Журнал - Проблемы криобиологии 2009 №1
Подождите немного. Документ загружается.

20
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
Согласно современным представлениям, извест-
ные АФП по эффективности действия можно раз-
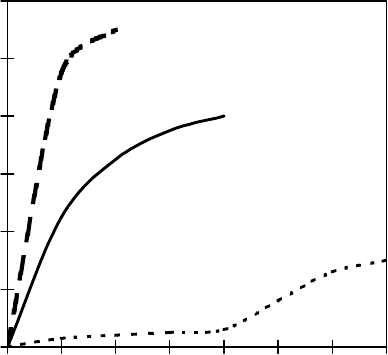
делить на 3 группы. На рис. 3 приведена зависи-
мость активности АФП от их концентрации в
растворе [17]. Несмотря на установленные сущест-
венные различия в активности гиперактивных и
умеренных АФП, пока отсутствуют данные о бел-
ках с промежуточной активностью.
1. “Очень слабые” АФП, основная функция
которых – ингибирование рекристаллизации,
вследствие их чрезвычайно слабой термогисте-
резисной активности. К представителям этой груп-
пы относится белок паслёна горько-сладкого
Solanum dulcamara [9]. На С-конце он содержит
10 последовательных повторов, что является
общей чертой большинства АФП. Анализ показал,
что структура этих АФП содержит 2 консерва-
тивные области длиной по 56 и 57 аминокислотных
остатков, которые организованы в 2 “цинковых
пальца”. Другой представитель этой группы – бе-
лок из многолетнего плевела Lolium perenne [16],
содержащий углеводную часть. При исследовании
гликозилированных и негликозилированных форм
выявлено, что с кристаллом связывается полипеп-
тидный остов. Вторичная структура этого АФП
представляет собой β-складчатый слой. Несмотря
на некоторую термогистерезисную активность, к
данной группе белков также относят АФП с моле-
кулярной массой 36 кДа моркови Daucus carota,
выделенный из апопласта корней [18]. Этот белок
также содержит углеводную часть на N-конце.
2. Умеренно активные АФП. К этой группе
относится большинство известных АФП I–III
типов и антифризные гликопротеиды (АФГП) рыб,
бактерий и т.д. Подробная характеристика предста-
вителей этой многочисленной группы будет пред-
ставлена в следующем сообщении, посвященном
распространению антифризных белков в природе.
В настоящей работе описаны основные отличия
этой группы белков от белков с иными характе-
ристиками активности.
3. Гиперактивные АФП, к которым относят
АФП камбалы, некоторые АФП бактерий и насеко-
мых. При охлаждении растворов, содержащих
умеренно активные АФП, ниже точки равновесия
начинается внезапный рост кристаллов льда вдоль
оси с (см. рис. 2) [17]. Этот процесс в присутствии
любого гиперактивного АФП остается контроли-
руемым при более низких температурах, а затем
начинается взрывообразный рост в направлении,
перпендикулярном к оси с. При этом образуются
кристаллы гексагональной формы с ярко выражен-
ными плоскими базальными гранями. Поэтому
авторы считают, что гиперактивные АФП блоки-
руют рост кристаллов за пределами базальных
поверхностей, а умеренно активные АФП остав-
of action may be divided into 3 groups. Fig. 3 demon-
strates the dependence of activity of AFPs on their
concentration in the solution [17]. In spite of the
established significant differences in the activity of
hyperactive and moderate AFPs there have been no
data about the proteins with intermediate activity yet.
1. “Very weak” AFPs, inhibiting the re-crystal-
lization due to their very weak thermohysteresis acti-
vity. To the representatives of this group the protein
of bitter nightshade Solanum dulcamara is referred
[9]. At the C-terminal it contains 10 consequent repeats
that is a common feature of the majority of AFPs. The
analysis has shown that the structure of these AFPs
possesses 2 conservative areas with the lengths of 56
and 57 amino acid residues, which are organized into
2 “zinc fingers”. Another representative of this group,
the protein from long-standing darnel Lolium perenne
[16] contains carbohydrate part. However during the
study of glycosylated and non-glycosylated forms it
has been found that polypeptide skeleton binds with
the crystal. The secondary structure of this AFP
represents β-sheet. In spite of some thermohysteresis
activity to this group of proteins the AFP with
molecular weight of 36 kDa of the carrot Daucus
carota isolated from apoplast of roots [18] is also
Рис. 3. Термогистерезисная активность гиперактивных
и умеренно активных АФП [17]: 1 – рекомбинантные
АФП Archips fumiferana и Marinomonas primoryensis,
натуральный АФП камбалы; 2 – рекомбинантный АФП
Tenebrio molitor и натуральный АФП снежной блохи; 3 –
умеренно активные натуральные, рекомбинантные и
синтетические АФП рыб I–III типов и АФГП.
Fig. 3. Thermal hysteresis activity of hyperactive and
moderately active AFPs [17]: 1 – recombinant AFPs of
Archips fumiferana and Marinomonas primoryensis, natural
flounder AFPs; 2 – recombinant AFPs of Tenebrio molitor
and natural AFPs of snow flea; 3 – moderately active natural,
recombinant and synthetic fish AFPs of I–III types and
AFGP.
0
1
2
3
4
5
6
12345678
Концентрация АФП, мМ
AFPs’ concentration, mM
Термогистерезис, °С
Thermal hysteresis, °С
21
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
ляют уязвимыми кончики бипирамидальных крис-
таллов с минимальной базальной поверхностью,
на которых начинается рост кристаллов при уме-
ренном переохлаждении. Гиперактивные АФП
предотвращают рост кристаллов льда на базальной
поверхности. Кристаллы, образующиеся в их
присутствии, имеют плоскую, гексагональную
форму с ярко выраженной базальной поверх-
ностью. Это предположение требует дальнейшей
проверки, например с помощью АФП, меченных
флуоресцентными зондами, по которым можно
точно определить место связывания АФП с крис-
таллом. Известно, что кончики кристалла не всегда
бывают стартовыми локусами нуклеации. При от-
сутствии АФП нормальный рост кристаллов льда
осуществляется вдоль оси а (см. рис. 2), так как
кинетический барьер для адсорбции молекул Н
2
О
в направлении оси с больше. В связи с этим можно
предположить, что АФП могут изменять термоди-
намику замерзания воды, однако до настоящего
времени не предложено единой логичной концеп-
ции, объединяющей все существующие представ-
ления о механизмах функционирования АФП.
К гиперактивным АФП относят богатый алани-
ном α-спиральный белок массой 16,7 кДа, выде-
ленный из плазмы крови камбалы [14], а также
рекомбинантные и натуральные АФП насекомых.
Причем после сравнения их активностей было
установлено, что наличие чужеродных участков не
объясняет их чрезвычайно высокую активность.
Необычно крупный (более 1 МДа) Са
2+
-зави-
симый АФП с высокой активностью был выявлен
у антарктической бактерии M. primoryensis [8].
Существовало мнение, что АФП необратимо
связываются с поверхностью льда в пределах
гистерезисного промежутка. Однако это противо-
речит ряду фактов: различные типы АФП отли-
чаются термогистерезисной активностью при эк-
вимолярных концентрациях; поверхность льда не
перегревается в присутствии АФП; кроме того,
сила связи с поверхностью льда недостаточна для
необратимого связывания. Существует гипотеза
[11], учитывающая обмен молекулами АФП между
льдом и раствором в точке таяния. За обратимой
ассоциацией АФП следует необратимая их ассо-
циация на вновь образованных поверхностях крис-
таллов льда при понижении температуры ниже
точки таяния. Необходимая сила связи достигается
при “вмерзании” АФП в поверхность кристалла
при субзамораживающих температурах. Различная
термогистерезисная активность разных видов
АФП объясняется особенностями их раствори-
мости во время фазы обратимой ассоциации:
низкая растворимость в воде приводит к увеличе-
нию доли молекул АФП, связанных с поверх-
ностью льда в точке таяния. При этом с пониже-
referred. This protein also contains the carbohydrate
part on the N-terminal.
2. Moderately active AFPs. The majority of the
known AFPs of I–III types and antifreeze glycopro-
teids (AFGPs) of fish, bacteria etc. are referred to this
group. Detailed characteristics of the representatives
of this numerous group will be presented in the
following report, devoted to the dissemination of
antifreeze proteins in nature. The present research
describes the main differences of this group of proteins
from those with other activity parameters.
3. Hyperactive AFPs, comprising the AFPs of
flounder, some AFPs of bacteria and insects. During
cooling of the solutions containing moderately active
AFPs below the balance point the abrupt growth of
ice crystals along the axis c starts (see Fig. 2) [17].
This process in presence of any hyperactive AFP re-
mains to be controlled at lower temperatures and then
the burst-like growth in the direction perpendicular to
the axis c begins. Herewith the crystals of hexagonal
shape with vividly manifested flat basal facets are
formed. Therefore the authors believe that hyperactive
AFPs block the crystal growth beyond the limits of
basal surfaces and moderately active AFPs remain
vulnerable the tips of bipyramidal crystals with mini-
mum basal surface whereon the growth of crystals at
moderate supercooling begins. Hyperactive AFPs pre-
vent ice crystal growth on basal surface. The crystals
forming in their presence have flat hexagonal shape
with vividly manifested basal surface. This supposition
demands further checking, for instance with AFPs la-
belled with fluorescent probes due to which the binding
site of AFP with crystal can be properly determined.
It is known that crystal tips not always be the start
loci of nucleation. At the absence of AFPs the normal
growth of ice crystals is accomplished along the axis
a (see Fig. 2), since kinetic barrier for adsorption of
H
2
O molecules towards the axis c is higher. In this
connection one may suppose that AFPs may alter the
thermodynamics of water freezing, but up to now no
logical concept, joining all the existing notions on the
mechanisms of functioning AFPs have been proposed.
An alanine-rich helical protein of 16.7 kDa isolated
from flounder blood plasma [14] is referred to hyper-
active AFPs as well as recombinant and natural AFPs
of insects. Herewith after comparison of their activities
the presence of alien sites has been established as not
explaining their quite high activity. An unusually big
(above 1 mDa) Ca
2+
-dependent AFP with a high acti-
vity was revealed in Antarctic bacteria M. primoryen-
sis [8].
There was a notion that AFPs are irreversibly
bound with ice surface within the limits of hysteresis
interval. However this contradicts some facts: different
types of AFPs differ by thermohysteresis activities at
equimolar concentrations; ice surface is not overheated
22
нием температуры повышается плотность необра-
тимо адсорбированных АФП и, как следствие,
растет термогистерезисная активность.
АФП найдены у позвоночных и беспозвоноч-
ных животных, растений, бактерий и грибов [2].
Различные АФП связываются с разными по-
верхностями кристаллов льда и, возможно, поэто-
му единого механизма, объясняющего специфич-
ность и аффинность связывания АФП со льдом,
предложено не было.
Исследование 4-х негомологичных АФП рыб и
насекомых путем сайт-направленного мутагенеза
показало, что сайты связывания во всех белках
относительно плоские и в связывание со льдом
вовлекается существенная часть белковой молеку-
лы [4]. Эти сайты частично гидрофобны, причем
в большей степени, чем предполагалось. Установ-
лено, что гидрофобная часть сайтов связывания
обращена к жидкой фазе. Однако количество гид-
рофобных участков больше, чем экспонируемых
в жидкую фазу. Ключевую роль в тесном связы-
вании, по-видимому, играет комплементарность
поверхности, которая обеспечивается, в первую
очередь, Ван-дер-Ваальсовскими взаимодействия-
ми, а во вторую – водородными связями.
В настоящее время существуют 3 гипотезы,
объясняющие активность АФП [12]:
– первая гипотеза основана на рентгено-
структурном анализе, компьютерном моделиро-
вании и других физических методах; предполагает-
ся ключевая роль комплементарности атомов
кислорода в решетке льда и группировок в молеку-
ле белка, ответственных за водородные связи;
– вторая гипотеза основана на сайт-направлен-
ном мутагенезе и компьютерном моделировании;
согласно данной гипотезе, гидрофобная часть ам-
фифильной спирали АФП 1 располагается на гра-
нице лёд-вода;
– третья гипотеза также основана на сайт-на-
правленном мутагенезе и компьютерном модели-
ровании; предполагается, что АФП, расположен-
ные на поверхности лёд-вода, сами влияют на
структуру плоскости кристалла, с которой далее
связываются.
Первая и вторая гипотезы подчеркивают факт
связывания или накопления белка на определён-
ных поверхностях кристалла, а согласно третьей
гипотезе, белок сам участвует в развитии места
связывания. В настоящее время ни одна из этих
гипотез не опровергнута. Отметим, что в [6] опуб-
ликовано сообщение о способности АФП III рыб
адсорбироваться не только на поверхности крис-
таллов льда, но и на поверхности частичек, являю-
щихся центрами гетерогенной нуклеации.
Таким образом, имеющиеся сведения пока не
позволяют сформулировать гипотезу о едином ме-
in the presence of AFPs; in addition, the strength of
the bond with ice surface is not essential for irrever-
sible binding. There is a hypothesis [11] taking into
account the exchange of AFPs molecules between ice
and solution in the melting point. AFPs irreversible
association follows reversible one on newly formed
ice crystal surfaces at temperature lowering below the
melting point. Necessary bond force is achieved at
freezing-in of AFPs into the surface of crystal under
subfreezing temperatures. Different thermohysteresis
activity of different types of AFPs is explained by the
peculiarities of their solubility during the phase of
reversible association: low solubility in water leads
to the rise in the share of AFPs bound with ice surface
in the melting point. Herewith with temperature
reduction the density of irreversibly adsorbed AFPs
increases and as a consequence the thermohysteresis
activity grows.
AFPs are found in vertebrates and invertebrates,
plants, bacteria and fungi [2].
Different AFPs are bound with various surfaces of
ice crystals and probably therefore no common mecha-
nism explaining specificity and affinity of AFPs’
binding with ice has been proposed.
The study of four non-homological fish and insect
AFPs by means of site-directed mutagenesis has shown
that the binding sites in all the proteins are quite flat
and significant part of protein molecule is involved
into binding with ice. These sites are partially hydro-
phobic, moreover in a greater extent than it was suppo-
sed [4]. It has been established that hydrophobic part
of the binding sites is orientated towards liquid phase.
However the number of hydrophobic sites is bigger
that those exposed into liquid phase. The key role in a
tight binding is likely played by complementarity of
the surface, which is first of all provided by van der
Waals forces and secondly by hydrogen bonds.
Today there are 3 hypotheses explaining the AFPs
activity [12]:
– the first hypothesis is based on X-ray structure
analysis, computer modeling and other physical
methods; the key role of complementarity of oxygen
atoms in ice lattice and the arrangements in protein
molecule, responsible for hydrogen bonds is supposed;
– the second hypothesis is based on site-directed
mutagenesis and computer modeling; according to this
hypothesis hydrophobic part of amphiphilic helix of
AFP I is located at the ice-water interface;
– the third hypothesis is also grounded on site-di-
rected mutagenesis and computer modeling. It is suppo-
sed that AFPs located on ice-water surface affect the
structure of crystal plane, which is later bound with.
The first and second hypotheses establish the fact
of binding or accumulation of protein on certain crystal
surfaces, and according to the third one the protein
itself participates in development of binding site.
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
23
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
Литература
Аванов А.Л. Биологические антифризы и механизм их
активности // Молекулярная биология.– 1990.– Т. 24, №3.–
С. 581–597.
Barrett J. Thermal hysteresis proteins // Int. J. Biochem. Cell
Biol.– 2001.– Vol. 33, Issue 2.– P. 105–117.
Clarke C.J., Buckley S.L., Lindner N. Ice structuring proteins –
a new name for antifreeze proteins // Cryo Letters.– 2002.–
Vol. 23, N2.– P. 89–92.
Davies P.L., Baardsnes J., Kuiper M.J. et al. Structure and
function of antifreeze proteins // Phil. Trans. R. Soc. Lond. B.
Biol. Sci.– 2002.– Vol. 357, N1423.– P. 927–935.
Davies P.L., Hew C.L. Biochemistry of fish antifreeze proteins //
FASEB J.– 1990.– Vol.4.– P. 2460–2468.
Du N., Liu Y.X., Hew C.L. Ice nucleation inhibition: Mechanism
of antifreeze by antifreeze protein // J. Biol. Chem.– 2003.–
Vol. 278, N38.– P. 36000–36004.
Duman J.G. Antifreeze and ice nucleator proteins in terrestrial
arthropods // Annu. Rev. Physiol.– 2001.– Vol. 63.– P. 327–
357.
Gilbert J.A., Davies P.L., Laybourn-Parry J. A hyperactive,
Ca
2+
-dependent antifreeze protein in an Antarctic bacterium //
JFEMS Microbiol. Lett.– 2005.– Vol. 245, N1.– P. 67–72.
Huang T., Duman J.G. Cloning and characterization of a
thermal hysteresis (antifreeze) protein with DNA-binding
activity from winter bittersweet nightshade, Solanum
dulcamara // Plant. Mol. Biol.– 2002.– Vol. 48, N4.– P. 339–
350.
Kawahara H.J. The structures and functions of ice crystal-
controlling proteins from bacteria // Biosci. Bioeng.– 2002.–
Vol. 94, N6.– P. 492–496.
Kristiansen E., Zachariassen K.E. The mechanism by which
fish antifreeze proteins cause thermal hysteresis // Cryobiolo-
gy.– 2005.– Vol. 51, N3.– P. 262–280.
Madura J.D., Baran K., Wierzbicki A. Molecular recognition
and binding of thermal hysteresis proteins to ice // J. Mol.
Recognition.– 2000.– Vol. 13, Issue 2.– P. 101–113.
Margaritis A., Bassi A.S. Principles and biotechnological
applications of bacterial ice nucleation // Crit. Rev. Biotechnol.–
1991.– Vol. 11, N3.– P. 277–295.
Marshall C.B., Chakrabartty A., Davies P.L. Hyperactive
antifreeze protein from winter flounder is a very long rod-like
dimer of alpha-helices // J. Biol. Chem.– 2005.– Vol. 280, N18.–
P. 17920–17929.
Muryoi N., Sato M., Kaneko S. et al. Cloning and expression
of afpA, a gene encoding an antifreeze protein from the arctic
plant growth-promoting rhizobacterium Pseudomonas putida
GR12-2 // J. Bacteriol.– 2004.– Vol. 186, N17.– P. 5661–5671.
Pudney P.D., Buckley S.L., Sidebottom C.M. et al. The
physico-chemical characterization of a boiling stable antifreeze
protein from a perennial grass (Lolium perenne) // Arch.
Biochem. Biophys.– 2003.– Vol. 410, N2.– P. 238–245.
Scotter A.J., Marshall C.B., Graham L.A. et al. The basis for
hyperactivity of antifreeze proteins // Cryobiology.– 2006.–
Vol. 53, N2.– P. 229–239.
ханизме действия АФП и АФГП. Поскольку анти-
фризы у представителей далеких друг от друга сис-
тематических таксонов не обнаруживают гомоло-
гии и различны по своей третичной структуре [2],
логично допустить, что в процессе эволюции у них
установились различные способы реализации их
активности. В дальнейшем мы попытаемся привес-
ти классификацию различных АФП в зависимости
от их распространения в различных системати-
ческих таксонах.
Nowadays no one of these hypotheses is denied. It
should be noted that it has been reported [6] about the
ability of fish AFPs III to be adsorbed not only in ice
crystal surface, but also on the one of particles being
the centers of heterogeneous nucleation.
Thus the available data have not allowed yet to
specify the hypothesis about uniform action mecha-
nism of AFPs and AFGPs. There is logical admission
that during evolution in antifreezes there were set
different ways of their activity realization, since they
in the representatives of distant systematic taxons do
not reveal homology and vary on their tertiary structure
[2]. Later we will try to present the classification of
different AFPs depending on their variety in different
systematic taxons.
References
Avanov A.L. Biological antifreezes and mechanisms of their
activity// Molekulyarnaya Biologiya.– 1990.– Vol. 24, N3.–
P. 581–597.
Barrett J. Thermal hysteresis proteins // Int. J. Biochem. Cell
Biol.– 2001.– Vol. 33, Issue 2.– P. 105–117.
Clarke C.J., Buckley S.L., Lindner N. Ice structuring proteins –
a new name for antifreeze proteins // Cryo Letters.– 2002.–
Vol. 23, N2.– P. 89–92.
Davies P.L., Baardsnes J., Kuiper M.J. et al. Structure and
function of antifreeze proteins // Phil. Trans. R. Soc. Lond. B.
Biol. Sci.– 2002.– Vol. 357, N1423.– P. 927–935.
Davies P.L., Hew C.L. Biochemistry of fish antifreeze proteins //
FASEB J.– 1990.– Vol.4.– P. 2460–2468.
Du N., Liu Y.X., Hew C.L. Ice nucleation inhibition: Mechanism
of antifreeze by antifreeze protein // J. Biol. Chem.– 2003.–
Vol. 278, N38.– P. 36000–36004.
Duman J.G. Antifreeze and ice nucleator proteins in terrestrial
arthropods // Annu. Rev. Physiol.– 2001.– Vol. 63.– P. 327–
357.
Gilbert J.A., Davies P.L., Laybourn-Parry J. A hyperactive,
Ca
2+
-dependent antifreeze protein in an Antarctic bacterium //
JFEMS Microbiol. Lett.– 2005.– Vol. 245, N1.– P. 67–72.
Huang T., Duman J.G. Cloning and characterization of a
thermal hysteresis (antifreeze) protein with DNA-binding
activity from winter bittersweet nightshade, Solanum
dulcamara // Plant. Mol. Biol.– 2002.– Vol. 48, N4.– P. 339–
350.
Kawahara H.J. The structures and functions of ice crystal-
controlling proteins from bacteria // Biosci. Bioeng.– 2002.–
Vol. 94, N6.– P. 492–496.
Kristiansen E., Zachariassen K.E. The mechanism by which
fish antifreeze proteins cause thermal hysteresis // Cryobiolo-
gy.– 2005.– Vol. 51, N3.– P. 262–280.
Madura J.D., Baran K., Wierzbicki A. Molecular recognition
and binding of thermal hysteresis proteins to ice // J. Mol.
Recognition.– 2000.– Vol. 13, Issue 2.– P. 101–113.
Margaritis A., Bassi A.S. Principles and biotechnological
applications of bacterial ice nucleation // Crit. Rev. Biotechnol.–
1991.– Vol. 11, N3.– P. 277–295.
Marshall C.B., Chakrabartty A., Davies P.L. Hyperactive
antifreeze protein from winter flounder is a very long rod-like
dimer of alpha-helices // J. Biol. Chem.– 2005.– Vol. 280, N18.–
P. 17920–17929.
Muryoi N., Sato M., Kaneko S. et al. Cloning and expression
of afpA, a gene encoding an antifreeze protein from the arctic
plant growth-promoting rhizobacterium Pseudomonas putida
GR12-2 // J. Bacteriol.– 2004.– Vol. 186, N17.– P. 5661–5671.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
24
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
Smallwood M., Worrall D., Byass L. et al. Isolation and charac-
terization of a novel antifreeze protein from carrot (Daucus
carota) // Biochem. J.– 1999.– Vol. 340, Pt. 2.– P. 385–391.
Storey K.B. Living in the cold: freeze-induced gene responses
in freeze-tolerant vertebrates // Clin. Exp. Pharmacol. Physiol.–
1999.– Vol. 26, N1.– P. 57–63.
Поступила 10.06.2008
Рецензент Т.П. Линник
Scotter A.J., Marshall C.B., Graham L.A. et al. The basis for
hyperactivity of antifreeze proteins // Cryobiology.– 2006.–
Vol. 53, N2.– P. 229–239.
Pudney P.D., Buckley S.L., Sidebottom C.M. et al. The
physico-chemical characterization of a boiling stable antifreeze
protein from a perennial grass (Lolium perenne) // Arch.
Biochem. Biophys.– 2003.– Vol. 410, N2.– P. 238–245.
Smallwood M., Worrall D., Byass L. et al. Isolation and charac-
terization of a novel antifreeze protein from carrot (Daucus
carota) // Biochem. J.– 1999.– Vol. 340, Pt 2.– P. 385–391.
Storey K.B. Living in the cold: freeze-induced gene responses
in freeze-tolerant vertebrates // Clin. Exp. Pharmacol. Physiol.–
1999.– Vol. 26, N1.– P. 57–63.
Accepted in 10.06.08
18.
19.
16.
17.
18.
19.

25
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
УДК 57.043:579.2:57.086.8
È.Ô. ÊÎÂÀËÅÍÊÎ*, Ñ.Â. ÊÎÙÈÉ, Å.Â. ÒÈÌÎÔÅÅÂÀ, À.Â. ÑÀÊÓÍ,
Ñ.Å. ÊÎÂÀËÅÍÊÎ, È.Ï. ÂÛÑÅÊÀÍÖÅÂ, Ë.Ô. ÐÎÇÀÍÎÂ
Ïðîíèöàåìîñòü ìåìáðàí êëåòîê ÑÏÝÂ
äëÿ ìîëåêóë âîäû è ÄÌÑÎ
UDC 57.043:579.2:57.086.8
I.F. KOVALENKO*, S.V. KOSCHIY, E.V. TIMOFEYEVA, A.V. SAKUN,
S.YE. KOVALENKO, I.P. VYSEKANTSEV, L.F. ROZANOV
Permeability of SPEV Cell Membranes
for Water and DMSO Molecules
С использованием метода волюмометрии определены коэффициенты проницаемости мембран клеток СПЭВ для молекул
воды и ДМСО. Установлено, что при увеличении размеров клеток проницаемость их мембран для молекул ДМСО уменьшается.
Численное моделирование осмотического поведения клеток СПЭВ при замораживании в присутствии ДМСО показало, что
при охлаждении со скоростью 1С/мин дегидратация клеток СПЭВ происходит в диапазоне температур –7,5...– 13С, а увеличение
скорости охлаждения до 5С/мин и выше может стать причиной внутриклеточной кристаллизации.
Ключевые слова: коэффициенты проницаемости, клетки СПЭВ, волюмометрия.
З використанням методу волюмометрії визначені коефіцієнти проникності мембран клітин СПЕВ для молекул води та
ДМСО. Встановлено, що при збільшенні розмірів клітин проникність їх мембран для молекул ДМСО зменшується. Чисельне
моделювання осмотичної поведінки клітин СПЕВ при заморожуванні в присутності ДМСО показало, що при охолодженні зі
швидкістю 1С/хв дегідратація клітин СПЕВ відбувається в діапазоні температур –7,5...–13С, а збільшення швидкості
охолодження до 5С/хв і вище може бути причиною внутрішньоклітинної кристалізації.
Ключові слова: коефіцієнти проникності, клітини СПЕВ, волюмометрія.
Using the volumetry method there were defined the permeability coefficients of SPEV cell membranes for water and DMSO. It has
been established that with increasing the volumes of cell dimensions their membrane permeability for DMSO molecules reduces.
Numeric modeling of osmotic behavior of SPEV cells during freezing in DMSO presence has shown that during cooling with 1C/min
the dehydration of SPEV cells occurs within the temperature range of –7.5...–13C and the rise of cooling rate up to 5C/min and higher
may be the cause of intracellular crystallization.
Keywords: permeability coefficients, SPEV cells, volumetry.
* Àâòîð, êîòîðîìó íåîáõîäèìî íàïðàâëÿòü êîððåñïîíäåíöèþ:
óë.Ïåðåÿñëàâñêàÿ, 23, ã.Õàðüêîâ, Óêðàèíà 61015; òåë.:+38
(057) 373-31-19, ôàêñ: +38 (057) 373-30-84, ýëåêòðîííàÿ ïî÷òà:
cryo@online.kharkov.ua
Èíñòèòóò ïðîáëåì êðèîáèîëîãèè è êðèîìåäèöèíû
ÍÀÍ Óêðàèíû, ã. Õàðüêîâ
Institute for Problems of Cryobiology and Cryomedicine of the Na-
tional Academy of Sciences of Ukraine, Kharkov, Ukraine
* To whom correspondence should be addressed: 23,
Pereyaslavskaya str., Kharkov, Ukraine 61015; tel.:+380 57 373
3119, fax: +380 57 373 3084, e-mail:cryo@online.kharkov.ua
Перевиваемые клеточные культуры часто ис-
пользуются как модель для решения многих
проблем общебиологического значения, таких как
дифференцировка клеток, межклеточные контакты,
канцерогенез, передача наследственной информа-
ции, получение высокоэффективных вакцин и про-
тивовирусных препаратов в достаточном коли-
честве. Известно, что клетки в процессе длитель-
ного культивирования могут изменять свои исход-
ные свойства [2]. В связи с этим актуально созда-
ние криобанков перевиваемых клеточных культур,
а также разработка новых эффективных и надеж-
ных методов криоконсервирования этих клеток.
Большинство известных методов криоконсерви-
рования перевиваемых клеточных культур базиру-
ется на эмпирическом подходе, использовании ин-
туитивных представлений о путях оптимизации
условий криоконсервирования. Но такой метод не
Inoculated cell cultures are frequently used as the
model for solving nay tasks of general biological value,
such as cell differentiation, intercellular contacts, can-
cerogenesis, transfer of hereditary information, obtain-
ing of highly efficient vaccines and anti-viral formu-
lations in a sufficient quantity. Its is known that the
cells during long-term culturing may change their initial
properties [2]. In this connection the establish-ment of
cryobanks of inoculated cell cultures has become an
actual task, as well as the development of new effec-
tive and reliable cryopreservation protocols for these
cells.
The majority of available cryopreservation methods
for inoculated cell cultures is based on empirical appro-
ach using intuitive notions about the ways of optimizing
the cryopreservation conditions. However this method
does not answer the question about admissible limits
of varying different factors during cryopreservation.
26
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
даёт ответа на вопрос о допустимых пределах
варьирования различных факторов при криокон-
сервировании.
Основой поиска оптимальных методических
решений при разработке методов криоконсервиро-
вания может стать экспериментально-теорети-
ческий подход, использующий модифицированную
физико-математическую модель Кедем-Качальс-
кого [1]. Такой подход направлен на оптимизацию
процессов массообмена в системе “клетка окру-
жающая среда” в цикле криоконсервирования и тре-
бует конкретизации данных о составе вне- и
внутриклеточной среды, проницаемости и морфо-
метрических параметрах клеток. Актуальность
исследований процессов массообмена определя-
ется и тем, что они, согласно двухфакторной теории
криоповреждения, являются основными при опти-
мизации условий криоконсервирования [1].
Цель работы определение коэффициентов
проницаемости клеток СПЭВ для молекул воды и
диметилсульфоксида, а также моделирование их
осмотического поведения при криоконсервировании
с использованием полученных коэффициентов
проницаемости.
Ìàòåðèàëû è ìåòîäû
Объектом исследования служили клетки пере-
виваемой клеточной культуры СПЭВ (эмбриональ-
ная почка свиньи), диаметр которых в экспери-
менте составлял 14–22 мкм.
Перевиваемая клеточная линия СПЭВ была
выращена в культуральных матрасах в среде 199
с добавлением 10%-й эмбриональной телячьей
сыворотки и 100 ед/мл канамицина. Клетки инкуби-
ровали при 37С до образования монослоя, затем
переводили в суспензионное состояние, обрабаты-
вая монослой смесью раствора Версена с трипси-
ном (4:1) [3].
Исследования проводили на инвертированном
микроскопе МБИ-13 (“ЛОМО”). Для изучения
динамики осмотической реакции клеток СПЭВ на
добавление 1М раствора ДМСО суспензию клеток
фотографировали через определенные промежутки
времени. Полученные данные представляли в виде
зависимостей относительных объемов отдельных
клеток от времени экспозиции в растворе ДМСО.
Коэффициенты проницаемости плазматических
мембран клеток СПЭВ для молекул воды L
p
и
криопротектора K
p
определяли, сопоставляя экспе-
риментальные значения изменения относительного
объема клетки от времени экспозиции y(t) с реше-
ниями уравнений теоретической модели для задан-
ных экспериментальных условий.
Процессы массопереноса через клеточные
мембраны при наличии одного проникающего и
The basis of the search for optimal methodical
solutions during the development of cryopreservation
protocols may be a combined experimental and theoretical
approach using modified physical-mathematical model
of the Kedem-Katchalsky one [1]. This approach is
directed to optimize the processes of mass exchange
in the “cell-environment” system in cryopreservation
cycle and requires the specifying of the data on the
composition of extra- and intracellular medium, per-
meability and morphometric parameters of cells. The
actuality of studying the mass exchange processes is
determined by the fact that according to two-factor
theory of cryodamage they are the main ones during
optimization of cryopreservation conditions [1].
The research aim is examining the permeability
coefficients of SPEV cells for water and DMSO
molecules, as well as modeling their osmotic behavior
during cryopreservation using the obtained permeability
coefficients.
Materials and methods
The cells of SPEV, inoculated cell culture (porcine
embryonic kidney) served as the research object, their
diameter in the experiment made 14–22 m.
Inoculated cell line SPEV was grown in cultural
flask in medium 199 with adding 10% fetal calf serum
and 100 units/ml kanamycin. The cells were incubated
at 37C up to the formation of monolayer, afterwards
they were transferred into suspension state with
treating the monolayer with the mixture of Versene
solution and trypsin (4:1) [3].
The studies were performed with inverted micro-
scope MBI-13 (“LOMO”, Russia). To examine the
dynamics of osmotic reaction of SPEV cells on the
adding of 1M DMSO solution the cell suspension was
photographed in certain time periods. The obtained data
were represented as the dependences of relative volu-
mes of separate cells on exposure time in DMSO solu-
tion.
The permeability coefficients of plasma membranes
of SPEV cells for water molecules L
p
and cryopro-
tective agent K
p
were examined by comparing the
experimental values of the changes in cell relative
volume on exposure time y(t) with solving the equations
of theoretical model for the set experimental conditions.
The processes of mass transfer via cell membranes
in the presence of one penetrating agent and those non-
penetrating are described by the system of equations
[1]:
1
1
ˆˆ
1
111
0
ydt
dy
outin
ydt
dy
dt
d
inoutin
in
1
ˆˆˆ
1
ˆ
1111
1
1
,
27
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
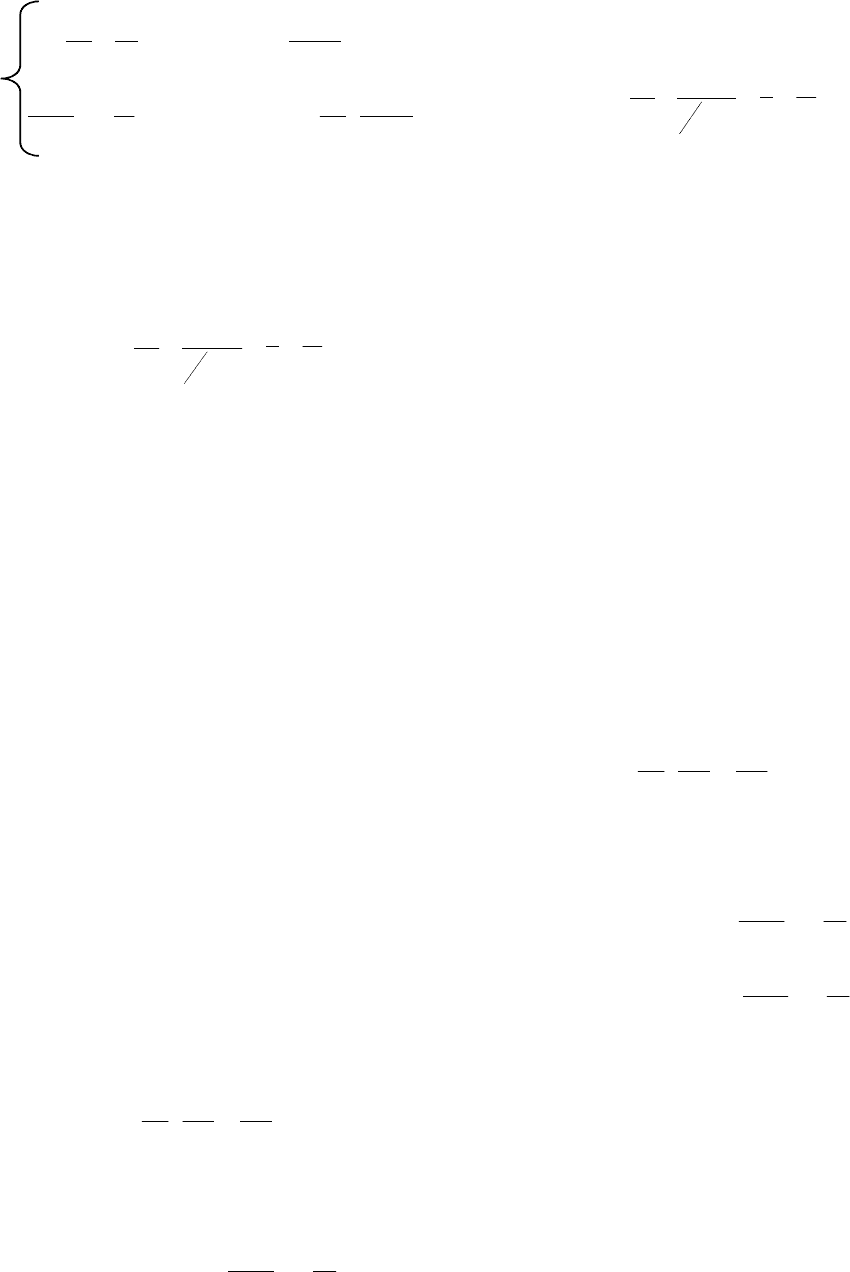
непроникающего веществa описываются системой
уравнений [1]:
1
1
ˆˆ
1
111
0
ydt
dy
outin
ydt
dy
dt
d
inoutin
in
1
ˆˆˆ
1
ˆ
1111
1
1
,
где у – относительный объём клетки (y = V/V
0
; V
0
начальный объём клетки);
0
и
1
комплексы
параметров
0
= 1/L
p
0
0
in
,
1
= 1/K
p
0
;
0
– поверх-
ностно-объёмное отношение клетки
3
2
0
0
0
3
4
4
r
r
V
S
D
r
63
;
1
– коэффициент отражения для проникающего
через мембрану вещества;
0
in
– осмотическое
давление изотонического раствора;
out
1
ˆ
и
in
1
ˆ
–
приведенное осмотическое давление первого раст-
ворённого вещества вне- и внутри клетки соот-
ветственно (
inoutout
011
/
ˆ
,
ininin
011
/
ˆ
);
–
объёмная доля осмотически неактивных веществ
внутри клетки.
Значение
определяли экстраполяцией нанесен-
ной на график зависимости относительного объема
клеток от
0
in
/
(
– осмотическое давление гипер-
тонического внеклеточного раствора хлористого
натрия) к нулевому значению аргумента.
Коэффициенты проницаемости мембран клеток
СПЭВ определяли, решая дифференциальные
уравнения. Коэффициент отражения клеточной
мембраны
1
во всех расчётах принимали равным
0,95.
Используемая нами физико-математическая
модель достаточно адекватно описывает и осмо-
тическое поведение клеток на этапе заморажи-
вания. Решениями модели при известных значе-
ниях L
p
и K
p
являются зависимости y(T),
1
in
(T) и
2
in
(T) от температуры. Такие решения несложно
получить для линейных режимов охлаждения,
перейдя к новым переменным:
dT
dy
dT
dT
dt
dy
,
где
– скорость охлаждения (
= dT/dt);
T
T
TR
U
TLTL
pp
0
00
0
0
1exp
;
where y is cell relative volume y = V/V
0
, here V
0
–
initial cell volume;
0
and
1
– the complexes of
parameters
0
= 1/L
p
0
0
in
,
1
= 1/K
p
0
, here
0
– cell
surface to volume ratio
3
2
0
0
0
3
4
4
r
r
V
S
D
r
63
;
1
– reflection coefficient for penetrating trough memb-
rane substance;
0
in
– osmotic pressure of isotonic
solution;
1
out
and
1
in
are values of normalized pressure
of the first dissolved substance out- and inside the cell,
correspondingly (
inoutout
011
/
ˆ
,
ininin
011
/
ˆ
);
– volumetric share of osmotically inactive substance
inside a cell.
The value
was found with extrapolation of plotted
dependence of relative volume of cells from
0
in
/
(
is osmotic pressure of hypertonic extracellular solu-
tion of sodium chloride) to zero value of the argument.
Permeability coefficients of SPEV cell membranes
were examined by solving the differential equations.
The reflection coefficient of cell membrane
1
in all
the calculations was assumed as equal to 0.95.
Used by us physical-mathematical model quite ade-
quately describes the osmotic behavior of cells at
freezing stage. The solutions of the model at the known
values L
p
and K
p
are the dependences у(T),
1
in
(T)
and
2
in
(T) on temperature. These solutions can be
easily obtained for linear cooling regimens changing to
new variables:
dT
dy
dT
dT
dt
dy
,
where
is cooling rate (
= dT/dt);
T
T
TR
U
TLTL
pp
0
00
0
0
1exp
;
T
T
TR
U
TKTK
pp
0
00
1
0
1exp
,
where T
0
– initial temperature of cell suspension; U
0
and U
1
– activation energies of transfer of water
molecules and dissolved substance through plasma
membrane, correspondingly; R
0
– universal gas constant.
Osmotic behavior of cells during freezing with
various rates was forecasted by the insertion of the
found values L
p
and K
p
into the model equation.
The system of differential equations describing the
kinetics of the change of cell relative volume and
concentration of penetrating through plasma membrane
substance inside a cell during extracellular crystal-
lization has the appearance [1]:
28
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
T
T
TR
U
TKTK
pp
0
00
1
0
1exp
,
где T
0
исходная температура клеточной суспен-
зии; U
0
и U
1
энергии активации процесса переноса
через плазматическую мембрану молекул воды и
растворённого вещества соответственно; R
0
уни-
версальная газовая постоянная.
Прогнозирование осмотического поведения кле-
ток при замораживании с различными скоростями
осуществляли, подставляя в уравнения модели
найденные значения L
p
и K
p
.
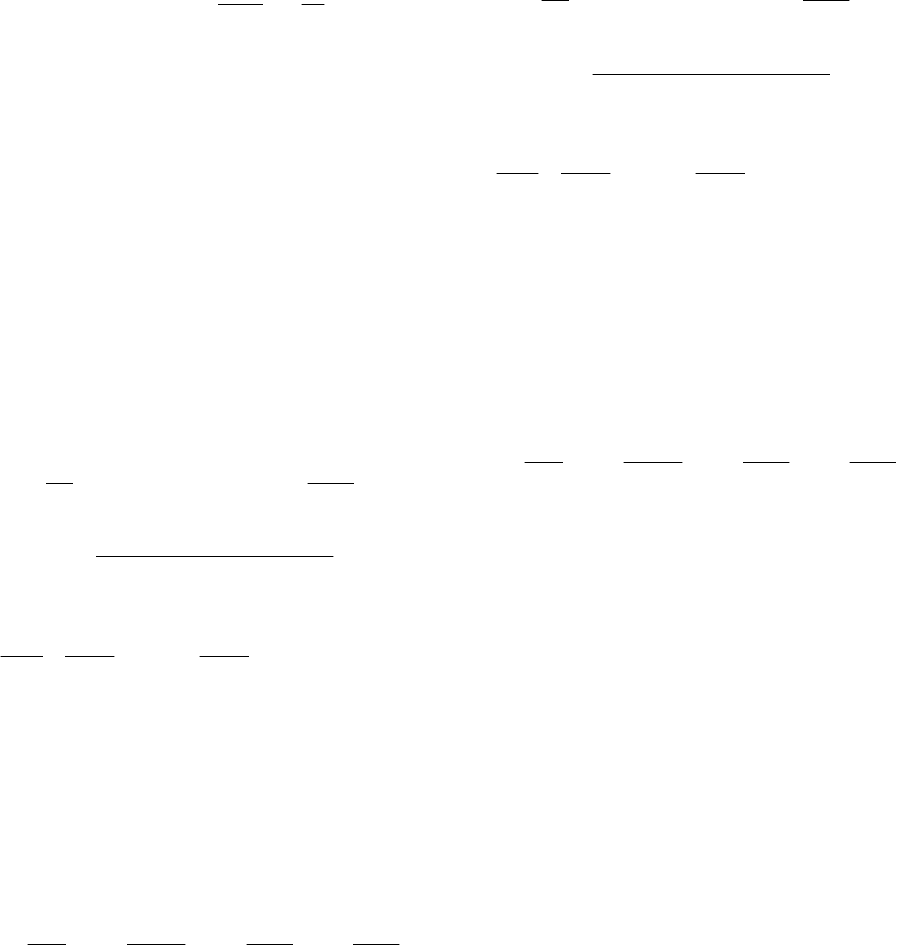
Система дифференциальных уравнений, описы-
вающая кинетику изменения относительного объе-
ма клетки и концентрацию проникающего через
плазматическую мембрану вещества внутрь
клетки в процессе внеклеточной кристаллизации,
имеет вид [1]:
1
ˆ
ˆ
/)1
ˆ
(exp
ˆ
11
in
y
TTap
Td
dy
;
ˆ
)1()1(
ˆ
)(
ˆ
)1(
ˆ
1
2
002
0101
1
out
in
gg
gyg
]
ˆ
)1(
1
ˆ
{[
ˆ
ˆ
2111
1
Ф
yy
p
Td
d
out
out
in
in
]
ˆˆ
)1[(
]
111
in
]};
ˆ
/)1
ˆ
(exp[)
ˆˆ
(]
ˆ
/)1
ˆ
(exp[
21
TTbФqTT
T
d
out
out
in
где
T
ˆ
относительная температура суспензии
(
0
/
ˆ
TTT
); g
0
– начальное значение отношения
суммарного объема клеток к полному объему
системы; p, q, a, b – безразмерные комплексы:
00
1
00
0
01
00
0
0
,,
)(
)(
,
TR
U
b
TR
U
a
T
T
q
T
p
.
Кинетику изменения концентрации внекле-
точного раствора в процессе замораживания зада-
вали при расчетах аналитически путем аппрокси-
мации фазовой диаграммы плавления водного
раствора ДМСО [2] в следующем виде:
C = 1,273·10
-5
×T
4
- 0,0128×T
3
+ 4,8324×T
2
–
– 801,939×T + 49674,1;
где Т – текущая температура.
Статистическую обработку результатов экспе-
риментов проводили по методу Стьюдента-Фишера.
Достоверность различий оценивали с помощью t-
критерия с уровнем значимости 5%.
1
ˆ
ˆ
/)1
ˆ
(exp
ˆ
11
in
y
TTap
Td
dy
;
ˆ
)1()1(
ˆ
)(
ˆ
)1(
ˆ
1
2
002
0101
1
out
in
gg
gyg
]
ˆ
)1(
1
ˆ
{[
ˆ
ˆ
2111
1
Ф
yy
p
Td
d
out
out
in
in
]
ˆˆ
)1[(
]
111
in
]};
ˆ
/)1
ˆ
(exp[)
ˆˆ
(]
ˆ
/)1
ˆ
(exp[
21
TTbФqTT
T
d
out
out
in
where
T
ˆ
– relative temperature of suspension
(
0
/
ˆ
TTT
); g
0
– initial value of the ratio of total cell
volume to bulk volume of the system; p, q, a, b –
dimensionless complexes:
00
1
00
0
01
00
0
0
,,
)(
)(
,
TR
U
b
TR
U
a
T
T
q
T
p
.
The kinetics of changes in concentration of extra-
cellular solution during freezing was determined when
calculating analytically by means of approximation of
phase diagram of melting for aqueous solution of DMSO
[2] as follows:
C = 1.273·10
-5
×T
4
– 0.0128×T
3
+ 4.8324×T
2
–
– 801.939×T + 49674.1;
where T – current temperature.
The results of experiments were statistically
processed using the method of Student-Fisher. The
statistical significance of differences was assessed by
means of t-criterion with 5% significance level.
Results and discussion
When processing the obtained dependences of
SPEV cell relative volume on exposure time in 1M
DMSO solution its has been found that the cells of
various sizes recover the volumes with different rates.
In this connection we attempted to use the differential
approach when defining the permeability coefficients
for the cells with different sizes.
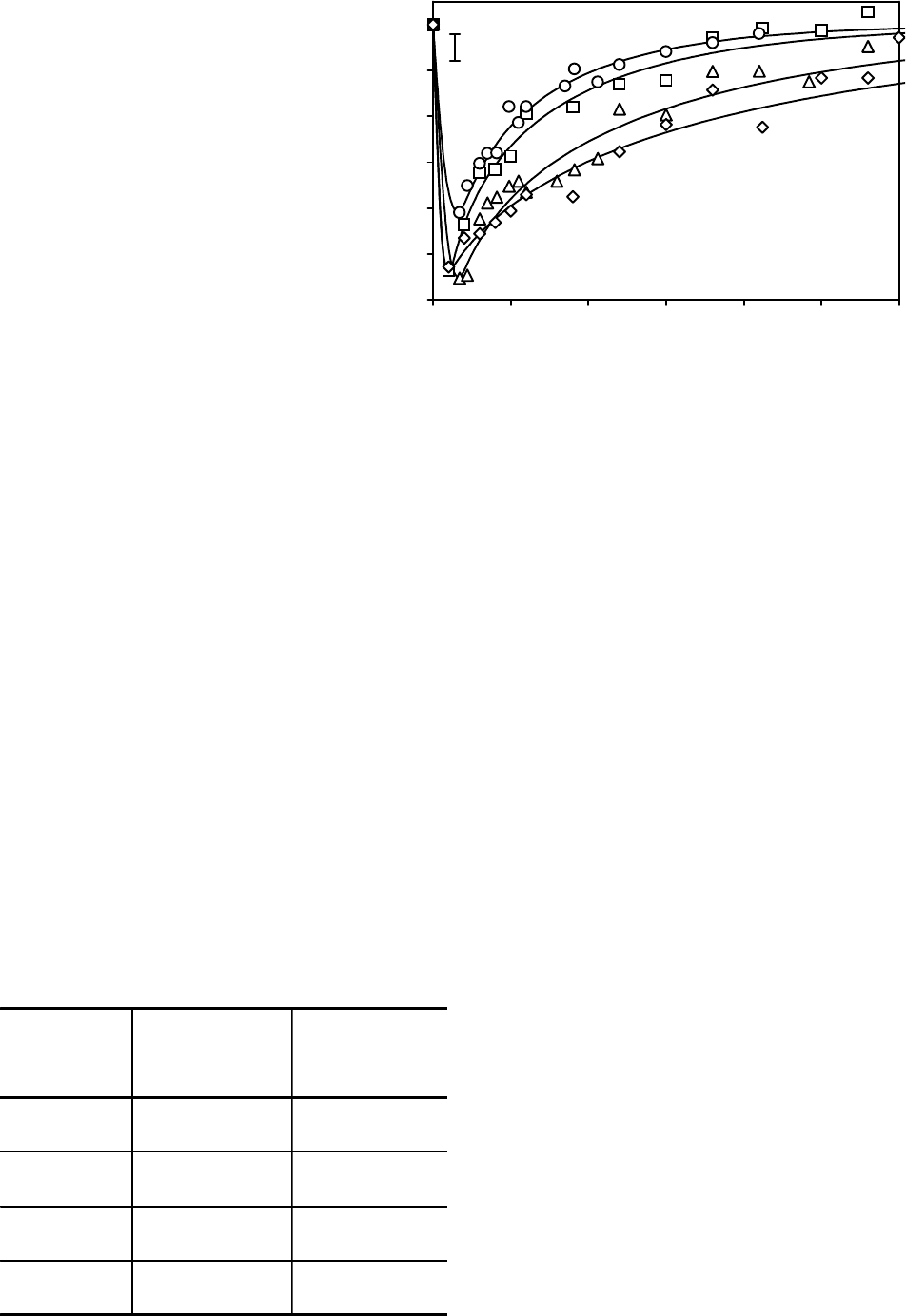
Fig. 1 shows the characteristic experimental depen-
dences of the volume changes for the cells with
different sizes vs. the contact time with DMSO soluti-
on and the theoretical curves, extrapolating osmotic
behavior of cells. It has been established that the cell
rehydration time with big initial volume after dehyd-
ration phase increases. According to calculations of
the permeability coefficients this dependence is
associated not only to lesser surface-volume ratio of
big size cells, but also likely to the change of properties
Ðåçóëüòàòû è îáñóæäåíèå
При обработке полученных зави-
симостей относительного объема
клеток СПЭВ от времени экспози-
ции в 1М растворе ДМСО установ-
лено, что клетки разных размеров
восстанавливают объем с разной
скоростью. В связи с этим мы пред-
приняли попытку подойти к опреде-
лению коэффициентов проницаемос-
ти клеток разных размеров диффе-
ренцированно.
На рис.1 представлены характер-
ные экспериментальные зависимос-
ти изменения объемов клеток раз-
ных размеров от времени контакта
с раствором ДМСО и экстраполи-
рующие осмотическое поведение
клеток теоретические кривые. Уста-
новлено, что время регидратации
клеток с большим исходным объе-
мом после фазы дегидратации уве-
личивается. Согласно расчетам
коэффициентов проницаемости такая
зависимость связана не только с
0,4
0,5
0,6
0,7
0,8
0,9
1
0 50 100 150 200 250 300
Относительный объем V/V
0
Relative volume V/V
0
Время экспозиции, с
Exposure time, s
Рис. 1. Характерные экспериментальные зависимости относительных
объемов клеток СПЭВ от времени контакта с раствором ДМСО и теоре-
тические кривые, экстраполирующие осмотическое поведение клеток
(средние диаметры клеток, мкм: 1 – 14,4; 2 – 15,9; 3 – 17,9; 4 – 20,3).
Fig. 1. Characteristic experimental dependences of relative volumes of SPEV
cells on time of contact with DMSO solution and theoretical curves extra-
polating osmotic behavior of cells, mean diameters of cells, m (1– 14.4; 2 –
15.9; 3 – 17.9; 4 – 20.3).
меньшим поверхностно-объемным отношением
клеток больших размеров, но и, по-видимому, с
изменением свойств, определяющих проницае-
мость мембран для молекул ДМСО.
В таблице приведены средние значения коэф-
фициентов проницаемости клеток разных размеров
для молекул воды и ДМСО. Коэффициент фильт-
рации мембран клеток разных размеров имеет
пределы (5,30 3,20)10
14
(14,3 5,2)10
14
м
3
/Нс.
При этом, хотя и имеются достоверные отличия
между отдельными группами клеток, зависимость
L
p
от размера клетки выражена неявно. В то же
время с увеличением размеров клеток коэффи-
determining the permeability of membranes for DMSO
molecules.
The table covers the mean values of permeability
coefficients of the cells with different sizes for water
and DMSO molecules. Filtration coefficient of cell
membra-nes of different sizes has the limits (5.30
3.20)10
14
(14.3 5.2)10
14
m
3
/Ns. Herewith though
there are significant differences between some groups
of cells, the L
p
dependence on cell size is slightly
manifested. At the same time with the rise of cells
sizes the permeability coefficient of their membranes
for DMSO molecules reduces, however the statistically
significant differences are found only for the cells, the
sizes of those significantly differ.
Using the calculated permeability coefficients and
corresponding geometrical cell parameters there was
modeled osmotic behavior of cells at the stage of their
freezing with different cooling rates.
Fig. 2 shows the dependence of relative volume of
SPEV cells with average sizes of 14.4; 15.9 and 20.3 m
on temperature during cooling with the rates of 1, 5
and 10C/min. The presented data testify to the fact
that with the rising of the cell size its dehydration rate
during freezing increases slightly. With cooling rate of
1C/min large cells approach the extreme dehydration
during cooling down to –7.5 and the small ones down
to –12.8C, with the cooling rate of 5C/min the dehyd-
ration period extends down to –23.8 and to –38.3C,
correspondingly, with the cooling rate of 10C/min the
dehydration rate of cells within the subzero temperature
range is insignificant.
Значения коэффициентов проницаемости клеток
разных диаметров для молекул воды и ДМСО
Values of permeability coefficients of cells of different
diameters for water and DMSO molecules
29
PROBLEMS
OF CRYOBIOLOGY
Vol. 19, 2009, ¹1
ÏÐÎÁËÅÌÛ
ÊÐÈÎÁÈÎËÎÃÈÈ
Ò. 19, 2009, ¹1
Диаметр клетки
D, мкм
Cell diameter
D, m
К оэффициент
фильтрации,
L
p
10
14
м
3
/Нс
Filtration coefficient,
L
p
10
14
m
3
/Ns
К оэффициент
проницаемости для
ДМСО, К
р
10
8
м/с
Permeability coefficient
for DMSO, K
p
10
8
m/s
14,4±0,47 5,41±0,58 12,00±4,00
15,9 ±0,50 8,28±2,57 7,36±2,07
17,9 ±0,74 5,30±3,20 4,61±0,50
20,3±0,82 14,3±5,2 3,80±0,60
