Zdunkowski W., Trautmann T., Bott A. Radiation in the atmosphere: A course in Theoretical Meteorology
Подождите немного. Документ загружается.

8.2 Molecular vibrations 285
x
y
x
3
y
3
x
1
y
1
α
α
ll
3
2
1
m
A
m
A
m
B
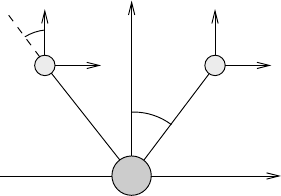
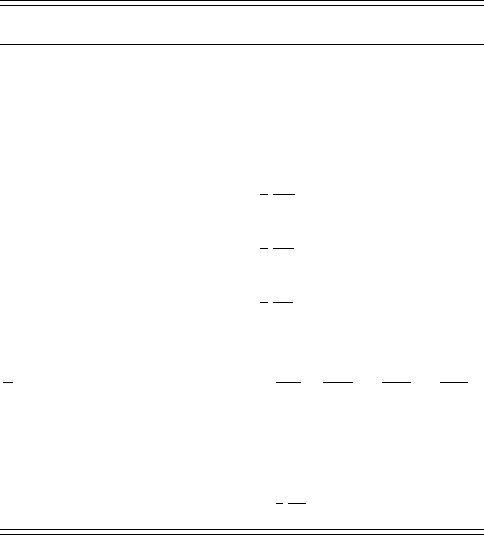
Fig. 8.7 Coordinates of the triangular molecule H
2
O.
of the dipole moment takes place along or perpendicular to the axis of symmetry
of the molecule which is the internuclear axis. The antisymmetric longitudinal or
the ν
3
-vibration induces a dipole moment parallel to the axis of symmetry. Thus
ν
2
- and ν
3
-vibrations are active in the infrared spectrum. The most important CO
2
band in the infrared spectral range is centered at the wave number ˜ν = 667.40 cm
−1
(λ = 15 µm) which results from the ν
2
-vibration. In contrast to the CO
2
molecule,
the diatomic gases N
2
and O
2
, abundantly occurring in the Earth’s atmosphere,
do not possess permanent electric dipole moments and, therefore, do not exhibit
infrared absorption bands.
We have now examined in some detail the normal vibrations of a linear triatomic
molecule. The actual internal vibrations of a semi-rigid system (small amplitude
vibrations) may be very complicated. However, the motion can always be decom-
posed into a sum of elementary motions described by the normal vibrational modes.
The frequency equations (8.22) and (8.29) are in exact agreement with the results
shown in the standard reference book Infrared and Raman Spectra by Herzberg
(1964b) where many details can be found.
8.2.4 Nonlinear triatomic molecules
It is not always as simple as in the case of the linear triatomic molecule to find the
normal coordinates. This will become apparent in the discussion of the nonlinear
triatomic H
2
O, see Figure 8.7. The three masses are labelled as 1, 2 and 3. The
equilibrium distance from the central mass m
B
to the masses m
A
is l.
Employing the conservation of the center of mass according to (8.15) we find
m
A
(x
1
+ x
3
) + m
B
x
2
= 0, m
A
(y
1
+ y
3
) + m
B
y
2
= 0 (8.31)
If we place ourselves in the resting position of the atom m
B
so that r
0,2
=0, noting
that |r
0,1
|=|r
0,3
|, we find that the conservation of angular momentum is given by
(y
1
− y
3
) sin α = (x
1
+ x
3
) cos α (8.32)
286 Absorption by gases
Details of the calculation will be left up to the exercises. Now we must find the
change of l due to stretching between the masses m
A
(mass point 1) and m
B
(mass
point 2) and between m
B
(2) and m
A
(3). The changes l
1
and l
3
are found by
projecting the vectors x
1
− x
2
and x
3
− x
2
on the directions of the lines connecting
the masses m
A
(point 1) and m
B
and m
B
and m
A
(point 3). A simple calculation
gives
δl
1
= (x
1
− x
2
) sin α + (y
1
− y
2
) cos α
δl
3
=−(x
3
− x
2
) sin α + (y
3
− y
2
) cos α
(8.33)
The change of the angle 2α is found by projecting the vectors x
1
− x
2
and x
3
− x
2
on the directions perpendicular to the lines connecting the points 1 and 2 and 2 and
3. The result is given by
δ =
1
l
[
(x
1
− x
2
) cos α − (y
1
− y
2
) sin α − (x
3
− x
2
) cos α − (y
3
− y
2
) sin α
]
(8.34)
Details of the calculations are left to the exercises.
The Lagrangian function is found as
L =
m
A
2
˙
x
2
1
+
˙
x
2
3
+
m
B
2
˙
x
2
2
−
k
1
2
(δl
1
)
2
+ (δl
2
)
2
−
k
2
2
(lδ)
2
(8.35)
where
˙
x
i
=
˙
x
i
i +
˙
y
i
j, etc. The third and fourth right-hand side terms describe the
potential energy of the extension of the springs connecting the masses and of the
bending of the molecule.
As motivated by the previous example, we introduce the new coordinates
q
a
= x
1
+ x
3
, q
s,1
= x
1
− x
3
, q
s,2
= y
1
+ y
3
=⇒
x
1
=
1
2
(q
a
+ q
s,1
), x
2
=−
m
A
m
B
q
a
, x
3
=
1
2
(q
a
− q
s,1
)
y
1
=
1
2
(q
s,2
+ q
a
cot α), y
2
=−
m
A
m
B
q
s,2
, y
3
=
1
2
(q
s,2
− q
a
cot α)
(8.36)
where we have employed the conservation of the center of mass. A simple but
tedious calculation gives the Lagrangian function of the system
L =
m
A
4
2m
A
m
B
+
1
sin
2
α
˙
q
2
a
+
m
A
4
˙
q
2
s,1
+
m
A
m
T
4m
B
˙
q
2
s,2
−q
2
a
k
1
4
2m
A
m
B
+
1
sin
2
α
1 +
2m
A
m
B
sin
2
α
−
q
2
s,1
4
(k
1
sin
2
α + 2k
2
cos
2
α)
−q
2
s,2
m
2
T
4m
2
B
(k
1
cos
2
α + 2k
2
sin
2
α) + q
s,1
q
s,2
m
T
2m
B
(2k
2
− k
1
) sin α cos α
(8.37)

8.2 Molecular vibrations 287
We immediately recognize that q
a
is a normal coordinate since no cross-term occurs
with the other coordinates. The coordinates q
s,1
and q
s,2
are not normal coordinates
due to the appearance of a cross-term. As we will see later, the suffixes a and s
stand for antisymmetric and symmetric vibrations. We omit the simple details in
the calculation of the equation of motion involving the coordinates
˙
q
a
and q
a
which
is decoupled from the remainder of the system. The solution of the q
a
equation of
motion is given by
ω
2
a
=
k
1
m
A
1 +
2m
A
m
B
sin
2
α
(8.38)
Using again the Lagrangian form of the equation of motion, after a few steps we
obtain
¨
q
s,1
+ A
1
q
s,1
+ A
2
q
s,2
= 0
¨
q
s,2
+ B
1
q
s,2
+ B
2
q
s,1
= 0
(8.39)
with
A
1
=
1
m
A
(k
1
sin
2
α + 2k
2
cos
2
α), A
2
=−
m
T
m
A
m
B
(2k
2
− k
1
) sin α cos α
B
1
=
m
T
m
A
m
B
(k
1
cos
2
α + 2k
2
sin
2
α), B
2
=−
1
m
A
(2k
2
− k
1
) sin α cos α
(8.40)
and m
T
= 2m
A
+ m
B
. It is seen that (8.39) is a coupled system of two second-order
linear differential equations. The solution to this system can be found by any one
of the standard methods. The operator method is particularly easy to apply since
the two equations are decoupled almost immediately. We leave it to the exercises
to verify that the characteristic equation is given by
ω
4
− ω
2
(A
1
+ B
1
) + (A
1
B
1
− A
2
B
2
) = 0 (8.41)
permitting us to determine the eigenfrequencies ω
s,1
and ω
s,2
of the normal vibra-
tions q
s,1
and q
s,2
.
We will now briefly discuss the normal modes of vibration. First we consider the
antisymmetric vibration described by (8.38) of the H
2
O molecule. Pure q
a
vibrations
exist if x
1
= x
3
and y
1
=−y
3
. Thus q
a
describes antisymmetric vibrations with
respect to the y-axis. This case is shown in Figure 8.8(a).
Inspection reveals that the vibrations corresponding to the coordinate q
s,1
and
q
s,2
are symmetric with respect to the y-axis as shown in parts (b) and (c) of the
figure. We set q
a
= 0 and find x
1
=−x
3
and from (8.36) follows that y
1
= y
3
.
A more exact but also more involved analysis is described in Herzberg (1964a,b)
who introduces an additional interaction coefficient. This changes slightly the fre-
quency equations (8.38) and (8.41). Setting this small interaction coefficient equal
288 Absorption by gases
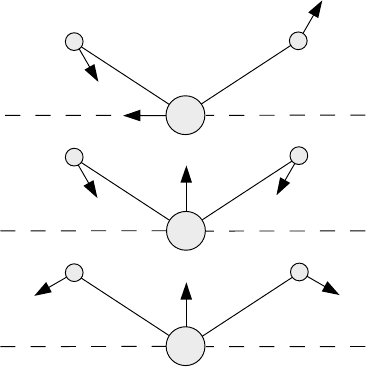
(a)
(b)
(c)
-vibration
-vibration
-vibration
ν
3
ν
2
ν
1
Fig. 8.8 Normal modes of vibration of the H
2
O molecule.
to zero results in the equations we have derived above. The inclusion of the inter-
action coefficient implies that the normal coordinates shown in Figure 8.8 also
change slightly. Particularly the ν
3
-vibration (Figure 8.8(a)), must be modified in
such a way that the arrows extending from the masses m
A
are nearly parallel to the
lines connecting them with m
B
. The remaining parts of the figure are qualitatively
correct. Part (b) is called the ν
2
-vibration and part (c) the ν
1
-vibration. None of
the normal modes of vibration we have shown is drawn to scale. The most impor-
tant absorption band in the infrared spectral range is centered at the wave number
˜ν = 1594.78 cm
−1
(λ = 6.27 µm) which results from the ν
2
-vibration.
The famous books Molecular Vibrations by Wilson et al. (1955) and Infrared
and Raman Spectra by Herzberg (1964b) describe in great detail the theory of
vibrational spectra. Discussion on normal coordinates are given in any textbook on
theoretical mechanics. Our reference goes to Greiner (1989), Mechanik, Volume 2,
who gives a number of examples on normal vibrations. A wealth of information
relevant to radiative transfer in the atmosphere is summarized in Goody (1964a).
8.3 Some basic principles from quantum mechanics
We shall not attempt a rigorous development of quantum mechanics, but we shall
merely state some of the basic results as they apply to the individual atom or atomic
systems. The quantum mechanical description of atomic or molecular systems
is carried out with the help of the wave function or the state function . This
function, in general, is a complex number and considered to be a function of all of
the configurational coordinates including time.

8.3 Some basic principles from quantum mechanics 289
According to the basic postulates of quantum mechanics, the square of the abso-
lute value ||
2
of the wave function is a measure of the probability that the consid-
ered system is located at the configuration corresponding to the particular values of
the coordinates. Sometimes the product
∗
or ||
2
is called the probability dis-
tribution function or the probability density. For example, if the system consists of a
single electron, then the probability that the electron is located somewhere between
x, y, z and x + dx, y + dy, z + dz is given by
∗
(x, y, z)(x, y, z) dxdydz.
From the interpretation of the wave function it follows that we cannot be certain
that the electron is located at any particular place. Only the probability of being
there within certain limits can be known. This interpretation is consistent with
Heisenberg’s uncertainty principle. Since the electron has to be somewhere in
space the total probability has to be unity as stated in
∞
−∞
∞
−∞
∞
−∞
∗
dx dy dz = 1 (8.42)
Functions satisfying (8.42) are classified as quadratically integrable normalized
functions.
8.3.1 Stationary and coherent states
We will now briefly discuss two particular states which are known as stationary
and coherent states.
(i) Stationary states
An eigenstate or characteristic state corresponds to a perfectly defined energy. A
given system may have many eigenstates each possessing, in general, a different
energy. If E
n
denotes the particular energy of one of its eigenstates, the complete
wave function can be written as
n
(x, y, z, t) = ψ
n
(x, y, z)exp
−
iE
n
t
¯h
with ¯h = h/(2π ) (8.43)
The first factor ψ
n
(x, y, z, ) depends on the space coordinates only while the
second factor gives the time dependency. The parameter h is Planck’s constant.
Multiplication of the wave function by its conjugate yields
∗
n
n
= ψ
∗
n
ψ
n
(8.44)
which indicates that the probability density is constant in time or stationary in the
sense that no changes at all are taking place with respect to the external surroundings.
Thus an eigenstate is also a stationary state. In this situation the system does not
radiate.

290 Absorption by gases
(ii) Coherent states
Suppose the system is in the process of changing from eigenstate
1
to
2
. During
the transition the state function is a linear combination of the two state functions
as shown in
= C
1
ψ
1
exp
−
iE
1
t
¯h
+ C
2
ψ
2
exp
−
iE
2
t
¯h
(8.45)
The time variation of the parameters C
1
and C
2
is slow in comparison with
the time variation of the exponential factors. A state of the type (8.45) is called
a coherent state. Inspection of this formula shows that the energy of a coherent
state is not well defined since two energies are involved. In contrast to the coherent
state, the energy of a stationary state is well defined. The probability density of the
coherent state is given by
∗
= C
∗
1
C
1
ψ
∗
1
ψ
1
+ C
∗
2
C
2
ψ
∗
2
ψ
2
+ C
∗
1
C
2
ψ
∗
1
ψ
2
exp(iωt)
+ C
∗
2
C
1
ψ
∗
2
ψ
1
exp(−iωt)
with ω = 2πν = (E
1
− E
2
)/¯h
(8.46)
The quantum mechanical description of a radiating atom may be stated in the
following way. During the change from one quantum state to another, the probabil-
ity distribution of the electron becomes coherent and oscillates sinusoidally. This
oscillation is associated with an oscillating electromagnetic field which constitutes
the radiation.
8.3.2 The Schr¨odinger equation
So far we have not given any information in which way we might find the wave
function . From classical physics we know that the time-dependent wave equation
can be written in the form
∂
2
∂t
2
= v
2
∇
2
(8.47)
where represents any wave function. Substituting the trial solution
= ψ(x, y, z) exp(iωt) (8.48)
assuming the usual sinusoidal time dependency of into (8.47) we obtain the
time-independent wave equation
∇
2
ψ +
2π
λ
2
ψ = 0 (8.49)
where λ is the wavelength.
8.3 Some basic principles from quantum mechanics 291
In order to find Schr
¨
odinger’s equation, we make use of Einstein’s mass–energy
relation
hν = mc
2
(8.50)
where c is the speed of light in empty space and m is the mass of the photon. The
linear momentum of the photon is denoted by
p = mc =
hν
c
=
h
λ
(8.51)
Just as light exhibits both wavelike and particle properties, De Broglie assumed that
a material particle m moving with speed v, also possesses a wave-like character as
stated in
λ =
h
p
=
h
mv
(8.52)
This assumption was later confirmed experimentally.
Replacing in (8.49) the wavelength λ by means of (8.52) we find
∇
2
ψ +
2π p
h
2
ψ = 0 (8.53)
If E stands for the total energy of the particle as given by E = mv
2
/2 + V , V is
the potential energy, the linear momentum may be expressed by
p
2
= 2m(E − V ) (8.54)
Substitution of (8.54) into (8.53) yields the famous Schr¨odinger equation
∇
2
ψ +
8π
2
m
h
2
(E − V )ψ = 0
(8.55)
permitting us to find the wave function ψ. In case that the physical system consists
of N particles, (8.55) must be replaced by
N
k=1
1
m
k
∂
2
ψ
∂x
2
k
+
∂
2
ψ
∂y
2
k
+
∂
2
ψ
∂z
2
k
+
8π
2
h
2
(E − V )ψ = 0
(8.56)
if Cartesian coordinates are used.
To obtain the wave function ψ from the Schr¨odinger equation can be very dif-
ficult. The solution procedure requires the specification of the potential function
V (x, y, z) of the physical system. Not all mathematical solutions of the Schr¨odinger
equation are acceptable since they may not be physically meaningful. To be an
acceptable solution, the function ψ must tend to zero for infinite values of the
coordinates in such a way that it is quadratically integrable. This requirement leads
292 Absorption by gases
Table 8.1 Examples of quantum mechanical operators
Variable Operator
Position
xx
yy
zz
Linear momentum
p
x
¯h
i
∂
∂x
p
y
¯h
i
∂
∂y
p
z
¯h
i
∂
∂z
Kinetic energy
1
2
m
v
2
x
+ v
2
y
+ v
2
z
−
¯h
2
2m
∂
2
∂x
2
+
∂
2
∂y
2
+
∂
2
∂z
2
Potential energy
V (x, y, z) V (x, y, z)
Total energy
E −
¯h
i
∂
∂t
to the result that acceptable functions can exist only if the energy E has definite
values. These allowed values of E, known as eigenvalues, are characteristic energy
levels of the system. The corresponding solutions are the eigensolutions. Simple
examples will be given later.
In quantum mechanics every variable, such as position or momentum is associ-
ated with an operator. If one of these variables is denoted by g and the corresponding
operator by G, then the operation on the wave function of the system by G gives,
in some cases, the value for the variable g times ψ, i.e.
Gψ = gψ (8.57)
Examples of some quantum mechanical operators are given in Table 8.1.
It is often convenient to employ a form of the Schr¨odinger equation that makes
use of a formal analogy between classical and quantum mechanics. From classical
mechanics (see Appendix 8.8.1) we know that for a conservative dynamical system
the sum of the kinetic energy K and the potential energy V is equal to the constant
E, i.e.
H = K + V = E (8.58)
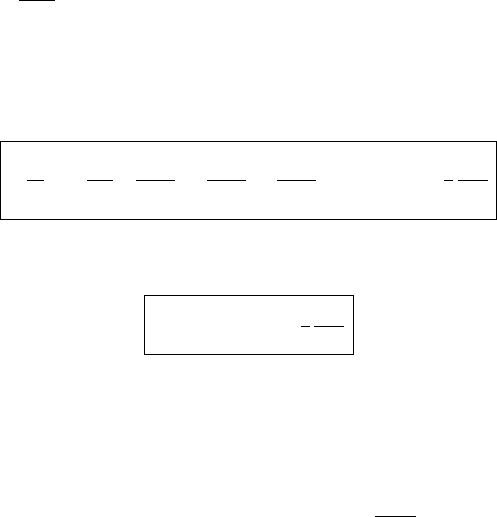
8.3 Some basic principles from quantum mechanics 293
The sum of K and V is called the Hamilton function H.If(x
k
, y
k
, z
k
) are the
coordinates of particle k and ( p
x
k
, p
y
k
, p
z
k
) the components of the linear momentum
of this particle, the Hamiltonian function H can be written in the form
H =
N
k=1
1
2m
k
p
2
x
k
+ p
2
y
k
+ p
2
z
k
+ V (x
1
, y
1
, z
1
,...x
k
, y
k
, z
k
,...) (8.59)
Replacing the momenta and E according to Table 8.1 and introducing the wave
function on which the operator is applied, we find the quantum mechanical
analogy. This gives the time-dependent Schr
¨
odinger equation
−
¯h
2
2
N
k=1
1
m
k
∂
2
∂x
2
k
+
∂
2
∂y
2
k
+
∂
2
∂z
2
k
+ V =−
¯h
i
∂
∂t
(8.60)
Utilizing the information listed in Table 8.1, the Schr¨odinger equation can also be
written as
H = E =−
¯h
i
∂
∂t
(8.61)
While we denote the Hamiltonian function by the symbol H we will use the
calligraphic print H to designate the analogous quantum mechanical Hamilton
operator.
The general solution to (8.61) is given by
=
n
a
n
n
=
n
a
n
ψ
n
exp
−
iE
n
t
¯h
(8.62)
In textbooks on quantum mechanics it is shown that any two eigenfunctions of an
atomic system belonging to different eigenvalues are orthogonal. Assuming that
the eigenfunctions are normalized we may write
∞
−∞
ψ
∗
m
ψ
n
dτ = δ
m,n
with dτ = dx dy dz (8.63)
If the wave function is normalized to 1, then the following relation must be valid
n
a
∗
n
a
n
=
n
|a
n
|
2
= 1 (8.64)
The latter equation implies that the product |a
n
|
2
= a
∗
n
a
n
represents the probability
of finding the system in a state of energy E
n
at time t.
In order to discuss radiation theory we need to find a suitable expression for the
Hamiltonian operator for a charged particle in an electromagnetic field. As before,
we begin our discussion with the classical Hamiltonian function.

294 Absorption by gases
8.3.3 Hamilton operator for a charged particle in an electromagnetic field
The reader may wish to refer to Appendix 2 of this chapter where we have briefly
summarized those relationships from electromagnetic theory which are needed
later. The interaction of a charged particle of mass m with an electromagnetic field
is described by the well-known Lorentz force equation. If E and B represent the
electric and the magnetic field vector, e and v the charge and the velocity of the
particle, then the Lorentz force equation can be written in the form
F = eE + ev × B
(8.65)
In deriving the classical Hamiltonian function it is more convenient to use the vector
potential A and the scalar potential φ rather than the field vectors themselves. The
basic relationships are
E =−∇φ −
∂A
∂t
, B =∇×A (8.66)
Hence the force equation assumes the form
F =−e
∂A
∂t
− e∇φ + ev × (∇×A) (8.67)
We will now briefly show that equation (8.67) can also be derived with the help
of Lagrange’s equation of motion if L is given by
L =
1
2
mv
2
+ e(v · A) −eφ
=
1
2
m(
˙
x
2
+
˙
y
2
+
˙
z
2
) + e(
˙
xA
x
+
˙
yA
y
+
˙
zA
z
) − eφ
(8.68)
where we have used Cartesian coordinates. The use of the Lagrangian equation
requires that we treat (x, y, z) and (
˙
x,
˙
y,
˙
z) as independent variables. For the x-
component we obtain
∂ L
∂x
= e
˙
x
∂ A
x
∂x
+
˙
y
∂ A
y
∂x
+
˙
z
∂ A
z
∂x
− e
∂φ
∂x
∂ L
∂
˙
x
= m
˙
x + eA
x
= p
x
(8.69)
so that Lagrange’s equation of motion can be generalized to
d
dt
(mv + eA) =∇L (8.70)
Using the vector identity
v × (∇×A) = (∇A) · v − v · (∇A) (8.71)
