
plified to a porous polymer/molecular sieve combination. Both this and the capil-
lary/FID system can be fitted into the same chromatograph
[7].
The sample
is
injected first into the packed column system for isothermal separation of
0,,
N,,
C,, CO, and C,, and then into the capillary for temperature programmed separa-
tion of the higher hydrocarbons. There is no backflush provision to remove the
heavier hydrocarbons from the packed columns, as they are forward eluted (but
not measured) during the temperature programmed part of the cycle. Figure 1.16
illustrates the column arrangement and Fig. 1.17 a typical chromatogram.
The analysis is comprehensive, but there is no provision for a bridge compo-
nent.
C,
and C, can be distinguished on the capillary column, but it is doubtful
whether C, could be measured with sufficient accuracy. Although the analysis
takes 30 min, the cycle time, allowing for cool down and re-stabilization, is
longer. In a similar application, three sample aliquots are injected, one onto mo-
lecular sieve for
O,/N,
separation, and from which C, and above are backflushed
to vent, one onto porous polymer for
(0,
+
N,),
C,, CO, and C,, and the third
onto a capillary for temperature programmed separation
[
81.
1.2.2.6
Separation
in
backflush
In a novel application intended for process use, the natural gas is injected onto
a single, long packed column containing porous polymer and operating with a
large pressure drop.
N,,
C, and CO, are measured normally, then the entire col-
umn is backflushed to the detector for measurement of the other components.
To
understand how this works requires careful consideration of the mechanism of
backflushing in gas chromatography.
Backflushing is assumed to recombine separated or partially separated com-
ponents because they have to travel equally far in the reverse direction from that
References
p.
40
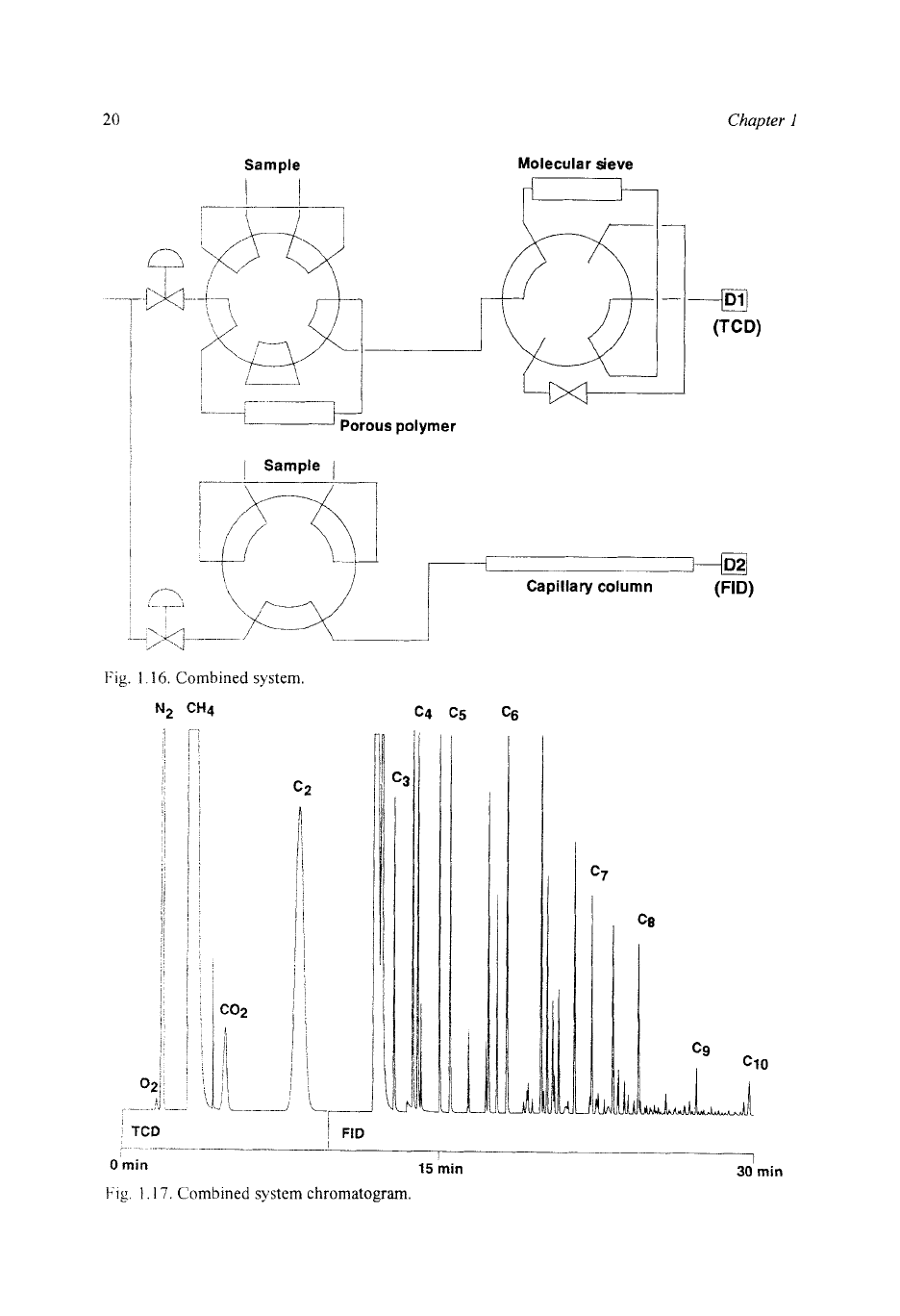
20
Sample
Chapter
1
Molecular sieve
Fig.
i
16.
Combined
system.
CH4 c4
c5
c6
Capillary column
(W
-
-
_____
I
I
15
min
30
min
0
min
Fig
1
17
Combined
s>
stem
chromatogram.

The analysis
of
hydrocarbon gases
21
in which they have been undergoing separation. This is an over-simplification. In
gas chromatography, the mobile phase is compressible, and is driven through the
column under the influence of a pressure gradient. Since each section of the col-
umn has the same mass flow of mobile phase passing through it at any time, the
actual volumetric flow at each point must vary according to the pressure at that
point.
As
the pressure decays from the beginning to the end of the column,
so
the
volumetric flow rate increases, and
so
does the linear velocity.
The mobile phase linear velocity controls the rate of travel of components,
and
so
if this increases along the length of the column, components, even in
iso-
thermal analysis, will accelerate between injection and detection. Looked at an-
other way, a component with a retention time of
x
will not have reached the mid
point of the column at
an
elapsed time of
x/2.
The larger the pressure gradient,
the greater the acceleration.
When the whole column is backflushed, the pressure gradient which exists in
forward flow is reversed, and
so
those parts of the column where the components
travelled most slowly become the high velocity areas, and vice versa. A compo-
nent which has nearly reached the end of the column at the time of backflushing
will have experienced the full acceleration. It now finds itself in the high pres-
sure, low velocity region, and has to do it all over again. Its time to elute in
backflush will be almost identical to its forward flow time. By contrast, a com-
ponent which had travelled only a short distance from the injection point by the
time of backflushing will have spent all the forward flow time in the low veloc-
ity region. After backfl ushing, it is in the high velocity region, and will be eluted
as a backflushed peak in a much shorter time than
it
spent travelling forward.
Hence there is a mechanism of separation, in reversed order, during back-
flushing. The fact that this is not evident when backflushing in applications such
as that illustrated in Figs.
1.10
and
1.12
is because in these, the backflushed sec-
tion is short. Hence, while it
is
subject to high pressure, low velocity on forward
flow, and vice versa on backflush, the pressure drop across the section itself
is
small, and backflush separation does not occur. Figure
1.18
is a chromatogram
showing the application
[9].
The advantages of this approach are that it requires
less hardware (a single ten-port valve would cover both injection and backflush-
ing) and the column can be long enough to give good
N,,
CH,,
CO,
separation
without a consequent time penalty for the heavier components. The main disad-
vantage is to do with the nominal
C,+
group. Since the backflush separation is
good enough to separate
C,
from
C,
and
C,
from
C,,
it is almost certainly per-
forming some separation of
C,, C,
and
C,.
This being
so,
the
C,+
group, which is
a sharp, distinct peak in Fig.
1.11,
becomes relatively “smeared out” and more
difficult to quantify.
I.
2.2.7
Rapid
analysis
Process natural gas analysers have analysis cycle times typically in the range
References p.
40
